Abstract
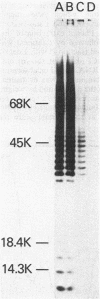
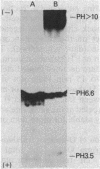
The repeating pentasaccharide of O-antigen from Escherichia coli O111 contains galactose, glucose, N-acetylglucosamine, and colitose, the latter representing the major antigenic determinant. Phenol extraction of this strain was previously shown to release two fractions (I and II) containing O-antigen carbohydrate, and both fractions were believed to be lipopolysaccharide. We have now characterized fractions I and II and conclude that only fraction II represents lipopolysaccharide. Fraction II contains phosphate, 2-keto-3-deoxyoctonate, beta-hydroxymyristic acid, and potent endotoxin activity, whereas fraction I was deficient in all of these properties of the lipid A and core oligosaccharide regions of lipopolysaccharide. Fractions I and II each represented 50% of the total cellular O-antigen, and both were present on the cell surface. Both fractions were metabolically stable, and no precursor-product relationship existed between them. Fraction II had a number-average molecular weight of 15,800, corresponding to an average of 12 O-antigen repeats per molecule. In contrast, fraction I had a number-average molecular weight of 354,000, corresponding to an average of 404 O-antigen repeats per molecule. Before heat treatment, cells of E. coli O111 are poorly agglutinated by O-serum; although this indicates the presence of a capsule, the corresponding K-antigen was never detected. We conclude that fraction I, when present on the cell surface, inhibits agglutination of unheated cultures of E. coli O111 by O-serum because: (i) a variant strain which lacks fraction I was agglutinated by O-serum without prior heating; (ii) erythrocytes coated with purified fraction I behaved like bacteria containing fraction I in showing inhibition of O-serum agglutination; and (iii) heat treatment released fraction I and rendered bacterial cells agglutinable in O-serum.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANACKER R. L., FINKELSTEIN R. A., HASKINS W. T., LANDY M., MILNER K. C., RIBI E., STASHAK P. W. ORIGIN AND PROPERTIES OF NATURALLY OCCURRING HAPTEN FROM ESCHERICHIA COLI. J Bacteriol. 1964 Dec;88:1705–1720. doi: 10.1128/jb.88.6.1705-1720.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beher M., Pugsley A., Schnaitman C. Correlation between the expression of an Escherichia coli cell surface protein and the ability of the protein to bind to lipopolysaccharide. J Bacteriol. 1980 Jul;143(1):403–410. doi: 10.1128/jb.143.1.403-410.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CYNKIN M. A., ASHWELL G. Estimation of 3-deoxy sugars by means of the malonaldehyde-thiobarbituric acid reaction. Nature. 1960 Apr 9;186:155–156. doi: 10.1038/186155a0. [DOI] [PubMed] [Google Scholar]
- Coleman W. G., Jr, Goebel P. J., Leive L. Genetic analysis of Escherichia coli O111:B4, a strain of medical and biochemical interest. J Bacteriol. 1977 May;130(2):656–660. doi: 10.1128/jb.130.2.656-660.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELBEIN A. D., HEATH E. C. THE BIOSYNTHESIS OF CELL WALL LIPOPOLYSACCHARIDE IN ESCHERICHIA COLI. I. THE BIOCHEMICAL PROPERTIES OF A URIDINE DIPHOSPHATE GALACTOSE 4-EPIMERASELESS MUTANT. J Biol Chem. 1965 May;240:1919–1925. [PubMed] [Google Scholar]
- Edstrom R. D., Heath E. C. The biosynthesis of cell wall lipopolysaccharide in Escherichia coli. VII. Studies on the structure of the O-antigenic polysaccharide. J Biol Chem. 1967 Sep 25;242(18):4125–4133. [PubMed] [Google Scholar]
- Goldman R. C., Leive L. Heterogeneity of antigenic-side-chain length in lipopolysaccharide from Escherichia coli 0111 and Salmonella typhimurium LT2. Eur J Biochem. 1980;107(1):145–153. doi: 10.1111/j.1432-1033.1980.tb04635.x. [DOI] [PubMed] [Google Scholar]
- Goldman R. C., White D., Leive L. Identification of outer membrane proteins, including known lymphocyte mitogens, as the endotoxin protein of Escherichia coli 0111. J Immunol. 1981 Oct;127(4):1290–1294. [PubMed] [Google Scholar]
- Gotschlich E. C., Fraser B. A., Nishimura O., Robbins J. B., Liu T. Y. Lipid on capsular polysaccharides of gram-negative bacteria. J Biol Chem. 1981 Sep 10;256(17):8915–8921. [PubMed] [Google Scholar]
- Hochstein H. D., Seligmann E. B., Jr, Marquina R. E., Rivera E. Limulus amebocyte lysate testing of nomral serum albumin (human) in the United States since 1975. Dev Biol Stand. 1979;44:35–42. [PubMed] [Google Scholar]
- Jann B., Jann K., Schmidt G., Orskov I., Orskov F. Immunochemical studies of polysaccharide surface antigens of Escherichia coli 0100:K?(B):H2. Eur J Biochem. 1970 Jul;15(1):29–39. doi: 10.1111/j.1432-1033.1970.tb00972.x. [DOI] [PubMed] [Google Scholar]
- Jann B., Reske K., Jann K. Heterogeneity of lipopolysaccharides. Analysis of polysaccharide chain lengths by sodium dodecylsulfate-polyacrylamide gel electrophoresis. Eur J Biochem. 1975 Dec 1;60(1):239–246. doi: 10.1111/j.1432-1033.1975.tb20996.x. [DOI] [PubMed] [Google Scholar]
- Jansson P. E., Lindberg A. A., Lindberg B., Wollin R. Structural studies on the hexose region of the core in lipopolysaccharides from Enterobacteriaceae. Eur J Biochem. 1981 Apr;115(3):571–577. doi: 10.1111/j.1432-1033.1981.tb06241.x. [DOI] [PubMed] [Google Scholar]
- Jansson P. E., Lindberg B., Lindberg A. A., Wollin R. Structural studies on the hexose region of the Enterobacteriaceae type R3 core polysaccharide. Carbohydr Res. 1979 Feb;68(2):385–389. doi: 10.1016/s0008-6215(00)83786-6. [DOI] [PubMed] [Google Scholar]
- KAUFFMANN F., DUPONT A. Escherichia strains from infantile epidemic gastro enteritis. Acta Pathol Microbiol Scand. 1950;27(4):552–564. doi: 10.1111/j.1699-0463.1950.tb04927.x. [DOI] [PubMed] [Google Scholar]
- Kent J. L., Osborn M. J. Further studies on enzymatic synthesis of O-antigen in Salmonella typhimurium. Biochemistry. 1968 Dec;7(12):4409–4419. doi: 10.1021/bi00852a037. [DOI] [PubMed] [Google Scholar]
- Kent J. L., Osborn M. J. Haptenic O-antigen as a polymeric intermediate of in vivo synthesis of lipopolysaccharide by Salmonella typhimurium. Biochemistry. 1968 Dec;7(12):4419–4422. doi: 10.1021/bi00852a038. [DOI] [PubMed] [Google Scholar]
- Kent J. L., Osborn M. J. Properties of the O-specific hapten formed in vivo by mutant strains of Salmonella typhimurium. Biochemistry. 1968 Dec;7(12):4396–4408. doi: 10.1021/bi00852a036. [DOI] [PubMed] [Google Scholar]
- Koplow J., Goldfine H. Alterations in the outer membrane of the cell envelope of heptose-deficient mutants of Escherichia coli. J Bacteriol. 1974 Feb;117(2):527–543. doi: 10.1128/jb.117.2.527-543.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANDY M., CEPPELLINI R. Production of O inagglutinability in erythrocytes coated with typhoid Vi and O antigens. Nature. 1955 Dec 31;176(4496):1266–1267. doi: 10.1038/1761266a0. [DOI] [PubMed] [Google Scholar]
- Leive L., Shovlin V. K., Mergenhagen S. E. Physical, chemical, and immunological properties of lipopolysaccharide released from Escherichia coli by ethylenediaminetetraacetate. J Biol Chem. 1968 Dec 25;243(24):6384–6391. [PubMed] [Google Scholar]
- Levy S. B., Leive L. Release from Escherichia coli of a galactosyltransferase complex active in lipopolysaccharide synthesis. J Biol Chem. 1970 Feb 10;245(3):585–594. [PubMed] [Google Scholar]
- Lewis M. S., Krieg L. C., Kirk W. D. The molecular weight and detergent binding of bovine rhodopsin. Exp Eye Res. 1974 Jan;18(1):29–40. doi: 10.1016/0014-4835(74)90041-4. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Leive L. Fractions of lipopolysaccharide from Escherichia coli O111:B4 prepared by two extraction procedures. J Biol Chem. 1975 Apr 25;250(8):2911–2919. [PubMed] [Google Scholar]
- NEU H. C., HEPPEL L. A. THE RELEASE OF RIBONUCLEASE INTO THE MEDIUM WHEN ESCHERICHIA COLI CELLS ARE CONVERTED TO SPEROPLASTS. J Biol Chem. 1964 Nov;239:3893–3900. [PubMed] [Google Scholar]
- Orskov I., Orskov F., Jann B., Jann K. Serology, chemistry, and genetics of O and K antigens of Escherichia coli. Bacteriol Rev. 1977 Sep;41(3):667–710. doi: 10.1128/br.41.3.667-710.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Rick P. D., Fung L. W., Ho C., Osborn M. J. Lipid A mutants of Salmonella typhimurium. Purification and characterization of a lipid A precursor produced by a mutant in 3-deoxy-D-mannooctulosonate-8-phosphate synthetase. J Biol Chem. 1977 Jul 25;252(14):4904–4912. [PubMed] [Google Scholar]
- SCHEIDEGGER J. J. Une micro-méthode de l'immuno-electrophorèse. Int Arch Allergy Appl Immunol. 1955;7(2):103–110. [PubMed] [Google Scholar]
- Schweizer M., Hindennach I., Garten W., Henning U. Major proteins of the Escherichia coli outer cell envelope membrane. Interaction of protein II with lipopolysaccharide. Eur J Biochem. 1978 Jan 2;82(1):211–217. doi: 10.1111/j.1432-1033.1978.tb12013.x. [DOI] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]