Abstract
Rationale: Severe respiratory syncytial virus (RSV) bronchiolitis has been associated with deficient IFN-γ production in humans, but the role of this cytokine in determining the outcome of reinfection is unknown.
Objectives: To define the role of IFN-γ in the development of RSV-mediated airway hyperresponsiveness (AHR) and lung histopathology in mice.
Methods: Wild-type (WT) and IFN-γ knockout mice were infected with RSV in the newborn or weaning stages and reinfected 5 weeks later. Airway responses were assessed on Day 6 after the primary or secondary infection.
Measurements and Main Results: Both WT and IFN-γ knockout mice developed similar levels of AHR and airway inflammation after primary infection. After reinfection, IFN-γ knockout mice, but not WT mice, developed AHR, airway eosinophilia, and mucus hyperproduction. Intranasal administration of IFN-γ during primary infection but not during reinfection prevented the development of these altered airway responses on reinfection in IFN-γ knockout mice. Adoptive transfer of WT T cells into IFN-γ knockout mice before primary infection restored IFN-γ production in the lungs and prevented the development of altered airway responses on reinfection. Treatment of mice with IFN-γ during primary neonatal infection prevented the enhancement of AHR and the development of airway eosinophilia and mucus hyperproduction on reinfection.
Conclusions: IFN-γ production during primary RSV infection is critical to the development of protection against AHR and lung histopathology on reinfection. Provision of IFN-γ during primary infection in infancy may be a potential therapeutic approach to alter the course of RSV-mediated long-term sequelae.
Keywords: respiratory syncytial virus, interferon-γ, asthma, airway hyperresponsiveness, mice
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Deficient IFN-γ production has been associated with respiratory syncytial virus (RSV) bronchiolitis. However, the role of IFN-γ in determining the outcome of reinfection with RSV remains unknown.
What This Study Adds to the Field
IFN-γ plays a critical role during initial infection that determines the outcome of reinfection with RSV. Provision of IFN-γ may interfere with the development of altered airway responses to reinfection in children.
Respiratory syncytial virus (RSV) infection is the most common cause of infant bronchiolitis. Up to two-thirds of infants are infected with RSV during the first year of life, and almost all children are infected at least once by 2 years of age (1, 2). In adults, RSV infection exacerbates asthma, chronic obstructive lung disease, and bronchitis, and may cause severe pneumonia and death in the elderly (3, 4). To some extent, the development of these disorders may be related to lower concentrations of antibody in the serum; however, the pathophysiology of the disease has not been fully delineated (4). The clinical and epidemiologic associations that exist between RSV lower respiratory tract infection in early life and the subsequent development of persistent wheezing and asthma are well documented (5–7). Repeated infection is common in all age groups, and previous infection does not prevent subsequent infections (8, 9). However, factors that govern the development of altered airway function on reinfection are not understood.
RSV infection of mice has provided unique opportunities to investigate the relationships between airway function and RSV infection at the immunologic level (10). We demonstrated that primary RSV infection in the mouse results in lung inflammation and the development of airway hyperresponsiveness (AHR) at both the neonatal and weaning ages of mice (11). In this study, mice were initially infected with RSV shortly after birth or at weaning and reinfected 5 weeks later. Initial infection of mice at weaning elicited an extensive inflammatory response that was protective against development of AHR upon reinfection. In contrast, initial infection of neonates resulted in the development of enhanced AHR associated with airway eosinophilia and mucus hyperproduction on reinfection. Interestingly, what distinguished the two age groups was the IFN-γ response to initial RSV infection. Compared with weanling or adult mice, neonates demonstrated lower IFN-γ responses to initial RSV infection.
The role of IFN-γ in the pathogenesis of RSV-mediated airway disease is not well established. A number of studies demonstrated some imbalance in Th1/Th2 cytokine production, with a predominant Th2 response after RSV infection (12, 13). For the most part, this imbalance was attributed to deficient IFN-γ production associated with RSV lower respiratory tract infection at an early age (14–17). On the basis of these observations, we hypothesized that IFN-γ may play a critical role during initial RSV infection, particularly in determining the airway response to subsequent RSV infection. To test this hypothesis, we used IFN-γ–deficient (IFN-γ−/−) mice and performed reconstitution experiments with recombinant IFN-γ and adoptive T-cell transfer in a model of RSV infection and reinfection. The results demonstrate that IFN-γ is required during initial RSV infection for the expression of protective responses against development of AHR and lung histopathology on subsequent reinfection. Some of the results of these studies have been previously reported in the form of an abstract (18).
METHODS
Additional detail on the methods is provided in the online supplement.
Animals
Wild-type (WT) and IFN-γ–deficient (IFN-γ−/−) C57BL/6 and BALB/c mice were obtained from Jackson Laboratories (Bar Harbor, ME). BALB/c mice were bred in the institution's animal facility. Animals were used under an experimental protocol approved by the National Jewish Medical and Research Center Animal Care and Use Committee.
Virus Preparation and Animal Inoculation
Human RSV (strain A2) was obtained from American Type Culture Collection. Preparation of purified stocks of virus and inoculation of animals were performed as previously described (11, 19).
Experimental Design
WT and IFN-γ−/− C57BL/6 mice received primary RSV infection at 6 to 8 weeks of age. Newborn BALB/c mice received the primary RSV infection at 2 to 4 days of age. The secondary RSV infection was administered 5 weeks after the primary infection. Unless otherwise stated, all assays were performed on Day 6 after the primary or the secondary infection.
To confirm the role of IFN-γ, both adult IFN-γ−/− mice and newborn BALB/c mice were treated with recombinant mouse IFN-γ (10 ng; R&D Systems, Minneapolis, MN) administered on Days 1, 3 and 5 after infection, either during the primary or the secondary infection. In other experiments, IFN-γ−/− mice were reconstituted by adoptive transfer of purified IFN-γ–sufficient T cells, isolated from spleens of uninfected donor WT mice. The cells (4 × 106/recipient) were adoptively transferred by intravenous injection 1 day before the primary or the secondary RSV infection.
Airway Function
A flexiVent small-animal ventilator (SCIREQ, Montreal, PQ, Canada) was used to assess airway function in anesthetized, mechanically ventilated animals, measuring changes in lung resistance (Rl) in response to increasing doses of inhaled methacholine (MCh).
Airway Inflammation and Lung Histopathology
Airway inflammation was assessed by total and differential counting of leukocytes recovered in the bronchoalveolar lavage (BAL) fluid, and by examination of lung tissue sections stained with hematoxylin and eosin. Mucus-producing goblet cells were detected by staining of tissue sections using the periodic acid-Schiff method.
Lung Viral Load
Lung tissue viral load was determined by quantitative real-time polymerase chain reaction using specific oligonucleotide primers designed to detect a 65-bp fragment of the RSV N gene. Lung viral titers were determined by immuno-plaque assay as previously described (11).
Isolation of Lung Leukocytes and Culture
Lung tissue–infiltrating leukocytes were isolated as previously described using collagenase digestion (20), followed by centrifugation of cells in a 35% Percoll-containing solution (21). The leukocytes recovered in the pellet were washed and cultured in RPMI 1640 medium in the presence of ultraviolet light (UV)–inactivated RSV or an immunodominant H-2b-restricted epitope (M187–195) from the RSV M protein (22). Culture supernatants were harvested after 4 days of culture and used for assessment of IFN-γ production by ELISA.
Intracellular Cytokine Staining
Intracellular IFN-γ was detected by immunofluorescent staining of lung CD4 and CD8 T cells, as previously described (23). Staining was analyzed by flow cytometry on a FACScalibur (Becton Dickinson Biosciences, San Diego, CA) using CellQuest software with gating on the CD3-positive lymphoid cell population.
Statistical Analysis
Data are expressed as mean ± SEM of six to eight animals in each group. Statistical significance at P < 0.05 was determined by analysis of variance. Statistical differences between the groups were detected by multiple comparisons using Fisher's protected least significant difference test. For viral load, the data were analyzed by nonparametric statistics using the Kruskal-Wallis test.
RESULTS
IFN-γ Deficiency Does Not Influence the Development of AHR after Primary RSV Infection
Figure 1A shows the changes in Rl in response to increasing doses of inhaled MCh for both WT and IFN-γ−/− mice. Primary RSV infection at 6 to 8 weeks of age led to the development of AHR in both WT and IFN-γ−/− mice, illustrated by significant MCh dose-dependent increases in Rl as compared with the respective sham-inoculated control groups. The degree of AHR was similar in both strains. After primary infection, there were also no statistically significant differences between the two groups in the numbers of inflammatory cells recovered in the BAL fluid (Figure 1B). IFN-γ was detected but only in the BAL fluid of RSV-infected WT mice (290 ± 81 pg/ml). None of the cytokines (IL-4, IL-5 or IL-13) was detected after primary RSV infection in the BAL fluids of WT or IFN-γ−/− mice. In tissue, RSV infection resulted in the development of peribronchial and perivascular tissue infiltration predominantly by mononuclear cells in both strains of mice (Figure 1C). No differences in mucus-producing (periodic acid-Schiff–positive) goblet cell numbers were detected in the airways between both strains of mice (Figure 1D). However, after primary RSV infection, viral load was significantly higher in the lungs of IFN-γ−/− mice compared with WT mice (Table 1). These data suggest that, although IFN-γ may play a role in limiting viral load in the lung, it does not appear to contribute to or regulate the degree of AHR or airway inflammation during primary RSV infection.
Figure 1.
Airway responsiveness (A), the inflammatory profile in bronchoalveolar lavage fluid (B), lung histopathology (C), and mucus goblet cell numbers (D) after primary respiratory syncytial virus (RSV) infection in wild-type (WT) and IFN-γ−/− mice. Groups of mice were infected with RSV (n = 8/group) or sham inoculated (n = 6/group). Responses were assessed on Day 6 after infection. Both mouse groups developed significant airway hyperresponsiveness (A) with lymphocyte accumulation in the airways (B), and exhibited peribronchial airway tissue inflammation (C, arrowhead) and mucus production (C, arrows, and D). *Statistical difference between the groups (P < 0.05). Scale bar in C represents 100 μm. BM = basement membrane; Eos = eosinophil; Lym = lymphocyte; Mac = macrophage; Neu = neutrophil; 1°RSV = primary RSV infection.
TABLE 1.
LUNG VIRAL LOAD
| WT Mice | IFN-γ−/− Mice | |
|---|---|---|
| 1°RSV (n = 8) | 12,296 ± 5,642 | 23,617 ± 11,712* |
| 2°RSV (n = 8) | 1,018 ± 294† | 18,463 ± 5,513* |
| 2°RSV, after reconstitution with | ||
| rIFN-γ, during 1°RSV (n = 8) | 2,611 ± 1,233‡ | |
| rIFN-γ, during 2°RSV (n = 8) | 4,457 ± 2,173‡ | |
| T cells, during 1°RSV (n = 6) | 3,027 ± 877‡ | |
| T cells, during 2°RSV (n = 6) | 4,445 ± 1,275‡ |
Definition of abbreviations: rIFN-γ = recombinant IFN-γ; WT = wild-type; 1°RSV = primary RSV infection; 2°RSV = secondary RSV infection.
Data are mean ± SEM of copy viral RNA/mg lung tissue. Viral load was assessed on Day 6 postinoculation.
Statistical difference (IFN-γ−/− mice vs. WT mice, on both 1°RSV and 2°RSV), P < 0.05.
Statistical difference (2°RSV vs. 1°RSV, in WT mice), P < 0.01.
Statistical difference, compared to 2°RSV in IFN-γ−/− mice, P < 0.05.
IFN-γ−/− Mice Develop Significantly Greater AHR after Reinfection with RSV
To define the pattern of airway response to secondary RSV infection, WT and IFN-γ−/− mice were initially infected at 6 to 8 weeks of age and reinfected 5 weeks later, after full recovery and when no significant AHR or airway inflammation could be detected after the primary infection in either strain of mice (Figures EA–EC of the online supplement), as previously described for BALB/c mice (24). Secondary RSV infection did not result in the development of AHR in WT mice despite an intense inflammatory response in the lungs (Figures 2A–2D). In contrast, IFN-γ−/− mice developed a significant increase in AHR (Figure 2A), and a pronounced BAL eosinophilia (Figure 2B), associated with marked airway tissue inflammation and enhanced mucus production (Figures 2C–2D). After reinfection, lung viral load was markedly reduced in WT mice but not in the IFN-γ−/− mice when compared with primary infection (Table 1).
Figure 2.
Airway responsiveness (A), the inflammatory profile in bronchoalveolar lavage (BAL) fluid (B), lung histopathology (C), and mucus goblet cell numbers (D) after reinfection in wild-type (WT) and IFN-γ−/− mice. Mice were initially infected with respiratory syncytial virus (RSV) (n = 8/group) and reinfected 5 weeks later; controls received sham inoculation (n = 6/group). Airway responses were assessed on Day 6 after reinfection. After reinfection, IFN-γ−/− mice developed significant airway hyperresponsiveness (A), associated with BAL eosinophilia (B) and enhanced airway mucus production (C, arrows, and D). By contrast, WT mice did not develop these altered responses after reinfection, despite increased airway inflammation. *Statistical difference between the groups (P < 0.05). Scale bar in C represents 100 μm. BM = basement membrane; Eos = eosinophil; Lym = lymphocyte; Mac = macrophage; Neu = neutrophil; 2°RSV = secondary RSV infection.
When lung mononuclear cells were isolated after the primary or secondary infection of WT mice and restimulated in vitro, both CD4+ and CD8+ T cells were identified as a major source of IFN-γ production (Figure 3). Natural killer (NK) cells were not found to be a significant source of IFN-γ after restimulation in vitro. Compared with primary infection, the secondary infection elicited further increases in numbers as well as in frequencies of IFN-γ–producing CD4+ and CD8+ T cells in the lungs of these animals (Figures 3A and 3B). Among the CD4+ T cells, the frequency of IFN-γ–producing cells increased from 6.1 ± 2.2% after primary infection to 8.9 ± 1.9% after secondary infection, but this increase did not reach statistical significance. Among CD8+ T cells, the frequencies of IFN-γ–producing cells increased from 15.1 ± 3.7% after primary infection to 26.3 ± 4.1% after secondary infection (P < 0.05).
Figure 3.
IFN-γ–producing lung T cells and natural killer (NK) cells. (A) Flow cytometry scatter plot showing IFN-γ–positive cells within gated CD4+ and CD8+ lung cell populations. (B) Numbers of IFN-γ–producing CD4 and CD8 T cells, and NK cells. CD4+ and CD8+ T cells were the predominant source of IFN-γ in the lung of respiratory syncytial virus (RSV)–infected wild-type mice. The number of these cells was significantly increased after reinfection of these mice. *Statistical difference between primary and secondary infection (P < 0.05).
Provision of IFN-γ during Primary Infection but Not Secondary Infection Restores Protection against the Development of AHR after Reinfection of IFN-γ−/− Mice
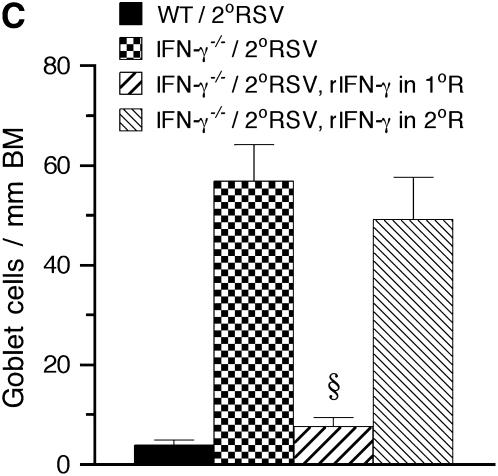
To determine whether reconstitution of IFN-γ in IFN-γ−/− mice would alter airway responsiveness to reinfection, we administered recombinant mouse IFN-γ during the primary or secondary RSV infection. Airway function, airway inflammation, and lung viral load were assessed in these animals on Day 6 after secondary infection and the results were compared with those obtained in WT and IFN-γ−/− mice that also were exposed to primary and secondary RSV infection. Administration of recombinant IFN-γ (rIFN-γ) during either the primary or the secondary infection was associated with significantly lower lung viral loads measured on Day 6 after reinfection (Table 1). However, when administered to IFN-γ−/− mice during secondary infection, rIFN-γ did not prevent the development of AHR (Figure 4A), BAL eosinophilia (Figure 4B), or mucus hyperproduction (Figure 4C). In striking contrast, administration of rIFN-γ during the primary infection resulted in significant attenuation of AHR, mucus production, and BAL eosinophilia on reinfection of these IFN-γ−/− mice (Figures 4A–4C). After reinfection, none of the Th2 cytokines (IL-4, IL-5, or IL-13) was detected in the BAL fluid of WT mice (Figure 4D). By contrast, significant levels of IL-5 and IL-13 were detected in the BAL fluid of IFN-γ−/− mice. IL-4 levels were below the detection limit (<8 pg/ml). Treatment of IFN-γ−/− mice with rIFN-γ, administered during the primary infection but not during the secondary infection, led to significant decreases in IL-5 and IL-13 levels in the BAL fluids of these mice. These results suggested that, to be effective, IFN-γ must be administered during primary RSV infection where it plays an essential role, leading to protection against the subsequent development of AHR and lung histopathology (eosinophilia and mucus hyperproduction) on reinfection.
Figure 4.
Effect of treatment with IFN-γ on airway responsiveness to reinfection in IFN-γ−/− mice. IFN-γ−/− mice were administered recombinant IFN-γ (rIFN-γ) during either the primary or the secondary respiratory syncytial virus (RSV) infection. Airway responses were assessed on Day 6 after reinfection. Compared with untreated IFN-γ−/− mice, mice administered rIFN-γ during the primary infection, but not during the secondary infection, developed a marked reduction in airway hyperresponsiveness (A), bronchoalveolar lavage (BAL) eosinophilia (B), mucus goblet cell metaplasia (C), and BAL Th2 (IL-5 and IL-13) cytokine levels (D) on reinfection. *Significant difference between the groups (P < 0.05); §significant difference compared with IFN-γ−/−/2°RSV group (P < 0.05). MCh = methacholine; WT = wild-type. See Figure 2 legend for definition of other abbreviations.
Reconstitution of T cells during Primary Infection Restores Protection against the Development of AHR after Reinfection of IFN-γ−/− Mice
Because T cells were identified as a major source of IFN-γ, the latter being required during primary infection to elicit protection against AHR on reinfection, we reasoned that transfer of T cells during the primary infection would restore protection in IFN-γ−/− mice. To this end, T cells were isolated from uninfected WT (IFN-γ–sufficient) donor mice and were adoptively transferred into recipient IFN-γ−/− mice before the primary or the secondary RSV infection. Airway responses were assessed on Day 6 after the secondary infection.
Adoptive transfer of T cells from uninfected donor WT mice into recipient IFN-γ−/− mice before primary or secondary RSV infection resulted in a significantly reduced lung viral load measure on Day 6 after reinfection (Table 1). Most important, adoptive transfer of T cells before the primary infection restored protection against the development of AHR, eosinophilic airway inflammation, and mucus hyperproduction on reinfection of the recipient IFN-γ−/− mice (Figures 5A–5C). Adoptive transfer of T cells before secondary infection did not prevent the development of these altered airway responses on reinfection of recipient IFN-γ−/− mice. After adoptive T-cell transfer (during either primary or secondary infection), the levels of IFN-γ recovered in the BAL fluid of recipient IFN-γ−/− mice were similar to those measured in BAL fluid of WT mice after the secondary infection (Figure 5D). However, the levels of IL-5 and IL-13 measured after reinfection in the BAL fluid of IFN-γ−/− mice were reduced only when T cells were transferred before the primary infection but not prior the secondary infection (Figure 5D). When lung leukocytes were isolated from the same recipient mice and stimulated in culture with UV-inactivated RSV or H-2b-restricted RSV peptide (M187–195), significant amounts of IFN-γ were produced (Figure 5E). These data clearly demonstrate that adoptively transferred IFN-γ–sufficient T cells restored IFN-γ production in the lungs of recipient IFN-γ−/− mice and mediated protection against the development of altered airway responses on reinfection of these animals but only when transferred during the primary infection.
Figure 5.
Effect of adoptive T-cell transfer on airway responsiveness to reinfection in IFN-γ−/− mice. IFN-γ–sufficient T cells, isolated from uninfected wild-type (WT) mice, were adoptively transferred into recipient IFN-γ−/− mice, before primary or secondary respiratory syncytial virus (RSV) infection. Airway responses were assessed on Day 6 after reinfection. (A) Airway responsiveness to inhaled methacholine (MCh); (B) bronchoalveolar lavage (BAL) cellularity; (C) mucus goblet cell metaplasia; (D) BAL cytokine levels; (E) in vitro IFN-γ production by isolated lung leukocytes. Transfer of IFN-γ–sufficient T cells before either primary or secondary RSV infection restored significant IFN-γ production in the lungs of recipient IFN-γ−/− mice (D, E). However, the development of airway hyperresponsiveness (A), BAL eosinophilia (B), and mucus goblet cell metaplasia (C) in these animals was only inhibited when IFN-γ–sufficient T cells were transferred before the primary but not the secondary RSV infection. *Significant difference between the groups (P < 0.05); §significant difference compared with IFN-γ−/−/2°RSV group (P < 0.05). BM = basement membrane; Eos = eosinophil; Lym = lymphocyte; Mac = macrophage; Neu = neutrophil; 1°R, 1°RSV = primary RSV infection; 2°R, 2°RSV = secondary RSV infection.
Provision of IFN-γ during Primary Neonatal Infection Prevents the Development of Enhanced AHR and Lung Histopathology on Reinfection with RSV
We have previously shown that, unlike weanling mice, newborn BALB/c mice produce very low levels of IFN-γ after primary RSV infection and develop enhanced AHR, airway eosinophilia, and mucus hyperproduction on reinfection (11). To further establish the importance of IFN-γ in determining the outcome of reinfection with RSV in newborn mice, we administered rIFN-γ to WT BALB/c mice during primary neonatal RSV infection or during the secondary RSV infection and assessed airway responses after the secondary infection.
As previously shown (11), mice initially infected as neonates developed enhanced AHR (Figure 6A), significant airway eosinophilia (Figure 6B), and mucus hyperproduction (Figures 6C and 6D) on reinfection with RSV. This response was also associated with increased levels of the Th2 cytokines IL-5 and IL-13 (IL-4 was below the detection limit of the assay), in addition to IFN-γ in the BAL fluid of these mice (Figure 6E).
Figure 6.
Effect of treatment with IFN-γ on airway responsiveness to reinfection in newborn mice. Newborn BALB/c mice were treated with recombinant IFN-γ (rIFN-γ), administered during primary neonatal infection or during reinfection. Airway responses were assessed on Day 6 after re-infection. (A) Airway responsiveness to inhaled methacholine (MCh); (B) bronchoalveolar lavage (BAL) cellularity; (C) lung histopathology; (D) mucus goblet cell metaplasia; (E) BAL cytokine levels; (F) lung viral titers; (G) in vitro cytokine production by peribronchial lymph node (PBLN) mononuclear cells. Treatment with rIFN-γ during primary neonatal infection, but not during reinfection, prevented the enhancement of airway hyperresponsiveness (A) and inhibited the development of airway eosinophilia (B), mucus hyperproduction (C, D), BAL Th2 cytokine levels (E), and Th2 cytokine production by PBLN cells on reinfection. IFN-γ levels (E, G) and lung viral titers (F) were not altered by IFN-γ treatment. *Significant difference compared with sham group (P < 0.05); §significant difference compared with 2°RSV group (P < 0.05). 1°RSV = primary RSV infection; 2°RSV = secondary RSV infection.
Administration of rIFN-γ during the primary neonatal infection but not during the secondary infection markedly inhibited the subsequent enhancement of AHR (Figure 6A) and development of airway eosinophilia (Figure 6B) and mucus production (Figures 6C and 6D), and reduced Th2 (IL-5 and IL-13) cytokine levels in the BAL fluid on reinfection of the newborn mice (Figure 6E). BAL IFN-γ levels (Figure 6E) as well as lung viral titers (Figure 6F) were not altered in these mice by treatment with rIFN-γ.
When mononuclear cells were isolated from peribronchial lymph nodes after reinfection of the mice initially infected as newborns and restimulated in culture with RSV, increased levels of Th2 cytokines (IL-4, IL-5, and IL-13) were detected in the culture supernatants as well as IFN-γ (Figure 6G). Interestingly, treatment of mice with rIFN-γ during the primary neonatal infection but not during the secondary infection resulted in significantly lower Th2 (IL-4, IL-5, and IL-13) cytokine production without altering IFN-γ production by these cells.
These data further demonstrate that deficiency of IFN-γ production during initial RSV infection predisposes to the development of altered and Th2-biased airway responses (enhanced AHR, airway eosinophilia, and mucus hyperproduction) on subsequent RSV infection. Administration of rIFN-γ during the primary neonatal RSV infection prevented the development of these altered responses, including the production of Th2 cytokines.
DISCUSSION
The objectives of this study were to define the role of IFN-γ in the induction of protective responses against the development of altered airway function after reinfection with RSV. Because recurrent RSV infection is frequent in humans, we hypothesized that the consequences of reinfection with RSV may be determined at the time of initial infection, where IFN-γ could play a key regulator of the response. In an earlier study (11), neonatal RSV infection was shown to predispose mice to develop more severe airway disease on reinfection. This amplified and altered response to reinfection was characterized by the development of significant AHR associated with a marked airway eosinophilia and mucus hyperproduction. By contrast, primary infection at later age (e.g., at weaning) elicited a protective airway response to reinfection characterized by an exuberant lymphocytic inflammatory response, but without development of AHR or airway eosinophilia and mucus hyperproduction. Interestingly, the most distinctive difference observed between the two ages in terms of response to primary RSV infection was lower IFN-γ production in the lungs of infected newborn mice. The present study determined that the outcome of reinfection with RSV (development of, or protection against, altered airway responses) is dependent on IFN-γ production during primary RSV infection.
The results of this study demonstrate that IFN-γ is not essential to the development of AHR during primary RSV infection, because IFN-γ−/− mice developed AHR to the same extent as did WT mice after primary RSV infection. This finding contradicts an early report, which suggested that IFN-γ might contribute to RSV-mediated AHR in mice (25); however, AHR was not documented in this study (e.g., by assessment of airway responsiveness to MCh) nor was the outcome of reinfection examined in IFN-γ−/− mice. Durbin and colleagues compared the responses of IFN-γ−/− and WT mice to primary RSV infection and found no differences in lung pathology, cytokine levels, or viral replication and rate of clearance between the two strains of mice even after inoculation with high doses of RSV (i.e., 107 PFUs [plaque-forming units]) (26). In contrast, mice lacking STAT1, a common signaling pathway shared by IFN-γ and IFN-α/β, developed marked lung pathology characterized by a prominent eosinophilia, a Th2-biased cytokine response, but no significant alteration in rate of viral clearance after primary RSV infection. Surprisingly, all these strains of mice including IFN-γ−/− mice appeared to be protected and did not develop signs of illness as defined by weight loss after reinfection with RSV. Nonetheless, it was concluded that STAT1 activation by both type I (α/β) and type II (γ) IFNs plays an important role in establishing a protective Th1-mediated immune response to RSV infection. In the present study, no significant differences were seen between IFN-γ−/− mice and WT mice in AHR or airway inflammation after primary RSV infection, which is not inconsistent with the findings reported by Durbin and colleagues (26). However, our data clearly show that IFN-γ−/− mice are not protected from, but rather predisposed to, developing altered airway responses (AHR, eosinophilia, and mucus hyperproduction) on reinfection, further establishing a critical role for IFN-γ in determining the outcome of reinfection with RSV. Interestingly, treatment of IFN-γ−/− mice by administration of rIFN-γ during the primary RSV infection prevented the subsequent development of altered airway responses on reinfection of these mice; treatment during reinfection was without effect. Likewise, adoptive transfer of IFN-γ–sufficient T cells into IFN-γ−/− mice during the primary infection but not during reinfection also prevented the development of altered airway responses on reinfection in IFN-γ−/− mice. These data support our hypothesis that deficient (or absence of) IFN-γ production during initial RSV infection predisposes to the development of altered airway responses on subsequent reinfection with this virus. This is further revealed in neonatal mice in which deficient IFN-γ production during initial RSV infection appears to be the basis for the subsequent development of altered and Th2-biased airway responses (enhanced AHR, airway eosinophilia, and mucus hyperproduction) on reinfection with RSV.
IFN-γ, a cytokine mainly produced by NK cells and activated CD4+ and CD8+ T cells, promotes cell-mediated immune responses to intracellular pathogens such as viruses and has well-characterized antiviral activity (27). The role of IFN-γ in RSV-mediated disease is not well understood, but there is an apparent association between severity of RSV bronchiolitis and IFN-γ production. Earlier studies reported that infants hospitalized for severe lower respiratory tract illness due to RSV infection and requiring ventilation had lower IFN-γ production by blood mononuclear cells compared with those with a milder illness (15, 28). Moreover, the levels of IFN-γ measured in nasopharyngeal aspirates were lower in infants hospitalized for severe RSV bronchiolitis compared with those exhibiting milder disease. In one study, deficient IFN-γ production by blood mononuclear cells at the time of RSV bronchiolitis was found to be an indicator of lower pulmonary function and increased airway responsiveness to histamine 5 months later, and appeared to predict the development of asthma in infants hospitalized for severe RSV bronchiolitis (14). A prospective study found that infants who developed lower respiratory tract disease (bronchiolitis) had lower levels of IFN-γ in nasal lavage fluids compared with those who developed upper respiratory tract disease alone after RSV infection (17). Recent findings from a study of hospitalized infants suggested that decreased IFN-γ production is more a characteristic of lower respiratory tract illness related to RSV than other respiratory viruses (29). Taken together, these human studies suggest an important role for initial IFN-γ production in determining the outcome of RSV-mediated airway disease.
IFN-γ production usually identifies a Th1-type response, which is often accompanied by a vigorous CD8+ cytolytic T lymphocyte (CTL) response to viral infection in animal models (30). By analogy, a weak IFN-γ and/or CD8+ CTL response could result in delayed virus clearance, leading in turn to an increased inflammatory response and more severe illness. Indeed, infants with a severe clinical course of RSV disease exhibit lower CD8+ T-cell and CD8+/CD25+ T-cell counts during the acute phase of illness (13). This is in accord with previous findings of RSV-specific cellular cytotoxic immune responses in infants with mild illness but not in those with the most severe disease (31), and with the findings of reduced numbers of CD8+ T cells in the peripheral blood of infants with severe disease, compared with infants with milder forms of illness caused by RSV (32).
There are several potential mechanisms by which impaired or lower IFN-γ production during primary RSV infection might be associated with enhanced AHR and airway inflammation after reinfection with RSV. Impaired IFN-γ at birth may facilitate skewing of T-cell differentiation toward a Th2 phenotype, thereby predisposing to subsequent development of atopic wheezing and asthma in children (33, 34). However, deficient IFN-γ may also reflect either decreased maturation of CD8+ T cells or decreased numbers of NK cells or Th1 cells. Previous in vitro studies have shown that addition of IFN-γ to activated T cells leads to acquisition of cytotoxic activities by CD8+ but not CD4+ T cells (35). In our study, the elevated viral load seen in mice lacking IFN-γ may reflect decreased cytolytic activity by CD8+ T cells, and the ability of IFN-γ–sufficient T cells to restore protection suggested that a T-cell source is sufficient for this protection. There is also evidence to suggest that both development and maturation of primary CD8+ T-cell responses are critically dependent on help from CD4+ T cells during priming (36, 37). Because both CD4+ and CD8+ T cells are a source of IFN-γ during RSV infection, interaction between both T-cell subsets is possibly required for mediating full protection. The nature of CD4 T-cell–mediated help is not clear but may involve production of IL-2 to facilitate CD8+ T-cell expansion and IFN-γ to promote the acquisition of cytotoxic activity by these cells. IFN-γ also regulates the production of IgG2a, a major class of complement-fixing and virus-neutralizing antibodies in mice; thus, a deficiency in IgG2a antibody production may also contribute to increased viral load in the lung. However, based on our findings, AHR appeared to correlate with lung immunopathology, not viral load.
Human RSV is known to elicit an adaptive (virus-specific) T-cell–mediated immune response in mice despite a limited viral replication in the lung of these animals, which may be explained by species-related differences in host permissiveness to the pathogen. Usually, no apparent clinical signs of disease are observed in mice after infection with doses up to 106 PFUs of human RSV. However, clinical signs of disease (e.g., ruffled fur and significant weight loss) are typically observed on Days 3–4 after infection of mice with higher doses (107 PFUs) of human RSV (38). In humans, there is a lack of information as to what extent RSV can replicate in vivo in the lower airways and it is unclear whether severity of RSV disease (bronchiolitis) can directly be related to the rate of viral replication in the lower respiratory tract of children. Previous studies have described marked differences between human primary nasal epithelial cells, bronchial epithelial cells, and alveolar macrophages in permissiveness to RSV infection and viral replication in vitro (39). Although both nasal and bronchial epithelial cells were equally permissive to RSV infection in vitro, nasal epithelial cells produced much more virus than bronchial epithelial cells (10-fold at 0.1 multiplicity of infection [MOI], 3-fold at 1 MOI). Alveolar macrophages were much less permissive and restricted viral replication. These observations suggest that RSV replication might also be limited (restricted) in human lower airways, the major site of RSV-induced bronchiolitis and altered airway function (wheezing).
Analysis of cells recovered by lavage from the airways of children with acute bronchiolitis revealed predominant airway neutrophilia in some studies (40), whereas lymphocytes appear to be more prominent in tissue (41). However, the role of neutrophils in RSV-mediated disease remains unclear. In one study, repeated BAL samples were obtained from both term and preterm infants hospitalized for 7 days for severe RSV bronchiolitis requiring ventilation (42). Although neutrophils predominated during the first 2 to 3 days after intubation, the numbers of these cells declined progressively, reaching baseline control values by Day 4. By contrast, despite the severity of RSV bronchiolitis, preterm infants did not exhibit such a prominent BAL neutrophilia (no difference compared with nonbronchiolitic control infant group). In the mouse model, neutrophils never predominate in the BAL fluid at the peak of RSV-induced lung inflammation (i.e., Days 6–7 postinfection). However, a transient BAL neutrophilia can be observed early (Days 1–3) after RSV infection in mice (unpublished observations). We are currently investigating this neutrophilic response in the mouse model to define its role in RSV-induced airway inflammation and altered airway function.
The exact mechanisms of AHR are not really clear and there is not a single common pathway that leads to this alteration, which can be triggered by allergen, virus, or pollution. Studies of murine models of allergic airway responsiveness have established a key role for IL-13 as a downstream mediator that regulates AHR and mucus hyperproduction and contributes to airway eosinophilia (reviewed in Reference 43). This pathophysiology is believed to be initiated during sensitization by an IL-4–dependent mechanism that drives the development of Th2 response. We have previously shown that IL-13 is not required for the development of AHR after primary RSV infection (44). However, similar to allergen-mediated AHR and lung histopathology, the pathophysiology (AHR, airway eosinophilia, and mucus hyperproduction) that develops on reinfection of IFN-γ–deficient mice (adult IFN-γ−/− or newborn mice) with RSV is likely driven by a Th2 (IL-13) response that develops due to the deficient (or the absence of) IFN-γ production during initial RSV infection. The fact that rIFN-γ did not alter the responses when administered during reinfection suggests that IFN-γ has no direct effect on AHR and cannot reverse established Th2 responses and lung histopathology. On the other hand, the effectiveness of IFN-γ treatment during the primary RSV infection in preventing the subsequent Th2-biased, altered airway responses that develop on reinfection further emphasizes the important role IFN-γ plays during initial infection in determining the outcome of reinfection with RSV. IFN-γ enhances Th1 responses and is an important counterregulator of Th2 development, particularly during priming (45). Conceivably, deficiency or lack of IFN-γ production during initial RSV infection (priming) may result in the unopposed differentiation of a Th2-biased response, leading to development of eosinophilia, mucus hyperproduction, and AHR on subsequent reinfection with this virus.
In conclusion, the results of this study demonstrate that IFN-γ production at the time of initial infection is a critical factor that determines the outcome of reinfection with RSV in mice. In conjunction with several clinical reports, these findings are important to our understanding of the pathogenesis of post-RSV wheezing and asthma and may provide novel insights for the development of immunotherapeutic approaches for the prevention of RSV-mediated long-term sequelae in infants with severe RSV bronchiolitis.
Supplementary Material
Acknowledgments
The authors thank Ms. Diana Nabighian and Lynn Cunningham for their assistance.
Supported by NIH grants HD053557, HL-61005, and HL-36577, and Environmental Protection Agency grant R825702.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200612-1890OC on October 25, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child 1986;140:543–546. [DOI] [PubMed] [Google Scholar]
- 2.Hall CB. Respiratory syncytial virus: a continuing culprit and conundrum. J Pediatr 1999;135:2–7. [PubMed] [Google Scholar]
- 3.Nicholson KG, Kent J, Ireland DC. Respiratory viruses and exacerbations of asthma in adults. BMJ 1993;307:982–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falsey AR, Walsh EE. Relationship of serum antibody to risk of respiratory syncytial virus infection in elderly adults. J Infect Dis 1998;177:463–466. [DOI] [PubMed] [Google Scholar]
- 5.Stein RT, Sherrill D, Morgan WJ, Holberg CJ, Halonen M, Taussig LM, Wright AL, Martinez FD. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet 1999;354:541–545. [DOI] [PubMed] [Google Scholar]
- 6.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med 2000;161:1501–1507. [DOI] [PubMed] [Google Scholar]
- 7.Schauer U, Hoffjan S, Bittscheidt J, Kochling A, Hemmis S, Bongartz S, Stephan V. RSV bronchiolitis and risk of wheeze and allergic sensitisation in the first year of life. Eur Respir J 2002;20:1277–1283. [DOI] [PubMed] [Google Scholar]
- 8.Hall CB, Walsh EE, Long CE, Schnabel KC. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis 1991;163:693–698. [DOI] [PubMed] [Google Scholar]
- 9.Simoes EA. Respiratory syncytial virus infection. Lancet 1999;354:847–852. [DOI] [PubMed] [Google Scholar]
- 10.Schwarze J, Hamelmann E, Bradley KL, Takeda K, Gelfand EW. Respiratory syncytial virus infection results in airway hyperresponsiveness and enhanced airway sensitization to allergen. J Clin Invest 1997;100:226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dakhama A, Park JW, Taube C, Joetham A, Balhorn A, Miyahara N, Takeda K, Gelfand EW. The enhancement or prevention of airway hyperresponsiveness during reinfection with respiratory syncytial virus is critically dependent on the age at first infection and IL-13 production. J Immunol 2005;175:1876–1883. [DOI] [PubMed] [Google Scholar]
- 12.Renzi PM, Turgeon JP, Yang JP, Drblik SP, Marcotte JE, Pedneault L, Spier S. Cellular immunity is activated and a TH-2 response is associated with early wheezing in infants after bronchiolitis. J Pediatr 1997;130:584–593. [DOI] [PubMed] [Google Scholar]
- 13.Roman M, Calhoun WJ, Hinton KL, Avendano LF, Simon V, Escobar AM, Gaggero A, Diaz PV. Respiratory syncytial virus infection in infants is associated with predominant Th-2-like response. Am J Respir Crit Care Med 1997;156:190–195. [DOI] [PubMed] [Google Scholar]
- 14.Renzi PM, Turgeon JP, Marcotte JE, Drblik SP, Berube D, Gagnon MF, Spier S. Reduced interferon-gamma production in infants with bronchiolitis and asthma. Am J Respir Crit Care Med 1999;159:1417–1422. [DOI] [PubMed] [Google Scholar]
- 15.Aberle JH, Aberle SW, Dworzak MN, Mandl CW, Rebhandl W, Vollnhofer G, Kundi M, Popow-Kraupp T. Reduced interferon-gamma expression in peripheral blood mononuclear cells of infants with severe respiratory syncytial virus disease. Am J Respir Crit Care Med 1999;160:1263–1268. [DOI] [PubMed] [Google Scholar]
- 16.Bont L, Heijnen CJ, Kavelaars A, van Aalderen WM, Brus F, Draaisma JM, Pekelharing-Berghuis M, van Diemen-Steenvoorde RA, Kimpen JL. Local interferon-gamma levels during respiratory syncytial virus lower respiratory tract infection are associated with disease severity. J Infect Dis 2001;184:355–358. [DOI] [PubMed] [Google Scholar]
- 17.Legg JP, Hussain IR, Warner JA, Johnston SL, Warner JO. Type 1 and type 2 cytokine imbalance in acute respiratory syncytial virus bronchiolitis. Am J Respir Crit Care Med 2003;168:633–639. [DOI] [PubMed] [Google Scholar]
- 18.Lee YM, Miyahara N, Takeda K, Balhorn A, Joetham A, Gelfand EW, Dakhama A. Role of IFN-γ in the development of airway hyperresponsiveness following respiratory syncytial virus infection in mice [abstract]. Am J Respir Crit Care Med 2007;175:A760. [Google Scholar]
- 19.Dakhama A, Park JW, Taube C, Chayama K, Balhorn A, Joetham A, Wei XD, Fan RH, Swasey C, Miyahara N, et al. The role of virus-specific immunoglobulin E in airway hyperresponsiveness. Am J Respir Crit Care Med 2004;170:952–959. [DOI] [PubMed] [Google Scholar]
- 20.Oshiba A, Hamelmann E, Takeda K, Bradley KL, Loader JE, Larsen GL, Gelfand EW. Passive transfer of immediate hypersensitivity and airway hyperresponsiveness by allergen-specific immunoglobulin (Ig) E and IgG1 in mice. J Clin Invest 1996;97:1398–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goossens PL, Jouin H, Milon G. Dynamics of lymphocytes and inflammatory cells recruited in liver during murine listeriosis: a cytofluorimetric study. J Immunol 1991;147:3514–3520. [PubMed] [Google Scholar]
- 22.Rutigliano JA, Rock MT, Johnson AK, Crowe JE Jr, Graham BS. Identification of an H-2D(b)-restricted CD8+ cytotoxic T lymphocyte epitope in the matrix protein of respiratory syncytial virus. Virology 2005;337:335–343. [DOI] [PubMed] [Google Scholar]
- 23.Miyahara N, Takeda K, Kodama T, Joetham A, Taube C, Park JW, Miyahara S, Balhorn A, Dakhama A, Gelfand EW. Contribution of antigen-primed CD8+ T cells to the development of airway hyperresponsiveness and inflammation is associated with IL-13. J Immunol 2004;172:2549–2558. [DOI] [PubMed] [Google Scholar]
- 24.Dakhama A, Park JW, Taube C, El Gazzar M, Kodama T, Miyahara N, Takeda K, Kanehiro A, Balhorn A, Joetham A, et al. Alteration of airway neuropeptide expression and development of airway hyperresponsiveness following respiratory syncytial virus infection. Am J Physiol Lung Cell Mol Physiol 2005;288:L761–L770. [DOI] [PubMed] [Google Scholar]
- 25.van Schaik SM, Obot N, Enhorning G, Hintz K, Gross K, Hancock GE, Stack AM, Welliver RC. Role of interferon gamma in the pathogenesis of primary respiratory syncytial virus infection in BALB/c mice. J Med Virol 2000;62:257–266. [DOI] [PubMed] [Google Scholar]
- 26.Durbin JE, Johnson TR, Durbin RK, Mertz SE, Morotti RA, Peebles RS, Graham BS. The role of IFN in respiratory syncytial virus pathogenesis. J Immunol 2002;168:2944–2951. [DOI] [PubMed] [Google Scholar]
- 27.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-gamma. Annu Rev Immunol 1997;15:749–795. [DOI] [PubMed] [Google Scholar]
- 28.Bont L, Heijnen CJ, Kavelaars A, van Aalderen WM, Brus F, Draaisma JT, Geelen SM, van Vught HJ, Kimpen JL. Peripheral blood cytokine responses and disease severity in respiratory syncytial virus bronchiolitis. Eur Respir J 1999;14:144–149. [DOI] [PubMed] [Google Scholar]
- 29.Aberle JH, Aberle SW, Rebhandl W, Pracher E, Kundi M, Popow-Kraupp T. Decreased interferon-gamma response in respiratory syncytial virus compared to other respiratory viral infections in infants. Clin Exp Immunol 2004;137:146–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munoz JL, McCarthy CA, Clark ME, Hall CB. Respiratory syncytial virus infection in C57BL/6 mice: clearance of virus from the lungs with virus-specific cytotoxic T cells. J Virol 1991;65:4494–4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Isaacs D, Bangham CR, McMichael AJ. Cell-mediated cytotoxic response to respiratory syncytial virus in infants with bronchiolitis. Lancet 1987;2:769–771. [DOI] [PubMed] [Google Scholar]
- 32.De Weerd W, Twilhaar WN, Kimpen JL. T cell subset analysis in peripheral blood of children with RSV bronchiolitis. Scand J Infect Dis 1998;30:77–80. [DOI] [PubMed] [Google Scholar]
- 33.Tang ML, Kemp AS, Thorburn J, Hill DJ. Reduced interferon-gamma secretion in neonates and subsequent atopy. Lancet 1994;344:983–985. [DOI] [PubMed] [Google Scholar]
- 34.Liao SY, Liao TN, Chiang BL, Huang MS, Chen CC, Chou CC, Hsieh KH. Decreased production of IFN gamma and increased production of IL-6 by cord blood mononuclear cells of newborns with a high risk of allergy. Clin Exp Allergy 1996;26:397–405. [PubMed] [Google Scholar]
- 35.Gromo G, Geller RL, Inverardi L, Bach FH. Signal requirements in the step-wise functional maturation of cytotoxic T lymphocytes. Nature 1987;327:424–426. [DOI] [PubMed] [Google Scholar]
- 36.Wang JC, Livingstone AM. Cutting edge: CD4+ T cell help can be essential for primary CD8+ T cell responses in vivo. J Immunol 2003;171:6339–6343. [DOI] [PubMed] [Google Scholar]
- 37.Khanolkar A, Fuller MJ, Zajac AJ. CD4 T cell-dependent CD8 T cell maturation. J Immunol 2004;172:2834–2844. [DOI] [PubMed] [Google Scholar]
- 38.Catsro SM, Guerrero-Plata A, Suarez-Real G, Adegboyega PA, Colasurdo GN, Khan AM, Garofalo RP, Casola A. Antioxidant treatment ameliorates respiratory syncytial virus-induced disease and lung inflammation. Am J Respir Crit Care Med 2006;174:1361–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Becker S, Soukup J, Yankaskas JR. Respiratory syncytial virus infection of human primary nasal and bronchial epithelial cell cultures and bronchoalveolar macrophages. Am J Respir Cell Mol Biol 1992;6:369–374. [DOI] [PubMed] [Google Scholar]
- 40.Everard ML, Swarbrick A, Wrightham M, McIntyre J, Dunkley C, James PD, Sewell HF, Milner AD. Analysis of cells obtained by bronchial lavage of infants with respiratory syncytial virus infection. Arch Dis Child 1994;71:428–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson JE, Gonzales RA, Olson SJ, Wright PF, Graham BS. The histopathology of fatal untreated human respiratory syncytial virus infection. Mod Pathol 2007;20:108–119. [DOI] [PubMed] [Google Scholar]
- 42.McNamara PS, Ritson P, Selby A, Hart CA, Smyth RL. Bronchoalveolar lavage cellularity in infants with severe respiratory syncytial virus bronchiolitis. Arch Dis Child 2003;88:922–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taube C, Dakhama A, Gelfand EW. Insights into the pathogenesis of asthma utilizing murine models. Int Arch Allergy Immunol 2004;135:173–186. [DOI] [PubMed] [Google Scholar]
- 44.Park JW, Taube C, Young ES, Joetham A, Balhorn A, Takeda K, Miyahara N, Dakhama A, Donaldson DD, Gelfand EW. Respiratory syncytial virus-induced airway hyperresponsiveness is independent of IL-13 compared with that induced by allergen. J Allergy Clin Immunol 2003;112:1078–1087. [DOI] [PubMed] [Google Scholar]
- 45.Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today 1996;17:138–146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.