Abstract
Background
Power spectral analysis of heart rate variability (HRV) has been used to indicate cardiac autonomic function. High-frequency power relates to respiratory sinus arrhythmia and therefore to parasympathetic cardiovagal tone; however, the relationship of low-frequency (LF) power to cardiac sympathetic innervation and function has been controversial. Alternatively, LF power might reflect baroreflexive modulation of autonomic outflows.
Objective
We studied normal volunteers and chronic autonomic failure syndrome patients with and without loss of cardiac noradrenergic nerves in order to examine the relationships of LF power with cardiac sympathetic innervation and baroreflex function.
Methods
We compared LF power of HRV in patients with cardiac sympathetic denervation, as indicated by low myocardial concentrations of 6-[18F] fluorodopamine-derived radioactivity or low rates of norepinephrine entry into coronary sinus plasma (cardiac norepinephrine spillover) to values in patients with intact innervation, at baseline, during infusion of yohimbine, which increases exocytotic norepinephrine release from sympathetic nerves, or during infusion of tyramine, which increases non-exocytotic release. Baroreflex-cardiovagal slope (BRS) was calculated from the cardiac interbeat interval and systolic pressure during the Valsalva maneuver.
Results
LF power was unrelated to myocardial 6-[18F] fluorodopamine-derived radioactivity or cardiac norepinephrine spillover. In contrast, the log of LF power correlated positively with the log of BRS (r=0.72, p<0.0001). Patients with low BRS (≤ 3 msec/mm Hg) had low LF power, regardless of cardiac innervation. Tyramine and yohimbine increased LF power in subjects with normal BRS but not in those with low BRS. BRS at baseline predicted LF responses to tyramine and yohimbine.
Conclusions
LF power reflects baroreflex function, not cardiac sympathetic innervation.
Keywords: heart rate variability, power spectral analysis, nervous system, sympathetic, fluorodopamine, baroreceptors
Spectral analysis of heart rate variability has been used widely as a non-invasive technique to examine sympathetic and parasympathetic nervous outflows to the heart. Low frequency (LF) and high frequency (HF) power have been used most commonly. Human and animal experiments have repeatedly confirmed the dependence of HF power on respiration-related alterations in parasympathetic cardiovagal outflow—respiratory sinus arrhythmia; however, whether LF power provides an indirect measure of cardiac sympathetic activity has been contentious. Pagani et al. reported that LF power (normalized to total spectral power) increased during states associated with sympathetic noradrenergic activation and that bilateral stellectomy in dogs reduced LF power (1). Alvarenga et al., however, reported that LF power was unrelated to all measures of norepinephrine kinetics in the heart (2); and in congestive heart failure, which is associated with a high rate of entry of norepinephrine into coronary sinus plasma (cardiac norepinephrine spillover) (3), LF power is decreased, not increased as might be expected if LF power reflected sympathetic activity (4–7).
Sleight and co-workers proposed an alternative explanation for the origin of LF power (10). In a small group of human subjects, power spectral analysis of HRV revealed that the amplitude of LF power was related to baroreflex gain and not to the level of sympathetic activity. Carotid sinus stimulation increased LF power only in individuals with normal baroreflex sensitivity and did not do so in those with depressed baroreflex gain. Therefore, results of power spectral analysis of LF power might reflect baroreflex-cardiovagal function (11).
Studies of patients with dysautonomias provide an unusual opportunity to examine neurocirculatory correlates of LF power. Some chronic autonomic failure syndromes feature cardiac sympathetic denervation, whereas others do not. Parkinson disease with neurogenic orthostatic hypotension and pure autonomic failure feature cardiac sympathetic denervation, whereas multiple system atrophy does not (12). All three diseases involve baroreflex-cardiovagal and baroreflex-sympathoneural failure (13). Chronic orthostatic intolerance syndromes (postural tachycardia syndrome, neurocardiogenic syncope) do not entail either cardiac sympathetic denervation or baroreflex failure (14).
For this report we carried out power spectral analyses of HRV on digitized electrocardiographic recordings from dysautonomia patients and normal volunteers, during supine rest, measurement of cardiac norepinephrine spillover, and during i.v. infusion of yohimbine and tyramine, two drugs that are known to release norepinephrine from cardiac sympathetic nerves (15,17). Cardiac sympathetic innervation was assessed by 6-[18F]fluorodopamine positron emission tomographic (PET) scanning (18).
We hypothesized that if LF power indicated cardiac sympathetic innervation and function, then patients with neuroimaging or neurochemical evidence of cardiac sympathetic denervation would have low LF power and attenuated increments in LF power in response to yohimbine and tyramine. Alternatively, if LF power was reflective of baroreflex function, alterations of LF power would be independent of cardiac sympathetic innervation status and correlate with changes in baroreflex gain.
METHODS
The study protocols were approved by the Intramural Research Board of the National Institute of Neurological Disorders and Stroke. All subjects were studied at the National Institutes of Health Clinical Center after giving informed, written consent.
Subjects
The study population consisted of a total of 98 subjects who participated in research protocols studying chronic orthostatic intolerance and chronic autonomic failure (Table 1). The subjects underwent autonomic function testing and had reviewable, digitized electrocardiographic data enabling retrospective power spectral analysis of HRV. ECG and blood pressure data were sampled at 1 k Hz.
Table 1.
Subject Groups
| Group | Innerv Nl BRS | Denerv Nl BRS | Innerv Low BRS | Denerv Low BRS | SUM |
|---|---|---|---|---|---|
| PD+NOH | 0 | 1 | 0 | 8 | 9 |
| MSA | 3 | 0 | 11 | 0 | 14 |
| PAF | 0 | 1 | 3 | 5 | 9 |
| PD No NOH | 12 | 2 | 2 | 17 | 33 |
| r/o CAF | 2 | 0 | 0 | 0 | 2 |
| Normal | 11 | 0 | 1 | 0 | 12 |
| COI | 12 | 0 | 7 | 0 | 19 |
| SUM | 40 | 4 | 24 | 30 | 98 |
| Group | Innerv Nl BRS | Denerv Nl BRS | Innerv Low BRS | Denerv Low BRS | SUM |
| YOH | |||||
| PD+NOH | 0 | 0 | 0 | 3 | 3 |
| MSA | 0 | 0 | 5 | 0 | 5 |
| PAF | 0 | 0 | 3 | 2 | 5 |
| PD No NOH | 0 | 0 | 0 | 0 | 0 |
| r/o CAF | 2 | 0 | 0 | 0 | 2 |
| Normal | 6 | 0 | 1 | 0 | 7 |
| COI | 0 | 0 | 0 | 0 | 0 |
| SUM | 8 | 0 | 9 | 5 | 22 |
| Group | Innerv Nl BRS | Denerv Nl BRS | Innerv Low BRS | Denerv Low BRS | SUM |
| TYR | |||||
| PD+NOH | 0 | 0 | 0 | 7 | 7 |
| MSA | 2 | 0 | 12 | 0 | 14 |
| PAF | 0 | 0 | 3 | 6 | 9 |
| PD No NOH | 5 | 0 | 0 | 1 | 6 |
| r/o CAF | 2 | 0 | 0 | 1 | 3 |
| Normal | 10 | 0 | 1 | 0 | 11 |
| COI | 0 | 0 | 0 | 0 | 0 |
| SUM | 19 | 0 | 16 | 15 | 50 |
Abbreviations: Innerv=innervated; Denerv=denervated; Nl BRS=normal baroreflex-cardiovagal slope; PD=Parkinson disease; NOH=neurogenic orthostatic hypotension; MSA=multiple system atrophy; PAF=pure autonomic failure; r/o CAF=rule out chronic autonomic failure; COI=chronic orthostatic intolerance.
The study subjects were separated into 4 groups, depending on their state of cardiac sympathetic innervation and baroreflex-cardiovagal slope (BRS, see below). There were 40 subjects with intact sympathetic innervation and normal BRS (Innervated-Normal BRS), 24 with intact sympathetic innervation and low BRS (Innervated-Low BRS), 4 with sympathetic denervation and normal BRS (Denervated-Normal BRS), and 30 with sympathetic denervation and low BRS (Denervated-Low BRS).
Autonomic Function Testing
Each subject was studied while supine with head on pillow after an overnight fast. Each patient had monitoring of the electrocardiogram and beat-to-beat blood pressure using either non-invasive devices (Finometer™ (Finapres Medical Systems, Amsterdam, Netherlands), Portapres™ (Finapres Medical Systems, Amsterdam, Netherlands), or Colin tonometer™ (Colin Medical Instruments, San Antonio, TX)) or a brachial intra-arterial catheter. We previously studied formally and reported excellent agreement between intra-arterial and these non-invasively obtained measures of beat-to-beat blood pressure (19). Continuous vital signs data were digitized and recorded using a PowerLab (AD Instruments Pty Ltd, Castle Hill, Australia) data acquisition system and stored for later analysis on an Apple PowerBook G4 computer (Apple, Cupertino, CA).
Following about a 10-minute baseline period, each subject performed a Valsalva maneuver (30 mm Hg for 12 secs) at least 3 times.
Baroreflex Function
As an index of baroreflex function we used the slope of the relationship between cardiac interbeat interval and systolic blood pressure during Phase II of the Valsalva maneuver (20). BRS, in units of msec/mm Hg, was calculated from the linear regression equation for the relationship between interbeat interval (with one beat delay) and systolic pressure. A BRS value of ≤3 msec/mm Hg was considered low (13).
Pharmacologic Testing
Pharmacologic testing was performed upon completion of the autonomic evaluation, using either tyramine or yohimbine. If a subject received both drugs, each drug administration was on a separate day. The durations of drug infusion were sufficient for heart rate and blood pressure to reach plateau values.
In a total of 22 subjects (Table 1), yohimbine was infused i.v. at 62.5 μg/kg over 3 minutes and then 0.5 μg/kg/min for 12 minutes. In a total of 50 subjects, tyramine was infused at a rate of 1 mg/min for 10 min. In patients with severe supine hypertension (systolic pressure more than 200 mm Hg) and orthostatic hypotension, the test drugs were infused during head-up tilting (15–30 degrees), to decrease baseline pressure, or else the drugs were not given.
HRV Analysis
Low frequency power (LF, 0.04–0.15 Hz), high frequency power (HF, 0.16–0.4 Hz), and total power (TP, 0.0–0.4 Hz) were calculated using Chart 5.4.2 and the HRV module version 1.03 (PowerLab, AD Instruments Pty Ltd,, Castle Hill, Australia). Stable heart rate epochs 3–5 minutes in duration were chosen for analysis. One epoch was sampled immediately prior to initiation of drug testing; the second followed attainment of steady state hemodynamic effects. Interbeat interval data were reviewed carefully to eliminate artifacts from noise and T waves, using segments with little to no premature beats. LF power and HF power were calculated as absolute power (msec2), with or without normalization for total power (0.04–0.4 Hz). Reported LF or HF power was integrated within their defined frequency bands.
Cardiac Sympathetic Neuroimaging
For cardiac sympathetic neuroimaging the subject was positioned supine, feet-first in a GE Advance™ scanner (General Electric, Milwaukee, WI), with the thorax in the gantry. After positioning the patient with the thorax in the scanner and transmission scanning for attenuation correction, 6-[18F] fluorodopamine (usual dose 1 mCi, specific activity 1.0–4.0 Ci/mmole, in about 10 cc normal saline) was infused i.v. at a constant rate for 3 min, and dynamic scanning data were obtained for thoracic radioactivity, with the midpoint of the scanning interval at 7.5 minutes after injection of the tracer (data collection interval between 5–10 minutes). Cardiac sympathetic denervation was defined by low concentrations of 6-[18F] fluorodopamine-derived radioactivity in the interventricular septum (less than 5000 nCi-kg/cc-mCi) or left ventricular free wall (less than 4000 nCi-kg/cc-mCi) corresponding to about 2 standard deviations below the normal means.
Cardiac Norepinephrine Spillover
Subgroups of subjects (3 PD+NOH, 3 MSA, 3 PAF, 5 normal volunteers) underwent right heart catheterization, for measurement of cardiac norepinephrine spillover. 3H-Norepinephrine was infused i.v., and arterial and coronary sinus blood was sampled and coronary sinus blood flow measured by thermodilution, for measurements of cardiac norepinephrine spillover as described previously (21). In some subjects, yohimbine was infused during cardiac catheterization. Patients with chronic autonomic failure received the doses described above; normal volunteers and patients with chronic orthostatic intolerance received twice the doses described above.
Data Analysis
Statistical analyses were performed using StatView version 5.0.1. (SAS Institute, Cary, NC). Mean values in the baseline condition for the several subject groups were compared using single factor analyses of variance (ANOVA). Responses to drugs were analyzed by dependent-means t tests. Differences in response to pharmacologic tests among subject groups were compared using repeated measures analyses of variance. Relationships between individual hemodynamic values were assessed by linear regression and calculation of Pearson correlation coefficients. Post-hoc testing consisted of Fisher’s PLSD test. Multiple regression analysis was done on the individual data, with the log of LF power as the dependent measure and the log of baroreflex slope and septal 6-[18F]fluorodopamine-derived radioactivity as independent measures. Mean values were expressed ± SEM.
RESULTS
Baseline
Across the 7 subject groups (N=98), LF power was unrelated to subject group (F=1.2). When individual subjects were stratified in terms of cardiac sympathetic denervation or innervation, based on concentrations of 6-[18F] fluorodopamine-derived radioactivity more than 2 standard deviations below the normal mean, then LF power was lower in the Denervated group (mean 221 ± 55 msec2/Hz, N=34) than in the Innervated group (516 ± 93 msec2/Hz, N=64, F=4.8, p=0.03). LF power normalized for total power, HF normalized for total power, and the ratio of LF:HF were not related to 6-[18F] fluorodopamine-derived radioactivity.
When subjects were stratified in terms of baroreflex-cardiovagal slope (BRS), then LF power was lower in the Low BRS group (223 ± 105 msec2/Hz, N=46) than in the Normal BRS group (617 ± 97 msec2/Hz, N=25, F=6.1, p=0.02). The Low BRS group did not differ from the Normal BRS group in normalized LF power (F=0.8).
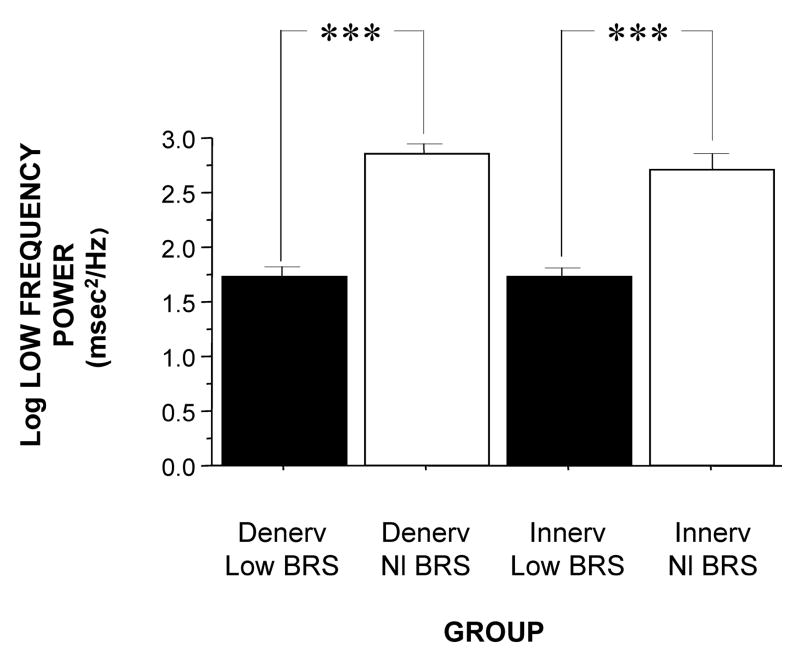
When individual subjects were stratified into 4 groups, based on both cardiac 6-[18F]fluorodopamine-derived radioactivity (Innervated or Denervated) and on baroreflex-cardiovagal slope (Normal BRS or Low BRS), then both LF power and the log of LF power varied highly significantly as a function of subject group (F=9.5, p<0.0001; F=4.6, p=0.0004). The Denervated-Low BRS group had lower LF power than did the Denervated-Normal BRS group (p=0.05), and the Innervated-Low BRS group had lower LF power than did the Innervated-Normal BRS group (p<0.0001). When level of baroreflex function was taken into account, the Innervated and Denervated groups did not differ in LF power (Figure 1).
Figure 1.
Mean (±SEM) values for the log of low-frequency power of heart rate variability in subjects groups with innervated (Innerv.) or denervated (Denerv.) hearts, as indicated by low 6-[18F]fluorodopamine-derived radioactivity, and normal (Nl) or low baroreflex-cardiovagal slope (BRS), as indicated by slope ≤ 3 msec/mm Hg during the Valsalva maneuver. (***) significant difference, p<0.001.
Values for HF power also varied with subject group, when individual subjects were stratified in terms of both cardiac sympathetic innervation and BRS (F=4.9, p=0.004; Table 2). The Innervated-Low BRS group had lower HF power than did the Innervated-Normal BRS group (p=0.003); however, the Denervated-Low BRS group did not differ from the Denervated-Normal BRS group in HF power. Normalization of LF and HF power for total power, and the ratio of low-to-high frequency did not reveal additional group differences (Table 2). In particular, the LF: HF ratio did not vary with the subject group (F=0.6).
Table 2.
Power Spectral Heart Rate Variability Parameters as a Function of Cardiac Sympathetic Innervation and Baroreflex Slope (BRS)
| Innervation | BRS | HF Power |
|---|---|---|
| Denervated | Low | 129 ± 57 |
| Denervated | Normal | 329 ± 69 |
| Innervated | Low | 59 ± 15 *** |
| Innervated | Normal | 489 ± 106 |
| Innervation | BRS | LF Nu |
|
| ||
| Denervated | Low | 53 ± 5 |
| Denervated | Normal | 65 ± 7 |
| Innervated | Low | 56 ± 5 |
| Innervated | Normal | 64 ± 3 |
| Innervation | BRS | HF Nu |
|
| ||
| Denervated | Low | 35 ± 3 |
| Denervated | Normal | 30 ± 6 |
| Innervated | Low | 33 ± 4 |
| Innervated | Normal | 32 ± 3 |
| Innervation | BRS | LF/HF |
|
| ||
| Denervated | Low | 2.3 ± 0.4 |
| Denervated | Normal | 2.7 ± 0.9 |
| Innervated | Low | 3.1 ± 0.6 |
| Innervated | Normal | 3.0 ± 0.4 |
Abbreviations: HF Power=high-frequency power; LF Nu=low-frequency power divided by summed power; HF Nu=high-frequency power divided by summed power; LF/HF=low-frequency power divided by high-frequency power.
Significant difference from Innervated-Normal BRS, p=0.0006.
Analysis of data from subjects during cardiac catheterization showed that LF power varied as a function of subject group (F=5.3, p=0.03, Figure 2). The Innervated-Low BRS group had lower LF power than did the Innervated-Normal BRS group (p=0.04), whereas the Denervated-Low BRS and Innervated-Low BRS groups did not differ in LF power. As expected, the Denervated-Low BRS group had lower cardiac norepinephrine spillover than the Innervated-Low BRS group.
Figure 2.
Mean (±SEM) values for (A) low frequency power of heart rate variability and (B) cardiac norepinephrine spillover during right heart catheterization in subjects groups with innervated (Innerv) or denervated (Denerv) hearts, as indicated by low 6-[18F]fluorodopamine-derived radioactivity, and normal (Nl) or low baroreflex-cardiovagal slope (BRS), as indicated by slope ≤ 3 msec/mm Hg during the Valsalva maneuver. (*) significant difference, p<0.05; (**) significant difference, p<0.01.
Individual values for LF power were positively correlated with BRS. When values for both variables were log-transformed, the log of LF power correlated positively with the log of BRS slope (r=0.72, p<0.0001, Figure 3). Individual values for the log of LF power were also correlated with the magnitude of fall in systolic pressure during performance of the Valsalva maneuver (r=−0.60, p<0.0001) and with the orthostatic change in systolic pressure (r=0.58, p<0.0001). In contrast, the log of LF power was unrelated to the septal myocardial concentration of 6-[18F]fluorodopamine-derived radioactivity, the plasma norepinephrine concentration, or cardiac norepinephrine spillover.
Figure 3.
Individual values for (A) the log of low-frequency (LF) power as a function of septal 6-[18F]fluorodopamine-derived radioactivity and (B) the log of baroreflex-cardiovagal slope.
From multiple regression analysis for the log of LF power as the dependent measure and the log of baroreflex slope and septal 6-[18F]fluorodopamine-derived radioactivity as independent measures, the regression coefficient for the log of baroreflex slope was 0.92 (p<0.0001), whereas the regression coefficient for 6-[18F]fluorodopamine-derived radioactivity was 3 × 10−6.
At baseline, the log of HF power correlated positively with the log of LF power (r=0.77, p<0.0001). HF power varied with the subject group (F=4.9, p=0.004). As with LF power, HF power was greater in the Innervated-Normal BRS than in the Innervated-Low BRS (p=0.001, Table 2). As expected, the log of HF power correlated positively with the log of BRS (r=0.60, p<0.0001). The log of HF power also correlated negatively with the magnitude of fall in systolic pressure during the Valsalva maneuver (r=−0.24, p=0.02) and positively with the orthostatic change in systolic pressure (r=0.40, p=0.004).
Yohimbine
Yohimbine infusion increased LF power (t=2.9, p=0.007). The group with cardiac sympathetic denervation did not differ from the group with intact cardiac innervation in terms of the change in LF power during yohimbine infusion (F=0.7). Yohimbine infusion increased LF power in the Innervated-Normal BRS group (t=2.8, p=0.01) but not in the innervated or denervated groups with low BRS (Figure 4). The Innervated-Normal BRS group had a larger increase in LF power during yohimbine infusion than did the Innervated-Low BRS group (p=0.02). Too few patients with cardiac denervation and normal BRS were studied to include in the ANOVA. The log of the change in LF power during yohimbine administration was positively correlated with the log of BRS at baseline (Figure 5).
Figure 4.
Mean (±SEM) values for the change in low-frequency power (ΔLF power) of heart rate variability during (A) i.v. infusion of yohimbine or (B) tyramine in groups with innervated (Innerv) or denervated (Denerv) hearts, as indicated by low 6-[18F]fluorodopamine-derived radioactivity, and normal (Nl) or low baroreflex-cardiovagal slope (BRS), as indicated by slope ≤ 3 msec/mm Hg during the Valsalva maneuver. (*) significant difference, p<0.05; (***) significant difference, p<0.001.
Figure 5.
Individual values for the log of change in low-frequency power (log ΔLF power) as a function of baroreflex-cardiovagal slope at baseline. Left: yohimbine infusion; right: tyramine infusion.
Yohimbine increased HF power in the Innervated-Normal BRS group (t=2.1, p=0.05) but not in the innervated or denervated groups with low BRS.
The change in LF power in response to yohimbine during cardiac catheterization, was unrelated to the change in cardiac norepinephrine spillover (r=−0.09, N=12).
Tyramine
Overall, tyramine infusion increased LF power (t=2.9, p=0.008). The group with cardiac sympathetic denervation did not differ from the group with intact cardiac innervation in terms of the change in LF power during tyramine infusion (F=1.7). Tyramine increased LF power in the Innervated-Normal BRS group but not in the Innervated-Low BRS or Denervated-Low BRS groups (Figure 4; data for 2 outliers excluded). The log of the change in LF power during tyramine administration was positively correlated with the log of BRS at baseline (Figure 5; data for 2 outliers excluded).
DISCUSSION
In this study, patients with neuroimaging evidence of cardiac sympathetic denervation had low LF power of heart rate variability. At first glance, this finding would seem to support the view that LF power can provide an index of cardiac sympathetic outflow. As explained below, several lines of evidence from the present study led us to infer that the association between low LF power and cardiac sympathetic innervation is determined mainly by concurrent baroreflex function.
Patients with low baroreflex-cardiovagal slope (BRS) had low LF power, and patients with normal BRS had normal LF power, regardless of the status of cardiac sympathetic innervation as assessed by 6-[18F]fluorodopamine scanning. Neither normalization of LF and HF power for total power nor use of the LF: HF ratio improved the association with indices of cardiac sympathetic innervation.
Neurochemical findings during cardiac catheterization supported the above results based on cardiac sympathetic neuroimaging. Among patients with innervated hearts who had normal cardiac norepinephrine spillover, LF power was decreased only in the group with low BRS and was normal in the group with normal BRS. As expected, cardiac norepinephrine spillover was decreased in patients with neuroimaging evidence of cardiac sympathetic denervation.
Effects of pharmacological manipulations that increase norepinephrine release from sympathetic nerves provided further support for an association between baroreflex failure and low LF power, independent of cardiac sympathetic function. Both tyramine and yohimbine increased LF power only in the subjects with normal BRS. In subjects with low BRS, neither drug increased LF power, even in the group with intact cardiac sympathetic innervation. Moreover, individual values for responses of the log of LF power to both drugs were correlated positively with the log of BRS at baseline.
The fact that HF power was positively correlated with LF power could also fit with the notion of baroreflex function acting as a common determinant of values of both variables. We cannot exclude concurrent parasympathetic cardiovagal and sympathetic denervation as an explanation for the association between HF and LF power. Inhibition of the effects of parasympathetic activity following atropine administration results in the almost complete absence of both LF and HF HRV, further suggesting a common determinant (22).
Several previous investigations have cast doubt on the validity of LF power as a measure of sympathetic activity, because of dissociations between LF power and cardiac norepinephrine spillover, directly recorded sympathetic nerve traffic, and plasma norepinephrine levels (4,6,23). Such dissociations are especially glaring in patients with congestive heart failure, which is characterized by decreased LF power (11) despite marked cardiac sympathetic activation (3).
Other pathophysiologic states do result in both decreased sympathetic nervous system activity and decreased LF power. In these pathophysiologic states, the possibility remains that low LF power might reflect failure of baroreflexive modulation of sympathetic neuronal outflows, rather than sympathoinhibition itself. For instance, Wiklund et al. noted low LF power in patients with palmar hyperhidrosis undergoing bilateral transthoracic sympathectomy (24); however, baroreflex-cardiovagal sensitivity also declines following thoracic sympathectomy (25).
Sleight et al. (10) suggested dependence of LF power on baroreflex function, based on effects of carotid baroreceptor stimulation in 3 patients, 1 with normal BRS, 1 with ischemic heart disease, congestive heart failure, and normal BRS, and 1 with ischemic heart disease, congestive heart failure, and initially low BRS who subsequently had an improved clinical state and BRS. In the baseline state both congestive heart failure patients had low LF power, despite a presumably hypernoradrenergic state. Direct baroreceptor stimulation at 0.1 Hz increased LF power in the normal subject and in the patient with congestive heart failure and normal BRS. The congestive heart failure patient with low BRS did not have an increase in LF power until BRS normalized. These data revealed an initial dissociation between cardiac noradrenergic state in the patients with congestive heart failure and LF power. During carotid sinus stimulation, LF power increased only when BRS was normal. Low BRS obviated this effect.
Since congestive heart failure is well known to be associated with baroreflex-cardiovagal inhibition (28–30), the finding of low LF power in heart failure also supports an association between LF power and BRS, independently of increased tonic release of norepinephrine from sympathetic nerves in the heart. Cevese et al. (31) inhibited noradrenergic vasomotor tone using an alpha-adrenoceptor blocker in human subjects, while maintaining mean blood pressure at control levels using angiotensin II. This drug combination, which would be expected to attenuate sympathetically mediated vasomotor tone and thereby decrease arterial baroreceptor input, markedly decreased or abolished LF power of HRV, suggesting that, at least under resting supine conditions, a baroreflex mechanism accounts almost entirely for LF power of HRV.
deBoer et al. (32) developed a beat-to-beat model of the human circulation using a set of differential equations and the following principles of operation: (1) the baroreflex regulates heart rate and peripheral vascular resistance; (2) Windkessel properties characterize the systemic arterial tree; (3) contractile properties of the ventricular myocardium follow Starling’s law; and (4) respiration exerts mechanical effects on BP (38). The model attributes LF power to a resonance in the circulatory control system, produced by a slow time constant for reflexive sympathetically mediated responses to beat-to-beat blood pressure changes. The resonance can be up- or down-regulated by the state of baroreflex activity. The model of deBoer et al. predicts that changes in BP would lead HR changes at 0.1 Hz through a delayed sympathetic response. Changes in HR would depend on summed effects of sympathetic and vagal effects, with the sympathetic response delaying the overall response. At the respiratory frequency (0.2 – 0.3 Hz), BP and HR changes would occur with little delay, because of fast parasympathetic control. In essence, the response of the sympathetic nervous system behaves as a low band pass filter, with a peak response at 0.1 Hz and little response at frequencies above 0.2 Hz. Systolic blood pressure would lead to changes in heart rate, via the baroreflex. In general the results of this study fit with the deBoer model.
In conclusion, LF power derived from the interbeat interval spectrogram predominantly reflects baroreflex-mediated, phasic changes in cardiovagal and sympathetic noradrenergic outflows. In the setting of baroreflex failure, baseline LF power is reduced, regardless of the status of cardiac sympathetic innervation.
Limitations
The combination of cardiac sympathetic denervation and normal baroreflex function seems quite rare. One must exercise caution in drawing inferences from the findings in the Denervated-Normal BRS group, which contained only 4 subjects, even though the difference in mean log-transformed LF power from the Denervated-Low BRS group was highly statistically significant.
All the testing in our study was with the subjects supine. LF power measured in other positions might have different sources and meaning.
Acknowledgments
Funding Sources: Intramural research funds, National Institute of Neurologic Disorders and Stroke, NIH, Bethesda, MD
List of Abbreviations
- BRS
baroreflex-cardiovagal slope
- HF
high-frequency
- LF
low-frequency
- HRV
heart rate variability
Footnotes
Conflicts of Interest: None for all authors
References
- 1.Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R, Pizzinelli P, Sandrone G, Malfatto G, Dell’Orto S, Piccaluga E. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ Res. 1986;59:178–93. doi: 10.1161/01.res.59.2.178. [DOI] [PubMed] [Google Scholar]
- 2.Alvarenga ME, Richards JC, Lambert G, Esler MD. Psychophysiological mechanisms in panic disorder: a correlative analysis of noradrenaline spillover, neuronal noradrenaline reuptake, power spectral analysis of heart rate variability, and psychological variables. Psychosom Med. 2006;68:8–16. doi: 10.1097/01.psy.0000195872.00987.db. [DOI] [PubMed] [Google Scholar]
- 3.Eisenhofer G, Friberg P, Rundqvist B, Quyyumi AA, Lambert G, Kaye DM, Kopin IJ, Goldstein DS, Esler MD. Cardiac sympathetic nerve function in congestive heart failure. Circulation. 1996;93:1667–76. doi: 10.1161/01.cir.93.9.1667. [DOI] [PubMed] [Google Scholar]
- 4.Notarius CF, Butler GC, Ando S, Pollard MJ, Senn BL, Floras JS. Dissociation between microneurographic and heart rate variability estimates of sympathetic tone in normal subjects and patients with heart failure. Clin Sci (Lond) 1999;96:557–65. [PubMed] [Google Scholar]
- 5.Scalvini S, Volterrani M, Zanelli E, Pagani M, Mazzuero G, Coats AJ, Giordano A. Is heart rate variability a reliable method to assess autonomic modulation in left ventricular dysfunction and heart failure? Assessment of autonomic modulation with heart rate variability. Int J Cardiol. 1998;67:9–17. doi: 10.1016/s0167-5273(98)00252-6. [DOI] [PubMed] [Google Scholar]
- 6.Kingwell BA, Thompson JM, Kaye DM, McPherson GA, Jennings GL, Esler MD. Heart rate spectral analysis, cardiac norepinephrine spillover, and muscle sympathetic nerve activity during human sympathetic nervous activation and failure. Circulation. 1994;90:234–40. doi: 10.1161/01.cir.90.1.234. [DOI] [PubMed] [Google Scholar]
- 7.van de Borne P, Montano N, Pagani M, Oren R, Somers VK. Absence of low-frequency variability of sympathetic nerve activity in severe heart failure. Circulation. 1997;95:1449–54. doi: 10.1161/01.cir.95.6.1449. [DOI] [PubMed] [Google Scholar]
- 8.Piccirillo G, Magri D, Naso C, di Carlo S, MoisE A, De Laurentis T, Torrini A, Matera S, Nocco M. Factors influencing heart rate variability power spectral analysis during controlled breathing in patients with chronic heart failure or hypertension and in healthy normotensive subjects. Clin Sci (Lond) 2004;107:183–90. doi: 10.1042/CS20030401. [DOI] [PubMed] [Google Scholar]
- 9.Galinier M, Pathak A, Fourcade J, Androdias C, Curnier D, Varnous S, Boveda S, Massabuau P, Fauvel M, Senard JM, Bounhoure JP. Depressed low frequency power of heart rate variability as an independent predictor of sudden death in chronic heart failure. Eur Heart J. 2000;21:475–82. doi: 10.1053/euhj.1999.1875. [DOI] [PubMed] [Google Scholar]
- 10.Sleight P, La Rovere MT, Mortara A, Pinna G, Maestri R, Leuzzi S, Bianchini B, Tavazzi L, Bernardi L. Physiology and pathophysiology of heart rate and blood pressure variability in humans: is power spectral analysis largely an index of baroreflex gain? Clin Sci (Lond) 1995;88:103–9. doi: 10.1042/cs0880103. [DOI] [PubMed] [Google Scholar]
- 11.Saul JP, Arai Y, Berger RD, Lilly LS, Colucci WS, Cohen RJ. Assessment of autonomic regulation in chronic congestive heart failure by heart rate spectral analysis. Am J Cardiol. 1988;61:1292–9. doi: 10.1016/0002-9149(88)91172-1. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein DS, Holmes C, Li ST, Bruce S, Metman LV, Cannon RO. Cardiac sympathetic denervation in Parkinson disease. Ann Intern Med. 2000;133:338–47. doi: 10.7326/0003-4819-133-5-200009050-00009. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein DS, Eldadah BA, Holmes C, Pechnik S, Moak J, Saleem A, Sharabi Y. Neurocirculatory abnormalities in Parkinson disease with orthostatic hypotension. Independence from levodopa treatment. Hypertension. 2005;46:1333–9. doi: 10.1161/01.HYP.0000188052.69549.e4. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein DS, Eldadah B, Holmes C, Pechnik S, Moak J, Sharabi Y. Neurocirculatory abnormalities in chronic orthostatic intolerance. Circulation. 2005;111:839–45. doi: 10.1161/01.CIR.0000155613.20376.CA. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein DS, Holmes C, Frank SM, Dendi R, Cannon RO, Sharabi Y, Esler MD, Eisenhofer G. Cardiac sympathetic dysautonomia in chronic orthostatic intolerance syndromes. Circulation. 2002;106:2358–65. doi: 10.1161/01.cir.0000036015.54619.b6. [DOI] [PubMed] [Google Scholar]
- 16.Furlan R, Jacob G, Snell M, Robertson D, Porta A, Harris P, Mosqueda-Garcia R. Chronic orthostatic intolerance: a disorder with discordant cardiac and vascular sympathetic control. Circulation. 1998;98:2154–9. doi: 10.1161/01.cir.98.20.2154. [DOI] [PubMed] [Google Scholar]
- 17.Lord SW, Clayton RH, Mitchell L, Dark JH, Murray A, McComb JM. Sympathetic reinnervation and heart rate variability after cardiac transplantation. Heart. 1997;77:532–8. doi: 10.1136/hrt.77.6.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldstein DS, Eisenhofer G, Dunn BB, Armando I, Lenders J, Grossman E, Holmes C, Kirk KL, Bacharach S, Adams R. Positron emission tomographic imaging of cardiac sympathetic innervation using 6-[18F]fluorodopamine: initial findings in humans. J Am Coll Cardiol. 1993;22:1961–71. doi: 10.1016/0735-1097(93)90786-z. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein DS, Tack C. Non-invasive detection of sympathetic neurocirculatory failure. Clin Auton Res. 2000;10:285–91. doi: 10.1007/BF02281111. [DOI] [PubMed] [Google Scholar]
- 20.Goldstein DS, Horwitz D, Keiser HR. Comparison of techniques for measuring baroreflex sensitivity in man. Circulation. 1982;66:432–9. doi: 10.1161/01.cir.66.2.432. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein DS, Brush JE, Jr, Eisenhofer G, Stull R, Esler M. In vivo measurement of neuronal uptake of norepinephrine in the human heart. Circulation. 1988;78:41–8. doi: 10.1161/01.cir.78.1.41. [DOI] [PubMed] [Google Scholar]
- 22.Koh J, Brown TE, Beightol LA, Ha CY, Eckberg DL. Human autonomic rhythms: vagal cardiac mechanisms in tetraplegic subjects. J Physiol. 1994;474:483–95. doi: 10.1113/jphysiol.1994.sp020039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saul JP, Rea RF, Eckberg DL, Berger RD, Cohen RJ. Heart rate and muscle sympathetic nerve variability during reflex changes of autonomic activity. Am J Physiol. 1990;258:H713–21. doi: 10.1152/ajpheart.1990.258.3.H713. [DOI] [PubMed] [Google Scholar]
- 24.Wiklund U, Koskinen LO, Niklasson U, Bjerle P, Elfversson J. Endoscopic transthoracic sympathicotomy affects the autonomic modulation of heart rate in patients with palmar hyperhidrosis. Acta Neurochir (Wien) 2000;142:691–6. doi: 10.1007/s007010070114. [DOI] [PubMed] [Google Scholar]
- 25.Kawamata YT, Kawamata T, Omote K, Homma E, Hanzawa T, Kaneko T, Namiki A. Endoscopic thoracic sympathectomy suppresses baroreflex control of heart rate in patients with essential hyperhidrosis. Anesth Analg. 2004;98:37–9. doi: 10.1213/01.ANE.0000094984.90178.33. [DOI] [PubMed] [Google Scholar]
- 26.Verlato G, Polati E, Speranza G, Finco G, Gottin L, Ischia S. Both right and left cervical cordotomies depress sympathetic indexes derived from heart rate variability in humans. J Electrocardiol. 2001;34:309–17. doi: 10.1054/jelc.2001.27843. [DOI] [PubMed] [Google Scholar]
- 27.Iellamo F, Legramante JM, Massaro M, Galante A, Pigozzi F, Nardozi C, Santilli V. Spontaneous baroreflex modulation of heart rate and heart rate variability during orthostatic stress in tetraplegics and healthy subjects. J Hypertens. 2001;19:2231–40. doi: 10.1097/00004872-200112000-00017. [DOI] [PubMed] [Google Scholar]
- 28.Goldstein RE, Beiser GD, Stampfer M, Epstein SE. Impairment of autonomically mediated heart rate control in patients with cardiac dysfunction. Circ Res. 1975;36:571–8. doi: 10.1161/01.res.36.5.571. [DOI] [PubMed] [Google Scholar]
- 29.Cody RJ, Franklin KW, Kluger J, Laragh JH. Mechanisms governing the postural response and baroreceptor abnormalities in chronic congestive heart failure: effects of acute and long-term converting-enzyme inhibition. Circulation. 1982;66:135–42. doi: 10.1161/01.cir.66.1.135. [DOI] [PubMed] [Google Scholar]
- 30.Creager MA. Baroreceptor reflex function in congestive heart failure. Am J Cardiol. 1992;69:10G–15G. doi: 10.1016/0002-9149(92)91250-8. discussion 15G-6G. [DOI] [PubMed] [Google Scholar]
- 31.Cevese A, Gulli G, Polati E, Gottin L, Grasso R. Baroreflex and oscillation of heart period at 0.1 Hz studied by alpha-blockade and cross-spectral analysis in healthy humans. J Physiol. 2001;531:235–44. doi: 10.1111/j.1469-7793.2001.0235j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.deBoer RW, Karemaker JM, Strackee J. Hemodynamic fluctuations and baroreflex sensitivity in humans: a beat-to-beat model. Am J Physiol. 1987;253:H680–9. doi: 10.1152/ajpheart.1987.253.3.H680. [DOI] [PubMed] [Google Scholar]