Abstract
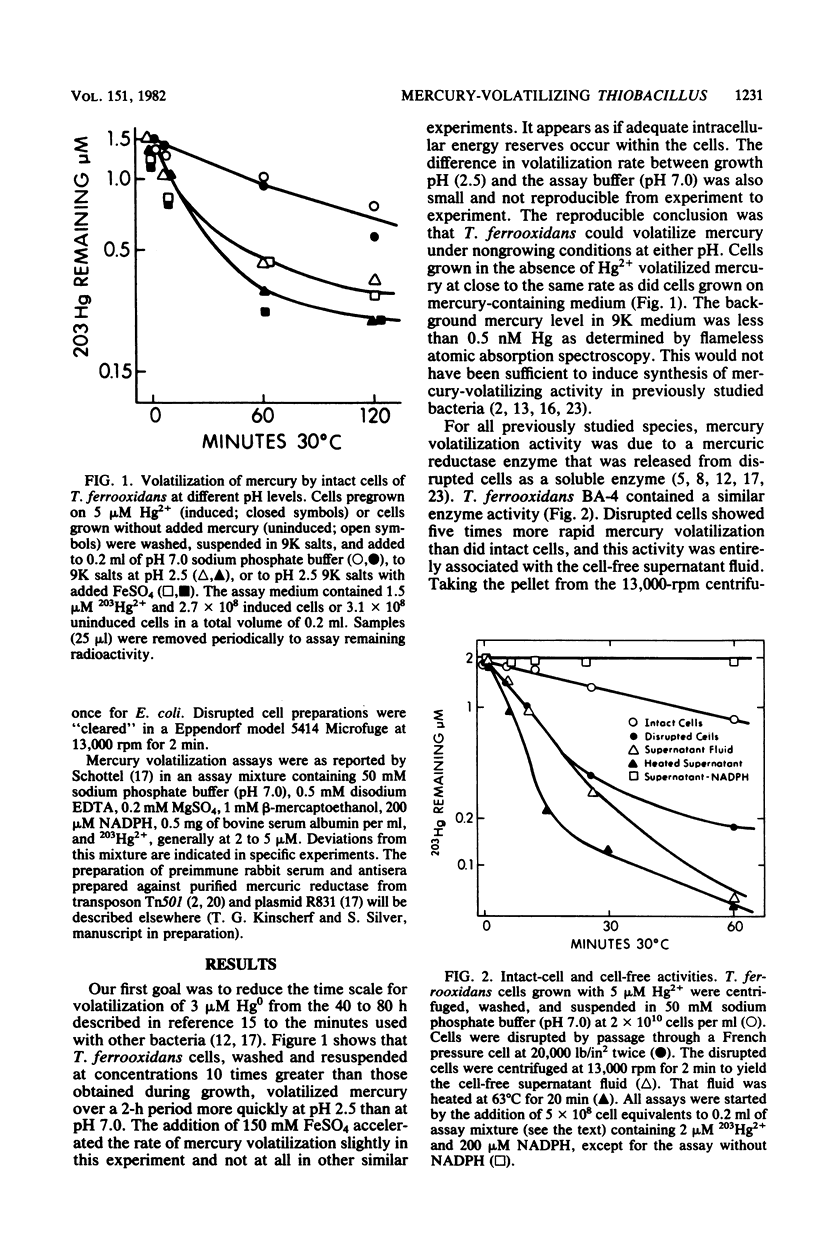
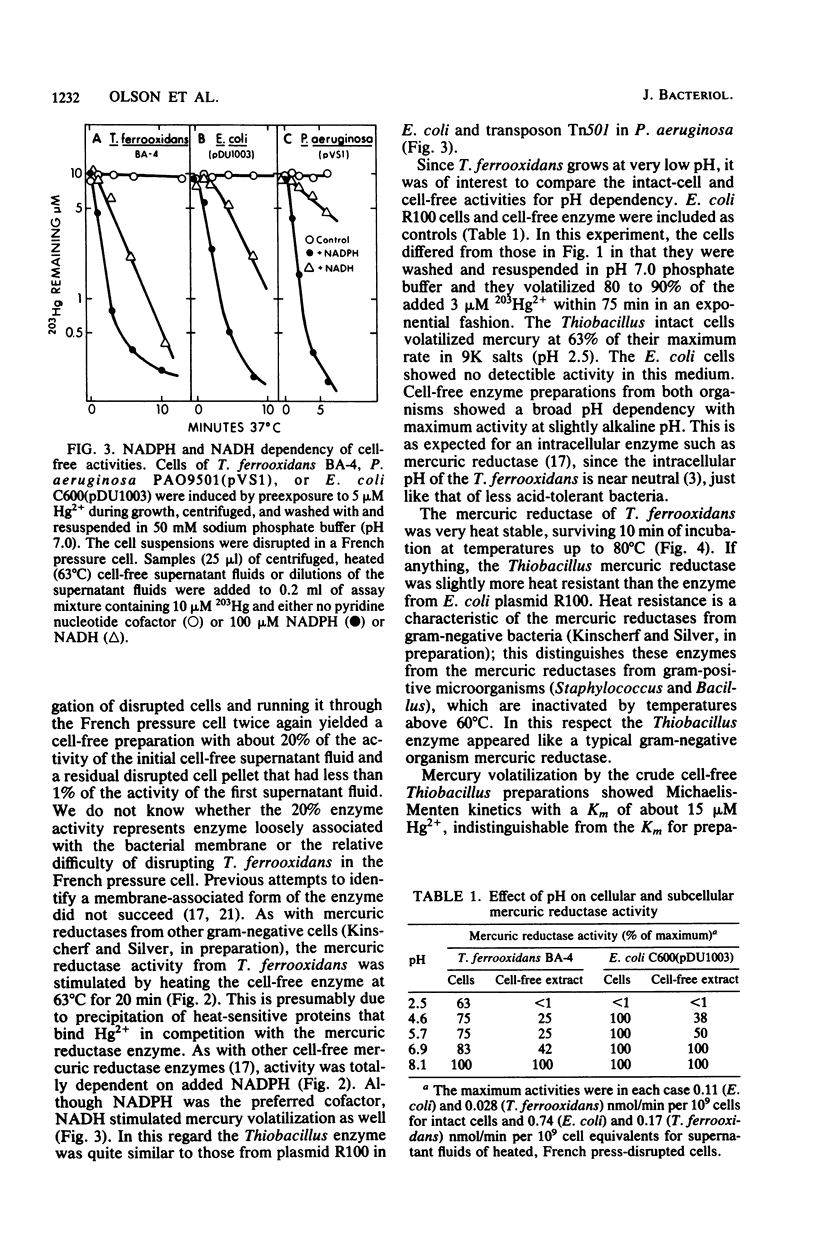
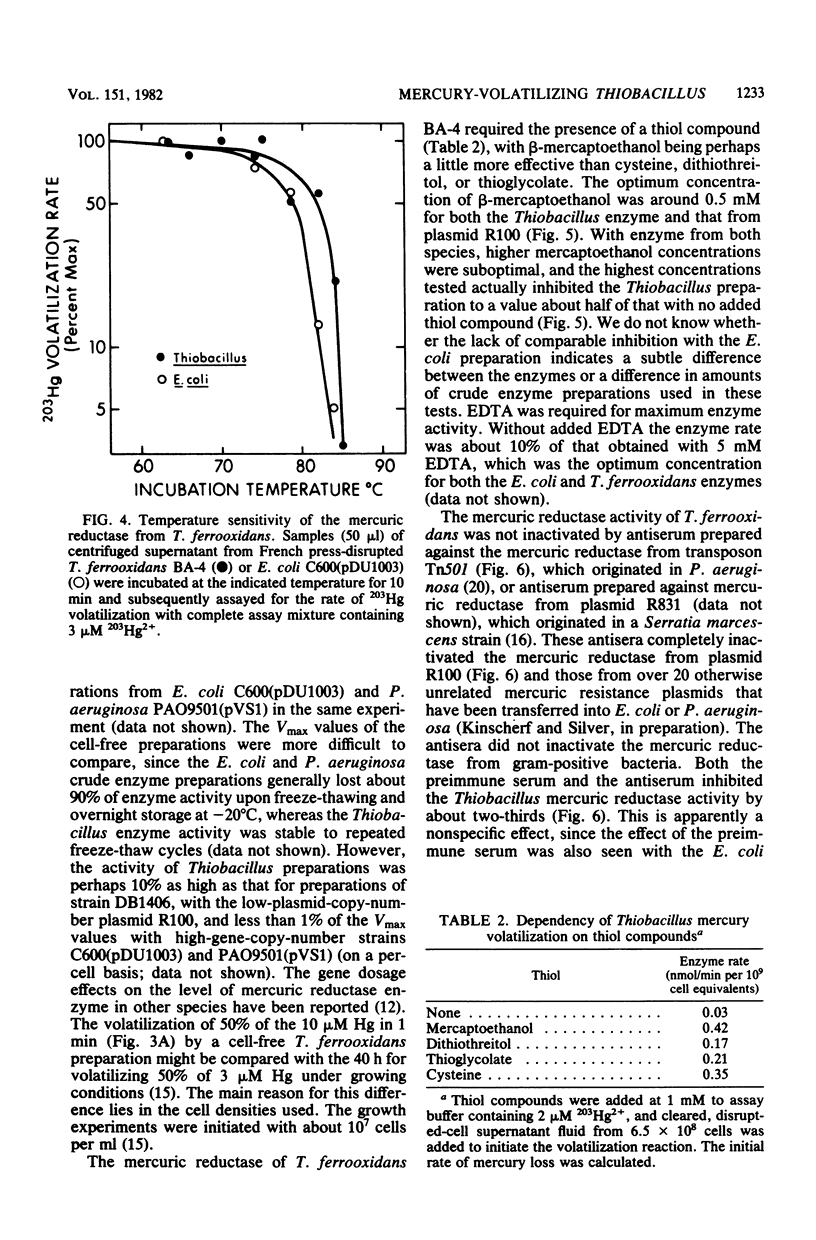
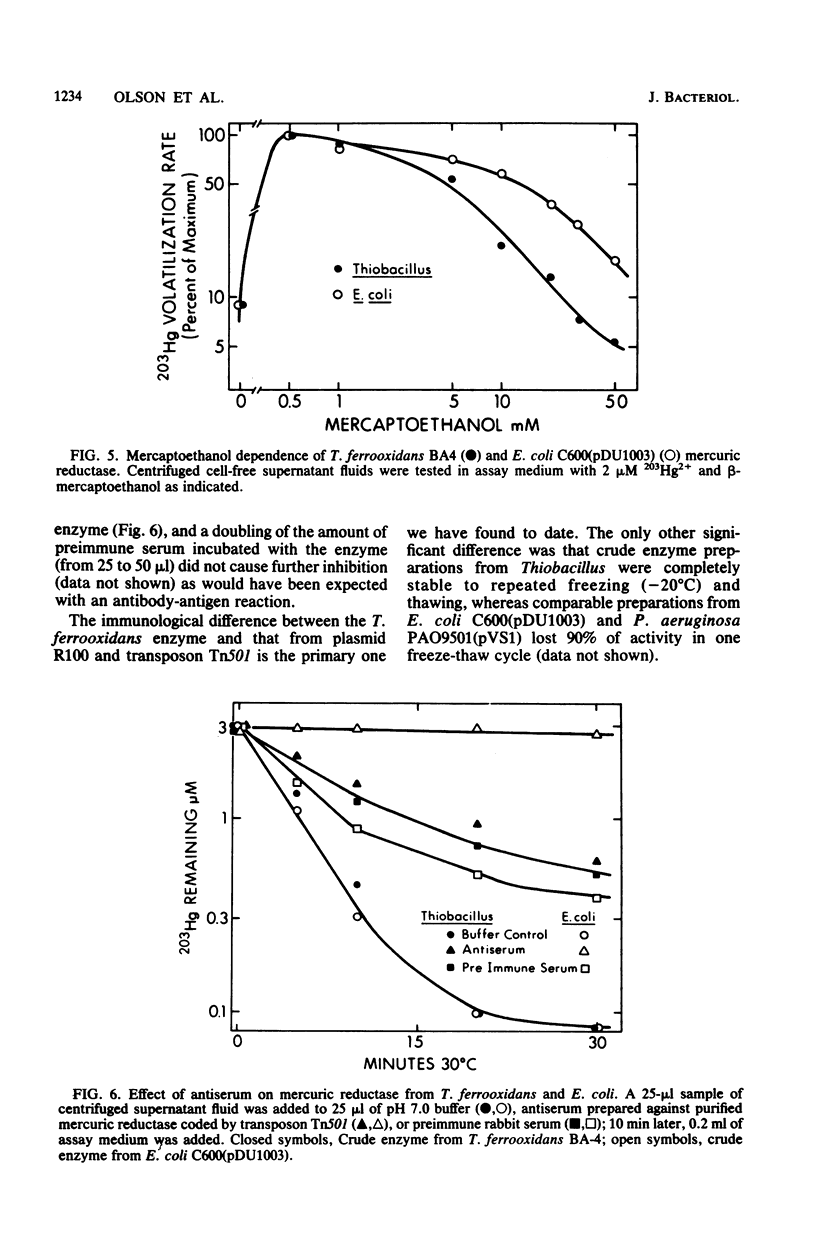
Cell-free mercury volatilization activity (mercuric reductase) was obtained from a mercury-volatilizing Thiobacillus ferrooxidans strain, and the properties of intact-cell and cell-free activities were compared with those determined by plasmid R100 in Escherichia coli. Intact cells of T. ferrooxidans volatilized mercury at pH 2.5, whereas cells of E. coli did not. Cell-free enzyme preparations from both bacteria functioned best at or above neutral pH and not at all at pH 2.5. The T. ferrooxidans mercuric reductase was a soluble enzyme that was dependent upon added NAD(P)H. The enzyme activity was stable at 80 degrees C, required an added thiol compound, and was stimulated by EDTA. Antisera against purified mercuric reductases from transposon Tn501 and plasmid R831 (which inactivated mercuric reductases from a wide range of enteric and pseudomonad strains) did not inactivate the enzyme from T. ferrooxidans.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brierley C. L. Bacterial leaching. CRC Crit Rev Microbiol. 1978;6(3):207–26I. doi: 10.3109/10408417809090623. [DOI] [PubMed] [Google Scholar]
- Cox J. C., Nicholls D. G., Ingledew W. J. Transmembrane electrical potential and transmembrane pH gradient in the acidophile Thiobacillus ferro-oxidans. Biochem J. 1979 Jan 15;178(1):195–200. doi: 10.1042/bj1780195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox B., Walsh C. T. Mercuric reductase. Purification and characterization of a transposon-encoded flavoprotein containing an oxidation-reduction-active disulfide. J Biol Chem. 1982 Mar 10;257(5):2498–2503. [PubMed] [Google Scholar]
- Hoffman L. E., Hendrix J. L. Inhibition of Thiobacillus ferrooxidans by soluble silver. Biotechnol Bioeng. 1976 Aug;18(8):1161–1165. doi: 10.1002/bit.260180811. [DOI] [PubMed] [Google Scholar]
- Izaki K., Tashiro Y., Funaba T. Mechanism of mercuric chloride resistance in microorganisms. 3. Purification and properties of a mercuric ion reducing enzyme from Escherichia coli bearing R factor. J Biochem. 1974 Mar;75(3):591–599. doi: 10.1093/oxfordjournals.jbchem.a130427. [DOI] [PubMed] [Google Scholar]
- Martin P. A., Dugan P. R., Tuovinen O. H. Plasmid DNA in acidophilic, chemolithotrophic thiobacilli. Can J Microbiol. 1981 Aug;27(8):850–853. doi: 10.1139/m81-133. [DOI] [PubMed] [Google Scholar]
- Nakahara H., Kinscherf T. G., Silver S., Miki T., Easton A. M., Rownd R. H. Gene copy number effects in the mer operon of plasmid NR1. J Bacteriol. 1979 Apr;138(1):284–287. doi: 10.1128/jb.138.1.284-287.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILVERMAN M. P., LUNDGREN D. G. Studies on the chemoautotrophic iron bacterium Ferrobacillus ferrooxidans. I. An improved medium and a harvesting procedure for securing high cell yields. J Bacteriol. 1959 May;77(5):642–647. doi: 10.1128/jb.77.5.642-647.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai A., Yanagisawa M., Imai Y., Konno S., Ishikawa S. [Surgical treatment of systemic atrioventricular valve insufficiency in corrected transposition]. Nihon Kyobu Geka Gakkai Zasshi. 1975 Nov;23(11):1354–1359. [PubMed] [Google Scholar]
- Schottel J. L. The mercuric and organomercurial detoxifying enzymes from a plasmid-bearing strain of Escherichia coli. J Biol Chem. 1978 Jun 25;253(12):4341–4349. [PubMed] [Google Scholar]
- Schottel J., Mandal A., Clark D., Silver S., Hedges R. W. Volatilisation of mercury and organomercurials determined by inducible R-factor systems in enteric bacteria. Nature. 1974 Sep 27;251(5473):335–337. doi: 10.1038/251335a0. [DOI] [PubMed] [Google Scholar]
- Stanisich V. A., Bennett P. M., Richmond M. H. Characterization of a translocation unit encoding resistance to mercuric ions that occurs on a nonconjugative plasmid in Pseudomonas aeruginosa. J Bacteriol. 1977 Mar;129(3):1227–1233. doi: 10.1128/jb.129.3.1227-1233.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers A. O., Silver S. Microbial transformations of metals. Annu Rev Microbiol. 1978;32:637–672. doi: 10.1146/annurev.mi.32.100178.003225. [DOI] [PubMed] [Google Scholar]
- Tuovinen O. H., Niemelä S. I., Gyllenberg H. G. Tolerance of Thiobacillus ferrooxidans to some metals. Antonie Van Leeuwenhoek. 1971;37(4):489–496. doi: 10.1007/BF02218519. [DOI] [PubMed] [Google Scholar]
- Weiss A. A., Murphy S. D., Silver S. Mercury and organomercurial resistances determined by plasmids in Staphylococcus aureus. J Bacteriol. 1977 Oct;132(1):197–208. doi: 10.1128/jb.132.1.197-208.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]



