Abstract
Different species maintain a particular body orientation in space (upright in humans, dorsal-side-up in quadrupeds, fish and lamprey) due to the activity of a closed-loop postural control system. We will discuss operation of spinal and supraspinal postural networks studied in a lower vertebrate (lamprey) and in two mammals (rabbit and cat).
In the lamprey, the postural control system is driven by vestibular input. The key role in the postural network belongs to the reticulospinal (RS) neurons. Due to vestibular input, deviation from the stabilized body orientation in any (roll, pitch, yaw) plane leads to generation of RS commands, which are sent to the spinal cord and cause postural correction. For each of the planes, there are two groups of RS neurons responding to rotation in the opposite directions; they cause a turn opposite to the initial one. The command transmitted by an individual RS neuron causes the motor response, which contributes to the correction of posture. In each plane, the postural system stabilizes the orientation at which the antagonistic vestibular reflexes compensate for each other. Thus, in lamprey the supraspinal networks play a crucial role in stabilization of body orientation, and the function of the spinal networks is transformation of supraspinal commands into the motor pattern of postural corrections.
In terrestrial quadrupeds, the postural system stabilizing the trunk orientation in the transversal plane was analyzed. It consists of two relatively independent sub-systems stabilizing orientation of the anterior and posterior parts of the trunk. They are driven by somatosensory input from limb mechanoreceptors. Each sub-system consists of two closed-loop mechanisms – spinal and spino-supraspinal. Operation of the supraspinal networks was studied by recording the posture-related activity of corticospinal neurons. The postural capacity of spinal networks was evaluated in animals with lesions to the spinal cord. Relative contribution of spinal and supraspinal mechanisms to the stabilization of trunk orientation is discussed.
1. Introduction
Different species maintain a particular body orientation in space (upright in humans, dorsal-side-up in quadrupeds, fish and lamprey) due to the activity of a closed-loop postural control system. This system responds to perturbations of body orientation, which are monitored by various sensory inputs, and causes corrections of posture (Deliagina et al., 2006a; Deliagina and Orlovsky, 2002; Horak and Macpherson, 1996). During locomotion, the postural system closely interacts with the locomotor system (Orlovsky et al., 1999; Zelenin et al., 2003).
In contrast to the locomotor system, which has been analyzed in considerable detail in a number of species (see, e.g., this volume), progress in studies of postural mechanisms, and especially in studies of the corresponding networks, is much slower mainly because of methodological problems. A traditional way to study complex neural mechanisms is to subdivide them into a number of smaller neuronal networks, each of which retains its normal function. This method is widely used to analyze central pattern generators, the networks capable of rhythmogenesis when isolated (Orlovsky et al., 1999). It is difficult to apply this method, however, to the closed-loop postural system, which needs the integrity of the brainstem and spinal networks, as well as the presence of sensory feedback for its normal function.
In this review, some data will be presented to demonstrate how these difficulties can be overcome by using different animal models (Deliagina and Orlovsky, 2002) and specially developed techniques. We will discuss operation of spinal and supraspinal postural networks studied in a lower vertebrate (lamprey) and in two mammals (rabbit and cat).
2. Postural control in lamprey
The lamprey swims due to the lateral body undulations that propagate in the rostro-caudal direction (Grillner and Kashin, 1976). During stationary swimming, orientation of the lamprey in the sagittal (pitch) and transversal (roll) planes (Fig. 1A) is stabilized by closed-loop control mechanisms driven by vestibular input (Deliagina et al., 1992a,b; Deliagina and Fagerstedt, 2000; Pavlova and Deliagina, 2002). Vestibular-driven mechanisms also contribute to stabilization of the direction of swimming in the horizontal (yaw) plane (Karayannidou et al., 2005). Any deviations from the stabilized body orientation are reflected in vestibular signals (Deliagina et al., 1992b), which cause corrective motor responses. In the pitch and yaw planes, the corrections occur due to the body bending in the corresponding plane (Fig. 1A, Pitch and Yaw) (McClellan and Hagevik, 1997; Ullén et al., 1995). In the roll plane, the corrections occur due to a change of the direction of locomotor body undulations, from lateral to oblique (Fig. 1A, Roll) (Zelenin et al., 2003). These motor responses are caused by four motoneuron pools in each segment that innervate the dorsal and ventral parts of a myotome on the two sides, respectively (Fig. 1B) (Tretjakoff, 1927; Wannier et al., 1998).
Figure 1.
(A) During regular swimming, the lamprey stabilizes its orientation in the sagittal (pitch) plane, transversal (roll) plane, and horizontal (yaw) plane. Deviations from the stabilized orientation in these planes (angles α, β, and γ, respectively) evoke corrective motor responses (large arrows) aimed at restoration of the initial orientation. (B) Commands for correcting the orientation are formed on the basis of vestibular information, and transmitted from the brainstem to the spinal cord by reticulospinal (RS) neurons; many RS axons reach the most caudal spinal segments. Motor output of each segment is generated by four motoneuron (MN) pools controlling the dorsal and ventral parts of a myotome on the two sides (d and v pools). (C–H) Roll and pitch control systems. Key elements of each system are two groups of RS neurons. Due to vestibular inputs, activities of these two antagonistic groups are position-dependent; they cause rotation of the lamprey in opposite directions (arrows). Each system normally stabilizes the orientation with equal activities of the two groups (D,G). However, the stabilized orientation (equilibrium point) can be changed by a tonic drive to one of the groups (E,H).
In the lamprey, commands for changing the body orientation are transmitted from the brainstem to the spinal cord mainly by reticulospinal (RS) neurons (Fig. 1B) (Brodin et al., 1988; Bussières, 1994; Deliagina et al., 2002; Ronan, 1989). These neurons constitute an essential part of the supraspinal postural network. Due to vestibular inputs (Fig. 1B), RS neurons respond to rotation in different planes in both the whole animal and in vitro preparation (Deliagina et al., 1992a; Deliagina and Fagerstedt, 2000). In each of the main planes (pitch, roll, yaw), there are two antagonistic groups of RS neurons responding to rotation in opposite directions (Deliagina and Fagerstedt, 2000; Deliagina et al., 1992a, 2006a; Karayannidou et al., 2005; Pavlova and Deliagina, 2002). These groups are shown in Figs. 1C and 1F for the roll and pitch control systems, and their tilt-related activities are presented in Figs. 1D and 1G, respectively. Thus, an important function of the supraspinal postural network is formation of the commands for postural corrections addressed to the spinal postural network.
The spinal postural network transforms RS commands into the output motor pattern of postural corrections. Until recently, there were no experimental data on the motor effects of RS neurons involved specifically in the roll, pitch or yaw control. To explain operation of these control systems, a hypothesis was advanced that each of the two groups of RS neurons (activated by rotation in a particular plane but in opposite directions), through the spinal network, causes rotation of the animal in the direction opposite to the initial turn (which activated the neurons), and the system will thus stabilize the orientation with equal activities of the two antagonistic groups (Deliagina et al., 1993, 2006a; Zelenin et al., 2000) (Equilibrium point in Fig. 1D,G). Normally, this occurs at the dorsal-side-up and horizontal orientation of the body in roll and pitch planes, correspondingly. However, the stabilized orientation in the lamprey can be gradually changed under the effect of some environmental factors. Unilateral eye illumination affects differently the two antagonistic groups of RS neurons of the roll control system and causes a shift of the equilibrium point, which results in a change of stabilized orientation (Fig. 1E) (Deliagina and Fagerstedt, 2000; Deliagina et al., 1993; Deliagina and Pavlova, 2002). In the pitch control system, the stabilized orientation can be changed by raising the water temperature, which affects differently the two groups and thus shifts the equilibrium point towards the nose-down orientation (Fig. 1H) (Pavlova and Deliagina, 2002).
The hypothesis that RS neurons of the roll control system cause rotation of the animal in the roll plane was supported by experiments on the neuro-mechaanical model (Zelenin et al., 2000). The model was driven by activity of corresponding antagonistic groups of RS neurons and was able to stabilize the body orientation of lamprey in roll plane as well as reproduce the behavioural fenomena caused by the shift of the equilibrium point.
Recently, we developed a technique which allowed to characterize both vestibular inputs and motor effects of individual RS neurons, and thus to correlate the characteristics of supraspinal and spinal postural networks (Zelenin et al., 2005). In the preparation consisting of the brainstem, spinal cord and vestibular organs, the activity of the RS neuron was recorded from its axon in the spinal cord, whereas the brainstem and vestibular organs were rotated in different planes to determine the control system (roll, pitch, yaw) to which the neuron belongs. Afterwards, the RS neuron was stimulated and its effects on motor output of the spinal cord were detected by means of the spike-triggered averaging technique (Fetz and Cheney, 1980). The effects were found to be similar along the whole extent of the axon (Zelenin et al. 2001), and they could be characterized by a combination of influences on the four motoneuron pools in any segment (Fig. 1B).
The majority of RS neurons responded to rotation in only one of the three main planes. Such neuron is shown in Fig. 2A. It responded to contralateral roll tilt, and did not respond to rotation in the yaw and pitch planes. Motor effects of this neuron included activation of motoneurons projecting to the ipsi-ventral and contra-dorsal myotomes, and inhibition of motoneurons projecting to the ipsi-dorsal and contra-ventral myotomes. In the swimming lamprey, this pattern would lead to a change of the direction of locomotor body undulations, from lateral to oblique, and to a roll turn in the direction opposite to the initial turn (Fig. 1A, Roll).
Figure 2.
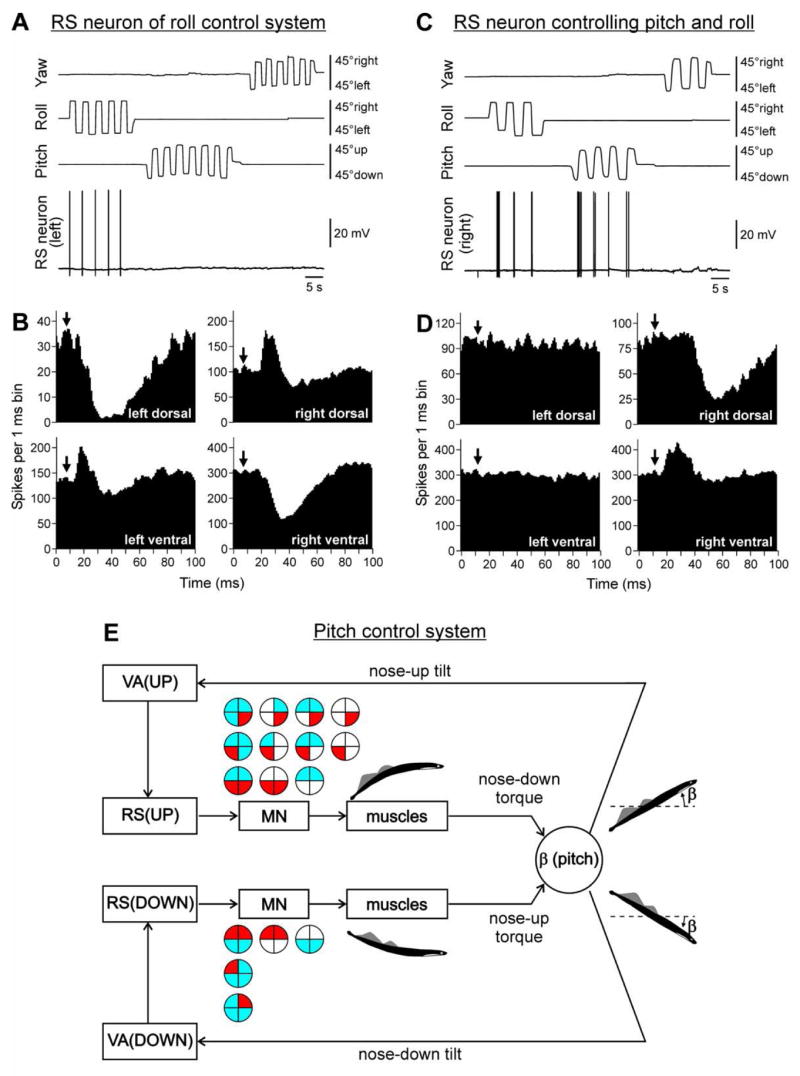
(A,B) An RS neuron that contributed only to stabilization of the roll angle. (A) The neuron fired spikes in response to right (contralateral) roll tilts only. (B) The neuron evoked excitation in the left (ipsilateral) ventral and right (contralateral) dorsal branches of the ventral roots and inhibition in the right ventral and left dorsal branches. Arrows indicate the time of arrival of the RS spike to segment 30 (where motor output was monitored). (C,D) An RS neuron that contributed to stabilization of both roll and pitch angles. (C) The neuron fired spikes in response to left (contralateral) roll tilts and nose-up pitch tilts. (D) The neuron evoked excitation in the ipsilateral ventral branch of the ventral root, and inhibition in the ipsilateral dorsal branch. In B,D, a post-RS-spike histogram was generated for the spikes of motoneurons recorded in the dorsal and ventral branches of the left and right ventral roots. The moment of RS spike occurrence at the stimulated site was taken as the origin of the time axis in the histogram. Typically, responses to a few thousand RS spikes (up to 20 min of stimulation at 10 Hz) were used for generation of a histogram. (E) Relationships between vestibular responses and motor effects in individual RS neurons of the pitch control system. The neurons were divided into RS(UP) and RS(DOWN) groups according to their inputs (vestibular responses). For each group, the patterns of motor effects in its neurons are shown as circle diagrams, with the quadrants representing the MN pools projecting to the corresponding parts of myotomes. Different colors designate the type of effect (excitation –red, inhibition – blue, no effect – white). Each RS neurons evoked a motor pattern (or a part of the pattern) opposing the initial turn that activated the neuron.
Some RS neurons responded to rotation in more than one plane. An example of such neuron is shown in Fig. 2C,D. This right RS neuron responded both to left (contralateral) roll tilts and to nose-up pitch tilts, but did not respond to tilts in the yaw plane. This neuron excited motoneurons projecting to the ipsi-ventral and inhibited motoneurons projecting to the ipsi-dorsal myotomes (Fig. 2D). Pitch tilts activate RS neurons on both sides of the nuclei. Thus, with rotation in nose-up direction, both right and left neurons of this type (each affecting only ipsilateral MNs) will be activated, and their collective effect will be bilateral excitation of the ventral MNs and inhibition of the dorsal MNs. In the swimming lamprey, this pattern would lead to a pitch turn in the direction opposite to the initial (nose-up) turn (Fig. 1A, Pitch). By contrast, tilting the body in the roll plane to the left will excite only the right RS neuron. That will lead to an increase of activity of the right ventral MNs and decrease of activity of the right dorsal MNs (Fig. 2D). During swimming, this RS neuron will contribute to a change of the direction of locomotor body undulations, from lateral to oblique, and to a roll turn in the direction opposite to the initial turn (Fig. 1A, Roll). It was found that the majority of RS neurons responding to rotation in more than one plane produced the motor pattern, which represented the common part of the patterns of postural corrections caused by tilting in corresponding planes.
In most RS neurons, a strong correlation between their vestibular inputs and motor effects was found. Usually, the neuron produced a motor pattern (or a part of the pattern) causing a torque, which would oppose the initial rotation that activated the neuron. Such closed-loop mechanisms, formed by individual neurons of a group, operate in parallel to generate the resulting motor responses (Fig. 2E).
Thus, the results of this study support the hypothesis (Deliagina et al., 1993, 2006a) suggesting a tight linkage between input and output characteristics of RS neurons. These results explain how individual RS neurons transform sensory information about the body orientation into the motor pattern that causes corrections of orientation.
To conclude, the spinal and supraspinal postural mechanisms in the lamprey have substantially different functions. The supraspinal mechanism generates commands for postural corrections on the basis of vestibular information about body orientation. It is also responsible for setting the equilibrium point of the postural systems. Sensory information of different modalities can affect the equilibrium point. The spinal mechanism does not receive sensory information about body orientation. Its function is transformation of supraspinal commands into the motor pattern of postural corrections.
3. Postural control in quadrupeds
3.1. Functional organization of postural system
We investigated the system maintaining the body orientation in the transversal plane in standing quadrupeds (cat, rabbit). This system usually operates as a functional unit and stabilizes both the head orientation and the trunk orientation. Lateral tilt of the supporting platform causes extension of the limbs on the side moving down and flexion of the limbs on the opposite side. Due to these limb movements, the body moves in relation to the platform, in the direction opposite to tilt, and the dorsal-side-up trunk orientation is stabilized (Fig. 3B,C). Simultaneously one can observe displacement of the dorso-ventral head axis toward the vertical. Under certain conditions, however, the system clearly dissociates into the subsystems controlling independently the head and the trunk (Barberini and Macpherson, 1998; Beloozerova et al., 2005; Berthoze and Pozzo, 1988; Boyle, 2001; Deliagina et al., 2000). These sub-systems are driven by sensory signals of different modalities: the head orientation is stabilized mainly on the basis of vestibular and visual information; for trunk stabilization, somatosensory inputs from limbs are most important (Beloozerova et al., 2003; Deliagina et al., 2000). In this respect, terrestrial animals strongly differ from aquatic ones, whose postural mechanisms are driven primarily by vestibular input (see section Postural control in lamprey).
Figure 3.
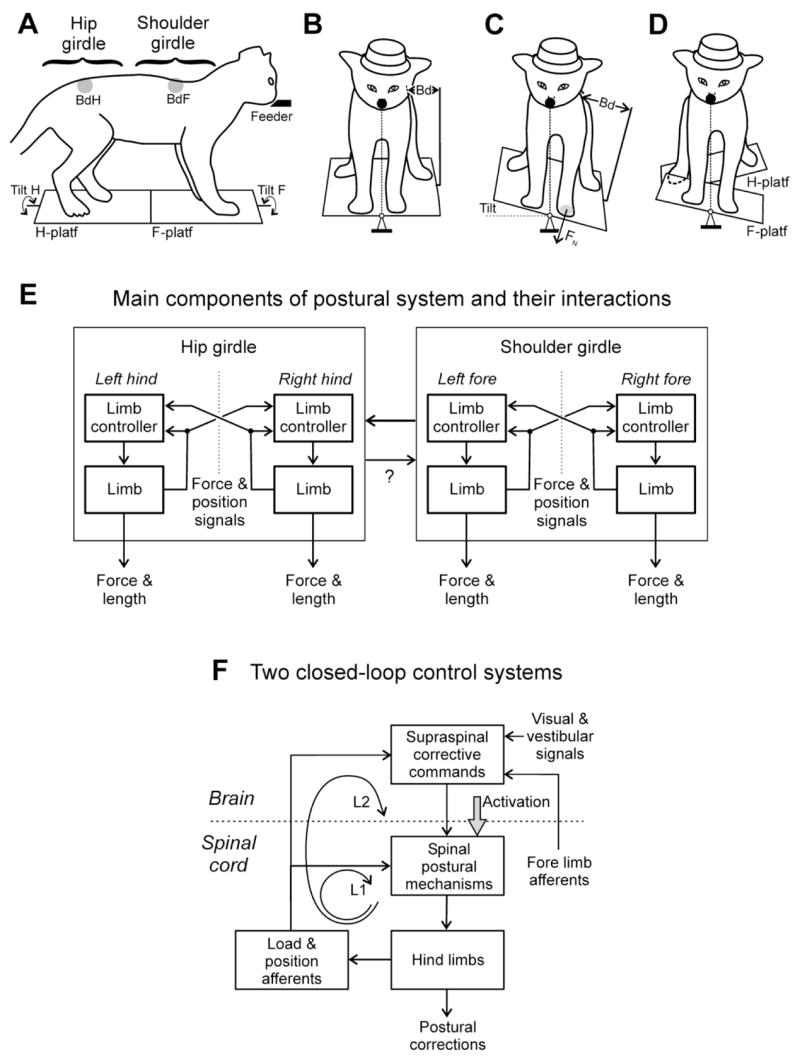
(A–D) Experimental design for testing postural responses to tilts in the cat. (A–C) the animal was standing on two platforms, one under the fore limbs and one under the hind limbs. Platforms could be tilted in the transverse plane (Tilt F and Tilt H) either in phase (C) or in anti-phase (D). Mechanical sensors BdF and BdH measured lateral displacements of the rostral and caudal parts of the trunk in relation to the corresponding platform. (E) Sensorimotor processing in the system stabilizing the back-up trunk orientation. The system consists of two sub-systems, one for the shoulder girdle and the other for the hip girdle (shown in A). They compensate for tilts of the anterior and posterior parts of the body, respectively. Each sub-system includes two controllers, one for the left limb and one for the right limb. Each limb controller contains a reflex mechanism driven by somatosensory input from its own limb. These local reflexes partly compensate for tilts. The limb controllers also receive somatosensory input from the contralateral limbs. The motor responses to these crossed influences are added to the local reflexes. The limb controllers exert influences on each other promoting their coordination. (F) Functional organization of the feedback mode of postural control in the hindquarters. Two closed-loop control systems (loops L1 and L2) stabilize the body orientation (see text for explanations).
In standing quadrupeds, each of the four limbs participates in supporting the body. When the animal’s posture is perturbed, each of the limbs contributes to the generation of a corrective motor response (Beloozerova et al., 2003; Jacobs and Macpherson, 1996; Lacquaniti et al., 1984; Macpherson, 1988a,b). To join the efforts of individual limbs, they must be accurately coordinated. One possible means for coordination was proposed by the hypothesis of a single regulated variable (see, e.g., Ghez, 1991; Massion, 1994; Massion et al., 1997). According to this idea, sensory information from individual limbs is processed and integrated to obtain a generalized characteristic of body posture, like a position of the centre of mass. With a deviation of this regulated variable from its desired value, specific commands are sent to individual limbs to elicit their coordinated movements.
It was found, however, that cats and rabbits are able to keep equilibrium in complicated postural tasks, when the anterior and posterior parts of the body are supported by two separate platforms tilted in anti-phase (Fig. 3D) (Beloozerova et al., 2003; Deliagina et al., 2006b). In this case, the centre of mass does not move, but postural corrections are present. To explain these results, it was suggested that the system stabilizing the trunk orientation in frontal plane in quadrupeds consists of two relatively independent sub-systems, stabilizing the anterior and posterior parts of the trunk, respectively. Each sub-system is driven by somatosensory input from corresponding limbs.
To characterize functional organization of sub-systems and their interactions, the experiments were carried out in which one, two, or three limbs were suspended and thus excluded from maintenance of body posture. By recording responses to tilt in such a “reduced” postural system, different components of the system were determined, and influences between them were evaluated (Fig. 3E) (Deliagina et al., 2006b). These experiments have shown that: (i) Each sub-system is capable to fully compensate for the lateral platform tilt when the limbs of another girdle do not participate in trunk stabilization. (ii) Coordination between the two sub-systems is based primarily on the influences of the forelimbs’ sub-system upon the hindlimbs’ sub-system. However, these influences do not necessarily determine the responses to tilt in the hindlimbs. In case of mismatch between the somatosensory input from hindlimbs and forelimbs, corrective movements are generated in response to somatosensory input from the hindlimbs. (iii) Each sub-system contains the mechanisms (limb controllers), which generate a part of the corrective movement of an individual limb in response to the tilt-related somatosensory information from the same limb. For the generation of corrective movement of full amplitude, the tilt-related somatosensory input from the contralateral limb of the girdle is necessary.
A similar functional organization, with semi-autonomous limb controllers influencing each other, was earlier suggested for the locomotor system of quadrupeds (von Holst, 1938; Orlovsky et al., 1999; Shik and Orlovsky, 1965). It seems likely that a control system consisting of semi-autonomous sub-systems better adapts to complicated environmental conditions (Gelfand and Zetlin, 1971).
3.2. Role of spinal and supraspinal mechanisms
Animals decerebrated at precollicular-premammillary level can stand, maintain equilibrium during locomotion, and generate postural corrections in response to lateral tilt of the supporting platform. When positioned on its side, the animal exhibits a set of righting reflexes and assumes the normal, dorsal-side-up posture (Bard and Macht, 1958; Magnus, 1924; Musienko et al., 2006). These findings indicate that an essential part of the nervous mechanisms responsible for the control of basic posture in quadrupeds is located below the decerebration level, that is, in the brain stem, cerebellum, and spinal cord.
Presumed interactions between the spinal and supraspinal levels of the postural system stabilizing trunk orientation are shown in Fig. 3F. For each of the girdles (shoulder and hip), there are two closed-loop nervous mechanisms (shown for the hindlimbs in Fig. 3F, see Lyalka et al., 2005). One of the mechanisms (loop L1) resides in the spinal cord. It is driven by input from limb mechanoreceptors, and contributes to compensation for postural disturbances by generating corrective motor responses. This mechanism is activated by tonic drive from some brain structures (Activation in Fig. 3F).
The other mechanism contributing to generation of postural corrections contains a “long” reflex loop (L2 in Fig. 3F) involving supraspinal centers. This mechanism receives sensory signals from hindlimb mechanoreceptors and, in addition, information about head orientation from visual and vestibular systems, as well as signals from forelimb mechanoreceptors. Output of this mechanism represents the phasic corrective signals, which are sent to the spinal cord via different descending pathways (reticulospinal, vestibulospinal, rubrospinal, corticospinal). These commands, along with spinal reflexes, contribute to corrections of posture.
A relative contribution of the spinal and supraspinal closed-loop mechanisms to the generation of postural corrections is not clear, however. On one hand, the animals with a complete transection of the spinal cord in the lower thoracic region exhibit very poor postural responses and, as a rule, are not able to maintain the dorsal-side-up orientation of their hindquarters (Macpherson et al., 1997; Macpherson and Fung, 1999). These results were interpreted as evidence to suggest a minor role for spinal reflexes (loop 1 in Fig. 3F) in postural control (Horak and Macpherson, 1996).
An alternative interpretation (Lyalka et al., 2005) is that spinal postural networks contribute significantly to generation of postural corrections. However, spinal cord transection deprives the networks of a necessary supraspinal tonic drive (Activation, Fig. 3F), which results in a dramatic reduction of their activity. Indirect evidence for this hypothesis was obtained in our studies on rabbits subjected to partial transection of the spinal cord (Lyalka et al., 2005). After ventral hemisection of the spinal cord (VHS) postural corrections were abolished and did not recover, suggesting that ventral descending pathways are crucial for postural control. By contrast, after the dorsal or lateral hemisection (DHS or LHS), rabbits exhibited a rapid recovery of postural corrections. Moreover, the temporal characteristics of their EMG patterns were similar to those in normal rabbits. Since these lesions obviously caused dramatic changes both in the ascending sensory signals and in the descending motor commands, it would be very difficult to explain the persistence of the principal features of postural responses in DHS and LHS animals by the operation of heavily damaged long-loop mechanisms (loop L2 in Fig. 3F). It seems more likely that the spinal postural mechanisms play an important role in postural control both under normal conditions and after DHS or LHS. After these lesions, the spinal postural circuits receive insufficient excitatory tonic drive through the remaining ventral descending pathways. We suggest that the recovery of postural corrections in DHS and LHS animals is associated with increased efficacy of this activating drive. If so, attempts to substitute the natural tonic drive, in subjects with spinal cord injury, by electrical or pharmacological stimulation of the cord below the lesion seem plausible. Such experiments would directly demonstrate the postural capacity of the spinal cord.
To assess the contribution of the brain motor centres in trunk stabilization, it is necessary to characterize the commands sent during postural corrections from the brain to the spinal cord through four main descending pathways (cortico-, rubro-, reticulo-, and vestibulospinal). To address this issue, we studied the main cortical output – pyramidal tract neurons (PTNs) from the limb representation of the motor cortex – in the cat maintaining equilibrium on a periodically tilting platform (as in Fig. 3A–C). We have found that the activity of PTNs strongly correlated with the platform tilts and with the postural corrections caused by these tilts (Fig. 4A, test 1) (Beloozerova et al., 2005). These experiments have demonstrated that the motor cortex is involved in postural control. To understand the functional role of cortical activity in the control of posture, one has to answer two questions: (1) What is the origin of posture-related cortical activity? (2) What are the motor effects of this activity?
Figure 4.

Involvement of the motor cortex in postural control. (A) Activity of the forelimb pyramidal tract neuron (PTN) is modulated in relation to sinusoidal lateral tilts of the platform (Tilt) and postural corrections (Bd, lateral position of the body) in control (Test 1. Control) and during lifting of the hindlimbs (Test 2. Lift Hind). (B) Activity of the PTN (from the left forelimb representation) during different postural tests: Test 1 – control; Test 2 - lifting of the hindquarters; Test 3 - lifting of the forequarters; Test 4 - anti-phase tilts of the platforms under the forelimbs and hindlimbs; Test 5R - lifting of the hindquarters and left forelimb; Test 5L -lifting of the hindquarters and right forelimb; Test 7R – lifting of the left forelimb; Test 7L –lifting of the right forelimb. A phase histogram of spike activity in the tilt cycle is shown for each test. The activity was averaged over all consecutive cycles of a given test.
In recent studies (Karayannidou et al., 2006), we addressed the first of these questions and tried to assess the origin of posture-related cortical activity in the framework of the functional model shown in Fig. 3E (Deliagina et al., 2006b). In these experiments, the cat was standing on the platform and maintained balance when the platform was periodically tilted in the frontal plane. During tilts, one, two, or three limbs were suspended and thus the somatosensory tilt-related input from them was abolished. The responses of individual PTNs to tilts were recorded in such a “reduced” postural system and then compared with responses in control.
An example of such testing of a forelimb PTN from the left motor cortex is shown in Fig. 4. The PTN was profoundly modulated in all cases when the right forelimb was standing on the platform (Fig. 4B, tests 1, 2, 4, 5R, 7R), and the modulation was considerably reduced when this limb was lifted (Fig. 4B, tests 3, 5L, 7L). We have found that the modulation in PTNs depended primarily on the tilt-related sensory input from the contralateral limb of the corresponding girdle. This input determined, to a large extent, the phase and the amplitude of the responses to tilts. Influences from the limbs of the other girdle on forelimb PTNs and on some hindlimb PTNs were weak. In other hindlimb PTNs, influences from the shoulder girdle contributed to modulation.
These findings suggest that, during maintenance of dorsal side-up trunk orientation, the PTNs are primarily involved in the feedback control of the contralateral limb of the corresponding girdle (intra-limb coordination), and thus constitute a part of the limb postural controller (Fig. 3E). Some hindlimb PTNs participate in coordination of activities of two girdles.
It is known that PTNs have specific afferent projections from the corresponding (contralateral) limb (“receptive fields”), which can be revealed in a quiescent animal. We estimated the contribution of input from the receptive field to the tilt-related modulation of PTNs (Karayannidou et al., 2006). In some PTNs (35 %), the response pattern well corresponded to the pattern which one could expect provided the PTN was driven by its receptive field input. One can suggest that these PTNs were controlled, at least in part, by their receptive field input. For a few PTNs, we managed to demonstrate directly that the receptive field input completely determines the PTN responses to tilts. In the majority of PTNs, however, the input from the receptive field afferents during tilts could not be responsible, even in part, for the generation of PTN reactions to tilts. We therefore conclude that sensory inputs to these PTNs, differing from the receptive field input, are used during postural tasks. Due to these newly formed sensory inputs, the PTNs generate appropriate responses to tilts (Karayannidou et al., 2006). A similar result was obtained earlier in our experiments in rabbits (Beloozerova et al., 2003).
There are data suggesting that other descending pathways also take part in transmitting postural corrective commands to the spinal cord. In preliminary experiments, we have recorded about twenty neurons of the red nucleus, whose axons comprise the rubrospinal tract. Many of them were modulated in the rhythm of tilts. Posture-related modifications of activity were also observed during locomotion in reticulospinal and vestibulospinal neurons of the cat (Matsuyama and Drew, 2000). Thus, all main supraspinal motor centres seem to participate in the control of a highly automatic motor activity – maintenance of the basic body posture.
To conclude, the supraspinal postural mechanism in quadrupeds generates commands for postural corrections on the basis of sensory information about body orientation. These commands are transmitted by a number of descending systems including the corticospinal tract. Neurons of this system participate in the intra- and interlimb postural coordination. The spinal postural mechanism responds to supraspinal commands and generates postural corrections. In addition, the spinal mechanism by itself receives sensory information about body orientation from limb mechanoreceptors. The capacity of the spinal networks to generate corrective movements in response to this input, without involvement of the supraspinal mechanism, has not been directly demonstrated yet, however.
Acknowledgments
The studies reviewed in this paper were supported by grants from NIH R01 NS-049884, the Swedish Research Council (no. 11554), the Royal Swedish Academy of Sciences, Gösta Fraenckels Foundation to TGD, and from NIH R01 NS-39340 to INB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barberini CL, Macpherson JM. Effect of head position on postural orientation and equilibrium. Exp Brain Res. 1998;122:175–184. doi: 10.1007/s002210050505. [DOI] [PubMed] [Google Scholar]
- Bard P, Macht MB. The behavior of chronically decerebrate cat. In: Wolstenholme GEW, O’Connor CM, editors. Neurological basis of behavior. Churchill; London: 1958. pp. 55–71. [Google Scholar]
- Beloozerova IN, Zelenin PV, Popova LB, Orlovsky GN, Grillner S, Deliagina TG. Postural control in the rabbit maintaining balance on the tilting platform. J Neurophysiol. 2003;90:3783–3793. doi: 10.1152/jn.00590.2003. [DOI] [PubMed] [Google Scholar]
- Beloozerova IN, Sirota MG, Orlovsky GN, Deliagina TG. Activity of pyramidal tract neurons in the cat during postural corrections. J Neurophysiol. 2005;93:1831–1844. doi: 10.1152/jn.00577.2004. [DOI] [PubMed] [Google Scholar]
- Berthoz A, Pozzo T. Intermittent head stabilization during postural and locomotory tasks in humans. In: Amblard B, Berthoz A, Clarac F, editors. Posture and Gait: Development, Adaptation and Modulation. Exerpta Medica; Amsterdam: 1988. pp. 189–198. [Google Scholar]
- Boyle R. Vestibular control of reflex and voluntary head movement. Ann NY Acad Sci. 2001;942:364–380. doi: 10.1111/j.1749-6632.2001.tb03760.x. [DOI] [PubMed] [Google Scholar]
- Brodin L, Grillner S, Dubuc R, Ohta Y, Kasicki S, Hökfelt T. Reticulospinal neurons in lamprey: transmitters, synaptic interactions and their role during locomotion. Arch Ital Biol. 1988;126:317–345. [PubMed] [Google Scholar]
- Bussières N. Les Systemes Descendants chez la Lamproie. Etude Anatomique et Functionnelle. Univ. of Montreal; Montreal: 1994. [Google Scholar]
- Deliagina TG, Fagerstedt P. Responses of reticulospinal neurons in intact lamprey to vestibular and visual inputs. J Neurophysiol. 2000;83:864–878. doi: 10.1152/jn.2000.83.2.864. [DOI] [PubMed] [Google Scholar]
- Deliagina TG, Orlovsky GN. Comparative neurobiology of postural control. Curr Opin Neurobiol. 2002;12:652–657. doi: 10.1016/s0959-4388(02)00376-8. [DOI] [PubMed] [Google Scholar]
- Deliagina TG, Pavlova EL. Modifications of vestibular responses of individual reticulospinal neurons in the lamprey caused by a unilateral labyrinthectomy. J Neurophysiol. 2002;87:1–14. doi: 10.1152/jn.00315.2001. [DOI] [PubMed] [Google Scholar]
- Deliagina TG, Orlovsky GN, Grillner S, Wallén P. Vestibular control of swimming in lamprey. II. Characteristics of spatial sensitivity of reticulospinal neurons. Exp Brain Res. 1992a;90:489–498. doi: 10.1007/BF00230931. [DOI] [PubMed] [Google Scholar]
- Deliagina TG, Orlovsky GN, Grillner S, Wallén P. Vestibular control of swimming in lamprey. 3 Activity of vestibular afferents Convergence of vestibular inputs on reticulospinal neurons. Exp Brain Res. 1992b;90:499–507. doi: 10.1007/BF00230932. [DOI] [PubMed] [Google Scholar]
- Deliagina TG, Grillner S, Orlovsky GN, Ullén F. Visual input affects the response to roll in reticulospinal neurons of the lamprey. Exp Brain Res. 1993;95:421–428. doi: 10.1007/BF00227134. [DOI] [PubMed] [Google Scholar]
- Deliagina TG, Beloozerova IN, Popova LB, Sirota MG, Swadlow H, Grant G, Orlovsky GN. Role of different sensory inputs for maintenance of body posture in sitting rat and rabbit. Motor Control. 2000;4:439–452. doi: 10.1123/mcj.4.4.439. [DOI] [PubMed] [Google Scholar]
- Deliagina TG, Zelenin PV, Orlovsky GN. Encoding and decoding of reticulospinal commands. Brain Res Rev. 2002;40:166–177. doi: 10.1016/s0165-0173(02)00199-6. [DOI] [PubMed] [Google Scholar]
- Deliagina TG, Orlovsky GN, Zelenin PV, Beloozerova IN. Neural bases of postural control. Physiology. 2006a;21:216–225. doi: 10.1152/physiol.00001.2006. [DOI] [PubMed] [Google Scholar]
- Deliagina TG, Sirota MG, Zelenin PV, Orlovsky GN, Beloozerova IN. Interlimb postural coordination in the standing cat. J Physiol. 2006b;573:211–224. doi: 10.1113/jphysiol.2006.104893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetz EE, Cheney PD. Postspike facilitation of forelimb muscle activity by primate corticomotoneuronal cells. J Neurophysiol. 1980;44:751–772. doi: 10.1152/jn.1980.44.4.751. [DOI] [PubMed] [Google Scholar]
- Gelfand IM, Zetlin ML. On the mathematical modeling of mechanisms of the central nervous system. In: Gelfand IM, Fomin SV, Zetlin ML, editors. Models of structural-functional organization of certain biological systems. MIT Press; Massachusets: 1971. pp. 2–23. [Google Scholar]
- Ghez C. Posture. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of Neural Science. Elsevier; New York: 1991. pp. 567–607. [Google Scholar]
- Grillner S. The motor infrastructure: from ion channels to neuronal networks. Nat Rev Neurosci. 2003;4:573–586. doi: 10.1038/nrn1137. [DOI] [PubMed] [Google Scholar]
- Grillner S, Kashin S. On the generation and performance of swimming in fish. In: Herman RM, Grillner S, Stein PSG, Stuart DG, editors. Neural Control of Locomotion. Plenum Press; New York: 1976. [Google Scholar]
- Horak FB, Macpherson JM. Postural orientation and equilibrium. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. Oxford UP; New York: 1996. [Google Scholar]
- Jacobs R, Macpherson JM. Two functional muscle groupings during postural equilibrium in standing cats. J Neurophysiol. 1996;76:2402–2411. doi: 10.1152/jn.1996.76.4.2402. [DOI] [PubMed] [Google Scholar]
- Karayannidou A, Orlovsky GN, Zelenin PV, Deliagina TG. Responses of descending neurons in the lamprey to lateral turns. Soc Neurosci Abstr. 2005;31:168.4. doi: 10.1152/jn.00912.2006. [DOI] [PubMed] [Google Scholar]
- Karayannidou A, Tamarova ZA, Sirota MS, Zelenin PV, Orlovsky GN, Deliagina TG, Beloozerova IN. Integration of sensory inputs from different limbs in postural responses of pyramidal tract neurons. Soc Neurosci Abstr. 2006;32:657.11. [Google Scholar]
- Lacquaniti F, Maioli C, Fava E. Cat posture on the tilted platform. Exp Brain Res. 1984;57:82–88. doi: 10.1007/BF00231134. [DOI] [PubMed] [Google Scholar]
- Lyalka FV, Zelenin PV, Karayannidou A, Orlovsky GN, Grillner S, Deliagina TG. Impairment and recovery of postural control in rabbits with spinal cord lesions. J Neurophysiol. 2005;94:3677–3690. doi: 10.1152/jn.00538.2005. [DOI] [PubMed] [Google Scholar]
- Macpherson JM. Strategies that simplify the control of quadrupedal stance. I. Forces at the ground. J Neurophysiol. 1988a;60:204–217. doi: 10.1152/jn.1988.60.1.204. [DOI] [PubMed] [Google Scholar]
- Macpherson JM. Strategies that simplify the control of quadrupedal stance. II. Electromyographic activity. J Neurophysiol. 1988b;60:218–231. doi: 10.1152/jn.1988.60.1.218. [DOI] [PubMed] [Google Scholar]
- Macpherson JM, Fung J. Weight support and balance during perturbed stance in the chronic spinal cat. J Neurophysiol. 1999;82:3066–3081. doi: 10.1152/jn.1999.82.6.3066. [DOI] [PubMed] [Google Scholar]
- Macpherson JM, Fung J, Lacobs R. Postural orientation, equilibrium, and the spinal cord. In: Seil FJ, editor. Neuronal Regeneration, Reorganization, and Repair. Advances in Neurology. Vol. 72. Lippincott-Raven Publishers; Philadelphia: 1997. pp. 227–232. [PubMed] [Google Scholar]
- McClellan AD, Hagevik A. Descending control of turning locomotor activity in larval lamprey: neurophysiology and computer modeling. J Neurophysiol. 1997;78:214–228. doi: 10.1152/jn.1997.78.1.214. [DOI] [PubMed] [Google Scholar]
- Massion J. Postural control system. Curr Opin Neurobiol. 1994;4:877–888. doi: 10.1016/0959-4388(94)90137-6. [DOI] [PubMed] [Google Scholar]
- Magnus R. Korperstellung. Springer; Berlin: 1924. [Google Scholar]
- Massion J, Popov K, Fabre J, Rage P, Gurfinkel V. Is the erect posture in microgravity based on the control of trunk orientation or center of mass position? Exp Brain Res. 1997;114:384–389. doi: 10.1007/pl00005647. [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Drew T. Vestibulospinal and reticulospinal neuronal activity during locomotion in the intact cat. II. Walking on an inclined plane. J Neurophysiol. 2000;84:2257–2276. doi: 10.1152/jn.2000.84.5.2257. [DOI] [PubMed] [Google Scholar]
- Musienko PE, Orlovsky GN, Zelenin PV, Lyalka VF, Deliagina TG. Postural performance in decerebrate rabbit. Soc Neurosci Abstr. 2006;32:558.2. doi: 10.1016/j.bbr.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlovsky GN, Deliagina TG, Grillner S. From mollusc to man. Oxford UP; Oxford: 1999. Neuronal control of locomotion. [Google Scholar]
- Pavlova EL, Deliagina TG. Responses of reticulospinal neurons in intact lamprey to pitch tilt. J Neurophysiol. 2002;88:1136–1146. doi: 10.1152/jn.2002.88.3.1136. [DOI] [PubMed] [Google Scholar]
- Ronan M. Origins of the descending spinal projections in petromyzontid and myxinoid agnathans. J Comp Neurol. 1989;281:54–68. doi: 10.1002/cne.902810106. [DOI] [PubMed] [Google Scholar]
- Shik ML, Orlovsky GN. Co-ordination of the limbs during running of the dog. Biophysics. 1965;10:1148–1159. [Google Scholar]
- Tretjakoff D. Das nervensystem des flussnevnauges. Z Wiss Zool. 1927;129:359–452. [Google Scholar]
- Ullén F, Deliagina TG, Orlovsky GN, Grillner S. Spatial orientation of lamprey. 1 Control of pitch and roll. J Exp Biol. 1995;198:665–673. doi: 10.1242/jeb.198.3.665. [DOI] [PubMed] [Google Scholar]
- von Holst E. Ûber relative Koordination bei Saugern und beim Menschen. Pfluegers Archiv. 1938;240:44–59. [Google Scholar]
- Wannier T, Deliagina TG, Orlovsky GN, Grillner S. Differential effects of reticulospinal system on locomotion in lamprey. J Neurophysiol. 1998;80:103–112. doi: 10.1152/jn.1998.80.1.103. [DOI] [PubMed] [Google Scholar]
- Zelenin PV, Deliagina TG, Grillner S, Orlovsky GN. Postural control in the lamprey: A study with a neuro-mechanical model. J Neurophysiol. 2000;84:2880–2887. doi: 10.1152/jn.2000.84.6.2880. [DOI] [PubMed] [Google Scholar]
- Zelenin PV, Grillner S, Orlovsky GN, Deliagina TG. Heterogeneity of the population of command neurons in the lamprey. J Neurosci. 2001;21:7793–7803. doi: 10.1523/JNEUROSCI.21-19-07793.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelenin PV, Grillner S, Orlovsky GN, Deliagina TG. The pattern of motor coordination underlying the roll in the lamprey. J Exp Biol. 2003;206:2557–2566. doi: 10.1242/jeb.00451. [DOI] [PubMed] [Google Scholar]
- Zelenin PV, Orlovsky GN, Grillner S, Deliagina TG. Motor effects of individual reticulospinal neurons match their vestibular inputs. Soc Neurosci Abstr. 2005;31:168.3. [Google Scholar]



