Abstract
Animal shoulder models are used to systematically investigate the factors influencing rotator cuff injury and repair. Each model has advantages and disadvantages that must be considered in the context of the specific research questions being asked. Herein we evaluate the utility of the canine model for studies of acute, full-thickness rotator cuff tendon injury and repair. We found that time zero failure load is dependent on the suture type and configuration used for repair. Acute, full-width tendon repairs fail anatomically within the first days after surgery in the canine model, regardless of suture type, suture configuration or post-operative protocol. Robust scar tissue forms in the gap between the failed tendon end and the humerus, which can be visually, mechanically and histologically misconstrued as tendon if an objective test of repair connectivity is not performed. We conclude that a full-width injury and repair model in the canine will provide a rigorous test of whether a new repair strategy or post-operative protocol (such as casting or temporary muscle paralysis) can maintain repair integrity in a high load environment. Alternatively, a partial-width tendon injury model allows loads to be shared between the tendon repair and the remaining intact portion of the infraspinatus tendon and prohibits complete tendon retraction. Thus a partial-width injury in the canine may model the mechanical environment of many single tendon tears in the human injury condition and warrants further investigation.
Introduction
Chronic rotator cuff tendon tears are a frequent cause for morbidity in the adult human population. It has been estimated that as much as 30% of the patient population seen by subspecialty shoulder surgeons may be related to rotator cuff pathology. Large, full-thickness cuff tears without surgical repair result in a persistent tendon defect and chronic exposure of the intra-articular joint surface and fluid. The unloaded muscle progresses to severe and irreversible atrophy and fibrosis40, and the affected muscle-tendon stiffens and becomes clinically difficult to mobilize and repair29.
Surgical repair of chronic tears is indicated when conservative treatment has failed to improve the patients' symptoms36. Repairs of large chronic cuff tears fail to heal in 20–95% of cases19, 20, 28. Several factors have been suggested to be responsible for this high failure rate. These include the size of tear11, 43, time from injury to repair7, tendon quality42, muscle quality26, biologic healing response27, and surgical technique30, 58.
Animal shoulder models are used to systematically investigate the factors influencing rotator cuff injury and repair. Each model has advantages and disadvantages that must be considered in the context of the specific research questions being asked. Features of the human injury condition that may be important to achieve in an animal model include: (a) an absence of spontaneous tendon healing or scar formation after tendon injury, (b) an intra-synovial injury environment, (c) a tendon size that allows for suture repair techniques used in humans, and (d) the ability to control post-operative rehabilitation. If investigation of chronic injury and repair is the object, then the animal model should also demonstrate (e) irreversible muscle atrophy, stiffening, and fatty infiltration of the associated muscle following chronic tendon injury (release).
The rat model developed by Soslowsky and colleagues is considered to have the greatest similarity to human based on anatomy (the presence of an acromial arch) and activity (overhead reaching)50. Because of the overlying acromial arch, the rat model has been particularly useful to study supraspinatus tendon injury mechanisms involved in the pathogenesis of rotator cuff disease50–52. These investigators have also used their model to study acute tendon to bone repair54, and the effect of post-operative activity levels (casting, free cage activity, exercise) on the acute healing response55. The rat model has also been used to investigate the pervasive clinical problem of chronic rotator cuff repair8, 24, 25. However, the absence of muscle fat accumulation in the chronic rat shoulder model is a significant departure from the human condition8. Further, the small size of the rat shoulder tendons makes the study of repair techniques utilized in human rotator cuff repair challenging.
Large animal models facilitate accuracy and reproducibility of injury and repair manipulations10. Rotator cuff injury and repair has been investigated in large animal models including rabbit15, 17, 18, 33–35, 37, 45, 57, goat16, 53, sheep12, 21, 23, 38, 39, 46 and dog1, 3, 4, 13, 31, 32, 41. We have chosen to further investigate the dog as a model for rotator cuff injury and repair as dogs tolerate casting14, 47, slinging48 and treadmill running2, allowing various post-operative rehabilitation protocols to be evaluated. It is our long-term research objective to investigate acute and chronic rotator cuff tendon injury and repair as well as tissue engineering strategies for enhancing tendon repair. The purpose of this initial study was to evaluate the utility of the canine model for studies of acute, full-thickness rotator cuff tendon injury and repair. We first investigated the time zero repair strength of various repair techniques in a cadaver model and subsequently evaluated several of these techniques (and two post-operative rehabilitation protocols) for in vivo repair of acute, full-thickness tendon injuries in the canine shoulder model. We hypothesized that an appropriate repair technique and post-operative rehabilitation protocol would result in successful healing of acute tendon injuries in the canine model.
Materials and Methods
Time zero mechanical properties of various repair techniques
Twenty-four cadaver shoulders from adult mongrel dogs (2 – 4 years, 25 – 35 kg) underwent infraspinatus tendon release and repair. Tendons were repaired via three transosseous bone tunnels using a variety of suture types (0-Ticron, 2-0 FiberWire®, 0-FiberWire®, 2- Orthocord™; DePuy Orthopaedics, Inc., Warsaw, IN) and configurations (simple, modified Mason Allen, Krakow) (Table I).
Table I.
Repair Biomechanics at Time Zero
| Canine Cadaver Samples | Suture Type | Suture Configuration | Failure Load (N) | Repair Gapping at 100 N (mm) | Repair Stiffness (between 50–100N) (N/mm) |
|---|---|---|---|---|---|
| n = 6 | 2-0 FiberWire® | 3X Simple | 88 ± 25a | Not Applicable | Not Applicable |
| n = 6 | 0-Ticron | 3X Modified Mason Allen | 151 ± 18b | 3.5 ± 0.2 | 25.4 ± 1.7 |
| n = 4 | 2-Orthocord™ | 2X Modified Mason Allen | 160 ± 19 | 2.9 ± 0.3 | 34.2 ± 4.5 |
| n = 2 | 0-FiberWire® | 2X Krakow 1X Modified Mason Allen | 333; 368 | Not Measured | Not Measured |
| n = 6 | 0-FiberWire® | 3X Modified Mason Allen | 236 ± 75a,b | 2.7 ± 1.8 (n=4) | 41.0 ± 24.3 (n=4) |
Denotes significant differences between groups with like notation using analysis of variance with a post-hoc Tukey test (p<0.05). No significant differences were detected among any groups for repair gapping or stiffness.
Biomechanical testing was used to quantify the repair gapping, stiffness and failure load of the various time-zero repair techniques. The bone-tendon-muscle complex was excised en bloc. The infraspinatus muscle, which envelops a large portion of the native tendon, was carefully dissected from the tendon, leaving a ~12 cm length of soft tissue for gripping. A custom cryo-clamp was used to grip the tendon approximately 35 mm from the bone interface. The humerus was secured in an aluminum pot with Cerrobend™ (Cerro-Bismuth Alloy; McMaster Carr, Chicago, IL). Two metal pins were placed in the humeral head as fixed markers at the lateral edge of the tendon repair site. Suture woven through the tendon tissue served as an optical marker on the tendon. Samples were positioned and tested in tension along their anatomic direction of pull. Because the cryo-clamp fixation precluded submersion of the samples in warm saline, samples were tested in air at room temperature and kept moist by spraying with saline. Repair samples were not preconditioned prior to failure tests, because of the limited strength of at least some of the repair methods. Samples were tested to failure on an MTS machine (MTS Systems Corp., Eden Prairie, MN) at 6 mm/min. A custom optical system, synchronized with the load data, was used to track the optical markers, and local displacements across the tendon-bone repair site were determined using custom texture correlation software9 (Matlab®, v 6.5; The MathWorks, Natick, MA). Repair stiffness was defined as the slope of the load versus (local) displacement data between 50 and 100N. Repair gapping was measured as the local displacement across the repair site at 100 N. Failure was defined as the lesser of either the maximum load point or the first decrease in load of at least 10 percent of the maximum load.
Acute repair with simple suture technique
In our first in vivo study, eight adult mongrel dogs (2 – 4 years, 25 – 35 kg) underwent infraspinatus tendon release and repair of the left shoulder. The infraspinatus tendon was sharply detached from its insertion at the greater tuberosity, however, the joint capsule was not compromised. The bone at the tendon insertion site was lightly decorticated. Similar to a repair technique described previously13, the detached infraspinatus tendon was repaired to the greater tuberosity bone bed utilizing three transosseous simple sutures (2–0 FiberWire®; Arthrex, Inc., Naples, FL). The tendon was also sutured to the underlying joint capsule with four simple sutures13. All wounds were irrigated, closed in layers and bandaged. Post-operatively, dogs were housed in individual cages and allowed unrestricted cage activity until sacrifice at 12 weeks. Biomechanical testing of the tendon-bone repairs was performed as described above except that these samples were preconditioned with five cycles from 0 – 50 N at 6 mm/min prior to failure testing. Eight, non-paired, normal infraspinatus tendon-bone samples were also tested for comparison to the repairs.
Acute repair with various repair techniques and post-operative rehabilitation protocols
In our second in vivo study, various repair techniques and post-operative protocols were investigated in a series of twelve adult mongrel dogs (2 – 4 years, 25 – 35 kg). Dogs underwent infraspinatus tendon release and repair of the left shoulder as described above except that two suture types (0-Ticron; Tyco Healthcare/Syneture, Norwalk, CT; 0-FiberWire®) and two configurations (modified Mason Allen, Krakow) were investigated (Table II). In this study, no sutures were placed between the tendon and the underlying, intact joint capsule. Post-operative slinging or low-ceiling housing was also investigated as means to reduce loading on the repairs (Table II). To objectively assess repair integrity, four small tantalum beads were implanted into the shoulder complex: two in the humeral head and two on the surface of the infraspinatus tendon near the myotendinous junction (Figure 1A). These beads were used to visualize the connectivity of the tendon-bone complex in vivo by 2D fluoroscopy at the time of surgery and within three weeks post-operatively (Figure 1B, 1C). Dogs were euthanized at one (n=2), two (n=3), three (n=2), six weeks (n=3) or twelve weeks (n=2) and gross observations of the repair were recorded. A sample of six week tendon-bone repair tissue from one dog was fixed in ten percent neutral buffered formalin, processed routinely, embedded in paraffin, sectioned to 6 microns and stained with hematoxylin and eosin for histologic analysis.
Table II.
Repair Method, Rehabilitation and Connectivity
| Dogs | Suture Type | Suture Configuration | Post-operative Rehabilitation | Repair Connectivity | Predominant Failure Mode |
|---|---|---|---|---|---|
| n = 3 | 0-Ticron | 3X Modified Mason Allen | Sling | Anatomic Failure (3 of 3 dogs) | Suture Break (8 of 9 sutures) |
| n = 3 | 0-FiberWire® | 3X Modified Mason Allen | Sling | Anatomic Failure (3 of 3 dogs) | Soft Tissue Amputate/Pullout (7 of 9 sutures) |
| n = 4 | 0-FiberWire® | 3X Modified Mason Allen | Low Ceiling (No Sling) | Anatomic Failure (4 of 4 dogs) | Soft Tissue Amputate/Pullout (6 of 6 sutures in n=2 dogs)* |
| n = 1 | 0-FiberWire® | 2X Krakow 1X Modified Mason Allen | Sling | Anatomic Failure (1 of 1 dog) | Soft Tissue Amputate/Pullout (3 of 3 sutures) |
| n = 1 | 0-FiberWire® | 2X Krakow 1X Modified Mason Allen | Low Ceiling (No Sling) | Anatomic Failure (1 of 1 dog) | Soft Tissue Amputate/Pullout (2 of 3 sutures) |
Two of the four dogs in this group were euthanized at twelve weeks; at this time the failure mode could not be determined because of the excessive scar tissue that had formed around the failure site.
Figure 1.
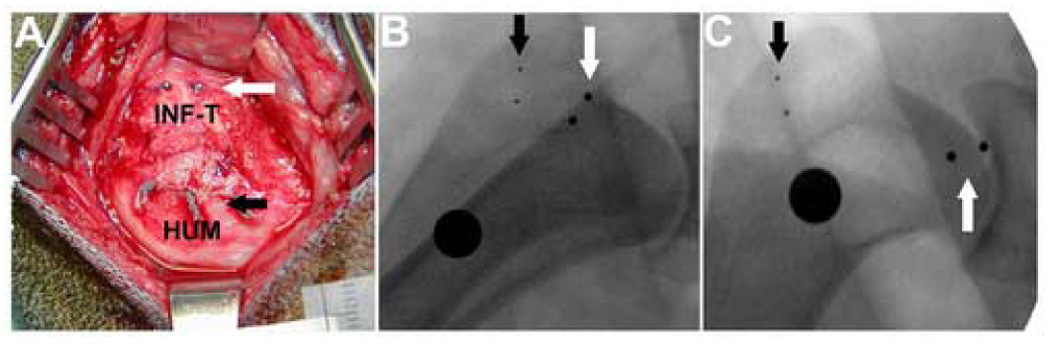
(A) To objectively assess repair connectivity, four small tantalum beads were implanted into the shoulder complex at the time of tendon repair: two embedded in the humerus (HUM) at the location of the black arrows and two on the surface of the infraspinatus tendon (INF-T) visualized at the white arrows. (B) Intra-operative fluoroscopy at the time of repair shows the tendon and bone beads as well as the calibration sphere (large black circle, OD = 9.5mm), (C) Follow-up fluoroscopy at 5 days post-op, demonstrates anatomic failure of the repair and approximately 2 cm retraction of the tendon stump.
Statistics
The mechanical properties of time zero repairs were compared using analysis of variance with a post-hoc Tukey test. The failure loads of 12-week acute infraspinatus repairs (using the simple suture technique) were compared to normal tendon using the Mann-Whitney Rank Sum test. Significance was set at α=0.05.
Results
Time zero biomechanical properties of various repair techniques
Time zero failure load is dependent on the suture type and configuration used for repair (Table I). The Krakow suture technique appeared to provide the highest time zero failure loads of all configurations tested; however, the suture severely strangulated and ultimately amputated the tendon. Hence we abandoned the Krakow technique after only two samples, and omitted the Krakow repair data from the statistical analysis. Repairs using three modified Mason-Allen sutures with 0-Fiberwire have approximately two-thirds the strength as the Krakow repairs at time zero, and are significantly stronger than the same repair technique with 0-Tricon or than repairs with three simple sutures (p<0.05). No significant differences were detected among repair groups for repair site gapping or stiffness (Table I).
Acute repair with simple suture technique
At sacrifice (12 weeks), well integrated, connective tissue was observed spanning muscle to bone in all cases (Figure 2). The regenerate tissue showed good integration to the humerus and joint capsule. However, careful inspection suggested that most of the repairs had not remained fully intact during the post-operative period. Repairs were classified as failed if any of the following criteria were met: (a) the in situ anatomical location of the lateral edge of the infraspinatus muscle was medial to the acromion, (b) the tendon stump was palpable medial to the repair site and/or (c) the infraspinatus muscle belly appeared fatty or atrophied compared to normal muscle. The repair construct conclusively met at least one of these failure criteria in 6 of 8 (75%) of the tendon repairs (e.g. Figure 2A vs. 2B). However, in all cases the tendon-like connective tissue spanning the original tendon defect from muscle to bone could be subjected to mechanical testing.
Figure 2.
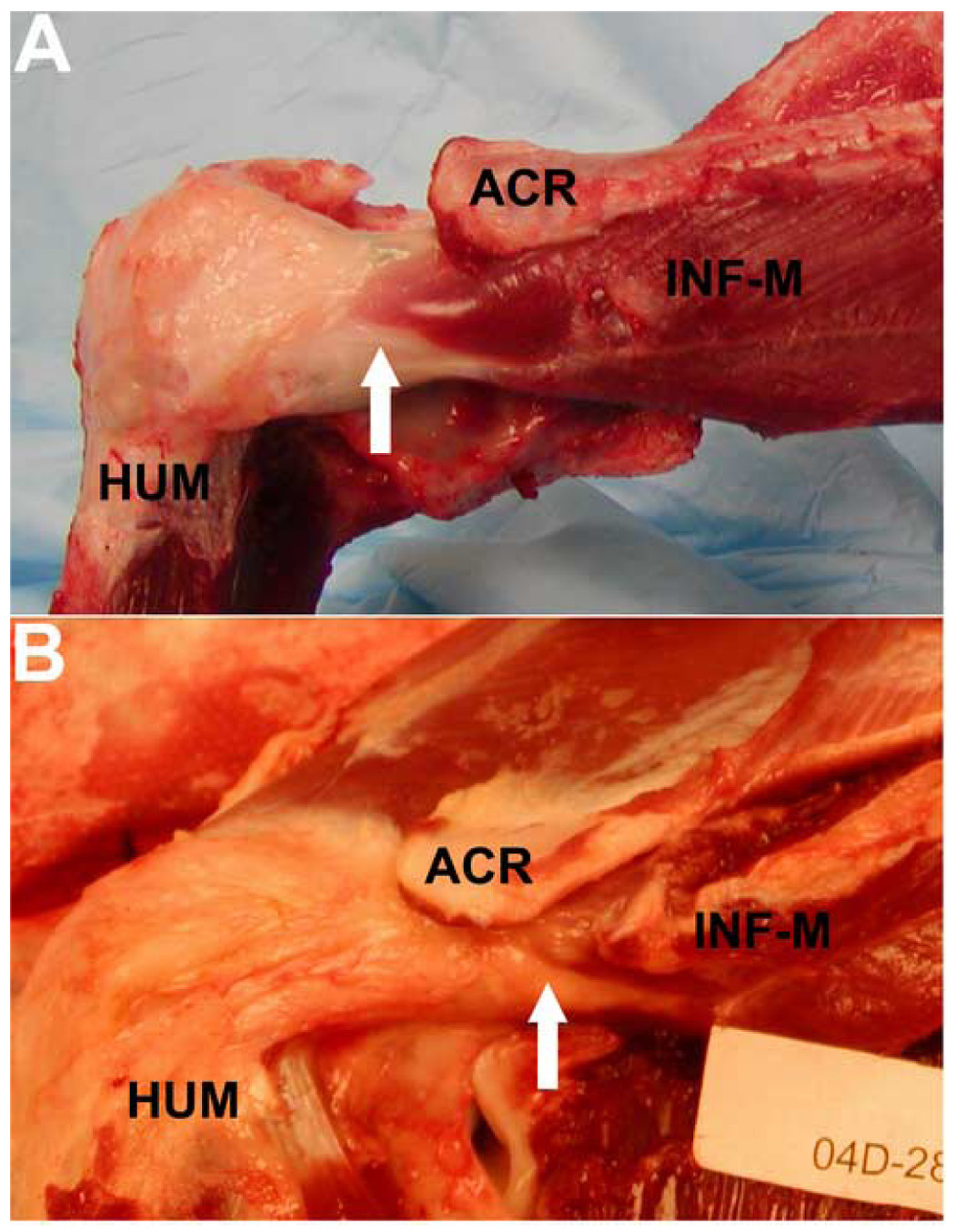
Acute infraspinatus repairs with simple sutures at sacrifice (12 weeks). A well integrated, connective tissue was observed spanning infraspinatus muscle (INF-M) to humerus (HUM) in all cases. Examples of a repair considered to have (A) remained intact or (B) failed during the post-operative period, based on the anatomic position of the lateral edge of the infraspinatus muscle (hashed arrows) relative to the acromion (ACR), are shown.
As a group, the failure loads of the repaired tendons were significantly less than normal at 12 weeks (Figure 3, p<0.05). Repair failure loads averaged 513.5 ± 524.9 N. Importantly, the two highest failure loads (1223.7; 1458.0) corresponded to the two samples considered to have remained intact during the 12 week post-operative period and were in the range of normal infraspinatus tendon-bone insertion (1349 ± 181 N, n = 8). These data demonstrate that the scar tissue that fills the gap between the failed tendon and its insertion site on the humerus is testable and yields moderate biomechanical properties, but a dramatically improved biomechanical outcome is achieved only if the repair remains intact.
Figure 3.
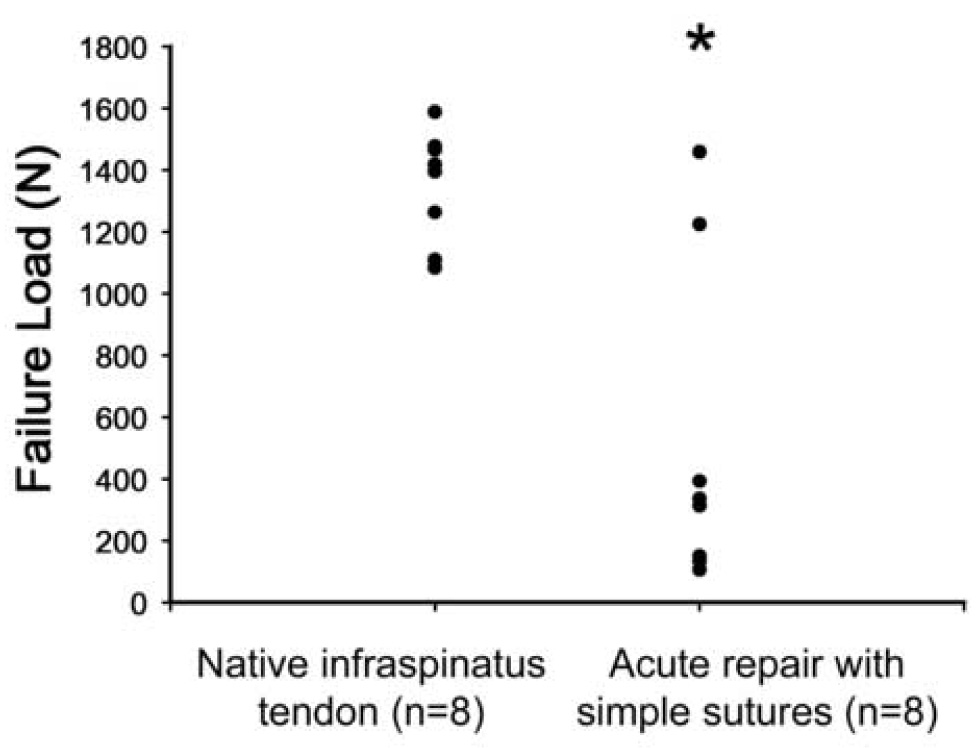
Failure loads for native infraspinatus tendon insertion compared to acute tendon injury and repair with simple sutures at 12 weeks (n = 8 in each group). The failure loads of the repaired tendons were significantly less than normal at 12 weeks (*, p<0.05).
Acute repair with various repair techniques and post-operative rehabilitation protocols
All of the tendon repairs, regardless of suture type, suture configuration or post-operative protocol, failed anatomically by the first post-operative x-ray, taken between 3 – 21 days (Table II). Figure 1 demonstrates the conclusive ability to identify repair failure with the x-ray technique. The failed tendon stump retracts 2 – 3 cm medially from the repair site. At sacrifice, the mode of failure was determined to be predominantly suture failure with Ticron suture, and soft tissue pullout or amputation with FiberWire® suture (Table II). No incidences of suture pulling through bone were observed.
Grossly, we observed that the retracted, failed tendon stump had adhered to surrounding soft tissues by two weeks Substantive scar tissue had formed in the 2–3 cm gap between the failed tendon end and the humerus by 3 weeks, which can be subjectively isolated as a tendon-like structure (Figure 4A). At six and twelve weeks, the gap scar tissue is robust (Figure 4B). In all cases, the failed tendon stump could be positively identified and discriminated from the scar tissue by locating the tantalum beads that had been placed on the tendon at the time of repair. Histologically, six week scar tissue demonstrated regions containing spindle-shaped nuclei and crimped, well-organized and oriented collagen (Figure 4C) as well as regions containing rounder nuclei and less-organized collagen.
Figure 4.
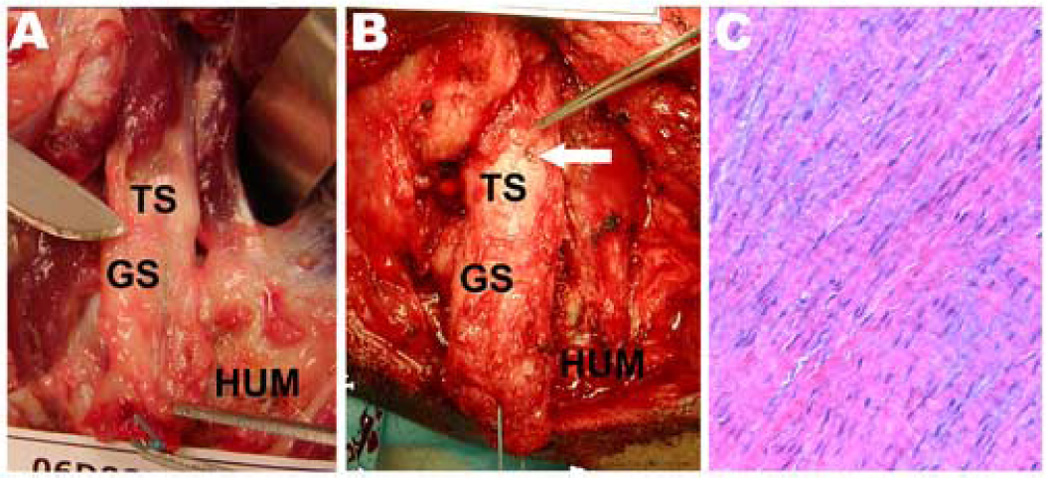
Robust, hypertrophic scar tissue formed in the 2–3 cm gap between the failed tendon stump (TS) and the humerus (HUM). Gap scar (GS) tissue at (A) three and (B) six weeks could be subjectively isolated as a tendon-like structure. The white arrow shows that one of the tantalum beads that had been placed on the tendon at surgery could be grossly visualized at dissection. (C) Histologically at six weeks, the isolated gap scar tissue demonstrates spindle-shaped nuclei and crimped, well-organized and oriented collagen (Hematoxylin-Eosin staining, 20X).
Discussion
The aim of this study was to evaluate the utility of the canine model for studies of acute, full-thickness rotator cuff tendon injury and repair. We noted that time zero failure load is dependent on the suture type and configuration used for repair. Repairs using the modified Mason-Allen technique have approximately two-thirds the strength as the Krakow repairs at time zero, but utilize less suture around and through the tendon stump than Krakow stitches. We feel that the modified Mason-Allen technique provides an appropriate balance of repair strength and tendon preservation, and recommend the use of a modified Mason-Allen technique with 0-FiberWire® for rotator cuff tendon repair in the canine model. This conclusion is supported by other large animal shoulder studies where the Mason-Allen suture technique has also been used12, 16, 22, 23, 46.
We also evaluated several repair techniques (and two post-operative rehabilitation protocols) for in vivo repair of acute, full-thickness tendon injuries in the canine shoulder. We hypothesized that an appropriate repair technique and post-operative rehabilitation protocol would result in successful healing of acute tendon injuries in this animal model. However, using a conclusive fluoroscopic technique to track beads implanted on the surface of the tendon repair, we found that acute, full-width tendon repairs fail anatomically within the first days after surgery in the canine model, regardless of suture type, suture configuration or post-operative protocol. Our results are supported by previous studies in other large animal shoulder models that have also reported repair failure (or gap formation) when animals were allowed full weight bearing16, 23, 46. In a comprehensive study of repair techniques in the sheep model, Gerber et al reported that “regardless of technique, none of the tested repairs would withstand the loads imposed by unprotected experimental conditions” and “protection from weight bearing [using a rubber ball affixed to the involved hoof or full body suspension by slinging] was essential”23. In our study, neither post-operative slinging nor housing in low-ceiling runs appear to protect the repairs from failure. Investigation of alternate methods to protect rotator cuff repairs in the early post-operative period in the canine model, such as casting14 or temporary muscle paralysis with botulinum toxin56 is warranted. Further, we are currently investigating the reparability of partial-width tendon injuries in this animal model. A partial-width injury model leaves a portion of the infraspinatus tendon intact such that load is shared with the repair and complete tendon retraction is disallowed-- conditions not dissimilar to many single tendon tears in the human injury condition.
In the canine shoulder model, robust scar tissue forms in the gap between the failed tendon end and the humerus that can be visually, mechanically and histologically misconstrued as tendon. Similar findings have been reported for failed repairs in the sheep model21, 23. These results may bring into question the interpretation of any previous study that used only a gross inspection of tendon-bone continuity to conclude that a repair remains intact post-operatively. Further, that different repair techniques in large animal models sometimes result in similar biomechanical outcomes46, 53 may be the consequence of testing gap scar tissue in all groups. It is clear that use of objective criteria to assess repair integrity in animal models is essential for accurate interpretation of outcomes. Fluoroscopic evaluation of beads implanted at the tendon repair site or even careful observation of the anatomic position of the myo-tendinous junction and muscle atrophy are simple and accurate means to assess repair integrity.
The data from our study show that the robust scar tissue associated with failed repairs has inferior biomechanical properties compared to intact repairs (Figure 3). In other words, an improved biomechanical outcome is achieved only if the repair remains intact. Hence, a full-width injury and repair model in the canine that tends to fail with standard techniques will provide a rigorous test of the ability of a new repair strategy or post-operative protocol to both maintain repair integrity and subsequently improve biomechanical outcomes. Further, if the amount of scar formation could be reduced (thus reproducing the human condition where a failed repair does not fill with scar tissue), then the canine model might demonstrate even greater biomechanical differences between failed and successful repair techniques and better predict outcomes for the human condition. Strategies to limit scar tissue formation at the repair site such as isolating the joint injury from the overlying deltoid and soft tissues using a silicone barrier21 are the subject of our current investigations.
Human rotator cuff tendons coalesce with the joint capsule near their humeral insertions, whereas canine shoulder tendons remain as distinct extra-articular structures (Figure 5A). Hence, human rotator cuff tears involve the joint capsule and result in exposure of the joint articular surfaces, whereas injury to the canine rotator cuff tendons neither involves the joint capsule nor compromises the joint when the tendon is released from its bony insertion. This anatomical difference between the human and canine shoulder can be abrogated by detaching the joint capsule from its humeral insertion and excising it back to the glenoid rim to create an intra-articular wound healing environment in the canine. Our ongoing studies are investigating whether a large joint capsule defect in the canine shoulder persists post-operatively, reduces scar formation and influences tendon-bone healing.
Figure 5.
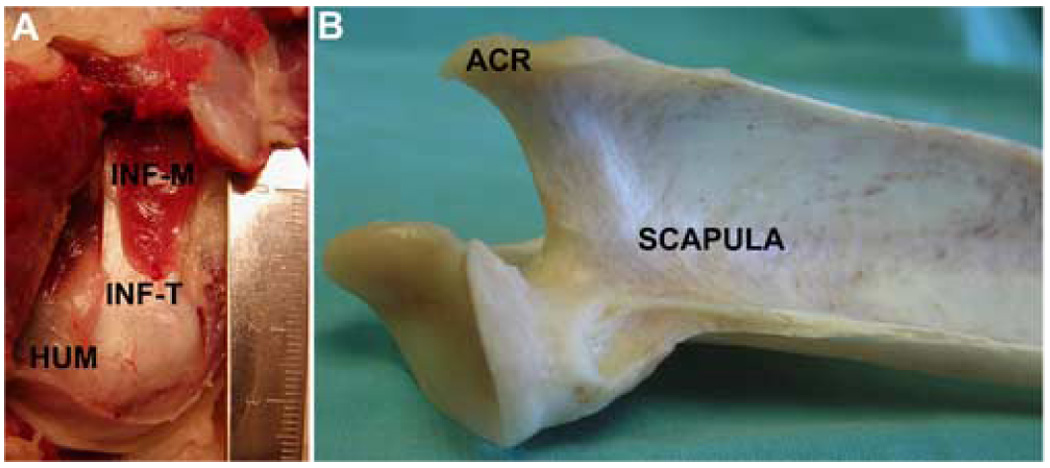
(A) The canine infraspinatus tendon (INF-T) connects the infraspinatus muscle (INF-M) to the humerus (HUM) and is a distinct extra-articular structure. (B) The canine scapula lacks a coracoid process. The canine acromion (ACR) is vestigial and does not cover the rotator cuff.
There are several limitations of the canine model of rotator cuff injury and repair that should be noted. First, there are unalterable differences between the bony anatomy of the canine and human shoulders. The canine acromion is vestigial and does not cover the rotator cuff (Figure 5B). The canine lacks a scapular coracoid process, a coraco-acromial ligament, and a clavicle. Second, there are differences in the biomechanics of the canine shoulder compared to the human. The canine is quadrupedal and its forelimb is weight bearing and used for gait with only limited overhead reaching. Canine shoulder stability is dependent primarily on the joint capsule and glenohumeral ligaments5, 6, 59 however, the periarticular musculature is also important for joint stability. The biceps tendon has been shown to be a primary passive stabilizer of the canine shoulder, while the infraspinatus tendon functions primarily as an active stabilizer49. Based on its bony anatomy and biomechanical loading, the canine shoulder model would not be appropriate to investigate the extrinsic factors contributing to the progression of rotator cuff disease (e.g., compression due to a reduction in the sub-acromial space). Furthermore, questions related to whether the presence of a coraco-acromial arch contributes to a decreased healing potential and/or an altered or complex loading environment for tendon repair can not be studied in the canine model. Finally, there is increased cost and management of the canine compared to smaller animals, which must be considered in the context of the research questions being asked.
In summary, the canine shoulder model has the advantage of being a practical size to facilitate accuracy and reproducibility of injury and repair manipulations. The canine lends itself to post-operative immobilization by sling or cast or rehabilitation protocols that involve treadmill running. Further, we have previously shown that the dog mimics the human condition in that muscle stiffness increases and atrophy and fat accumulation occurs and persists in chronically detached muscles44, demonstrating the canine model can be used to investigate treatment modalities of chronic muscle conditions. However, full-width acute tendon repairs fail in the early post-operative period using standard surgical techniques. Robust scar tissue forms in the gap between the failed tendon end and the humerus, which can be visually, mechanically and histologically misconstrued as tendon if an objective test of repair connectivity is not performed. We conclude that a full-width injury and repair model in the canine will provide a rigorous test of whether a new repair strategy or post-operative protocol (such as casting or temporary muscle paralysis) can maintain repair integrity in a high load environment. Alternatively, a partial-width tendon injury model that includes a clinically relevant joint capsule injury is currently being explored for acute and chronic tendon-bone healing in an intra-articular environment. A partial-width injury model allows loads to be shared between the tendon repair and the remaining intact portion of the infraspinatus tendon and prohibits complete tendon retraction. Thus a partial-width injury in the canine may model the mechanical environment of many single tendon tears in the human injury condition.
Funding Acknowledgements
NIH 1P30 AR-050953, NIH 1T32 AR-050959, NIH R21 PA-03-058
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Adams JE, Zobitz ME, Reach JS, Jr, An KN, Steinmann SP. Rotator cuff repair using an acellular dermal matrix graft: an in vivo study in a canine model. Arthroscopy. 2006 Jul;22(7):700–709. doi: 10.1016/j.arthro.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 2.Anderst WJ, Les C, Tashman S. In vivo serial joint space measurements during dynamic loading in a canine model of osteoarthritis. Osteoarthritis Cartilage. 2005 Sep;13(9):808–816. doi: 10.1016/j.joca.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 3.Aoki M, Miyamoto S, Okamura K, Yamashita T, Ikada Y, Matsuda S. Tensile properties and biological response of poly (L-lactic acid) felt graft: an experimental trial for rotator-cuff reconstruction. J Biomed Mater Res B Appl Biomater. 2004 Nov 15;71(2):252–259. doi: 10.1002/jbm.b.30084. [DOI] [PubMed] [Google Scholar]
- 4.Aoki M, Oguma H, Fukushima S, Ishii S, Ohtani S, Murakami G. Fibrous connection to bone after immediate repair of the canine infraspinatus: the most effective bony surface for tendon attachment. J Shoulder Elbow Surg. 2001 Mar;10(2):123–128. doi: 10.1067/mse.2001.111963. [DOI] [PubMed] [Google Scholar]
- 5.Bardet JF. Shoulder diseases in dogs. Vet Med. 2002;10:909–918. [Google Scholar]
- 6.Bardet JF. Diagnosis of shoulder instability in dogs and cats: a retrospective study. Journal of the American Animal Hospital Association. 1998 Jan;34(1):42–54. doi: 10.5326/15473317-34-1-42. [DOI] [PubMed] [Google Scholar]
- 7.Bartolozzi A, Andreychik D, Ahmad S. Determinants of outcome in the treatment of rotator cuff disease. Clin Orthop Rel Res. 1994 Nov;308:90–97. [PubMed] [Google Scholar]
- 8.Barton ER, Gimbel JA, Williams GR, Soslowsky LJ. Rat supraspinatus muscle atrophy after tendon detachment. J Orthop Res. 2005 Mar;23(2):259–265. doi: 10.1016/j.orthres.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 9.Bey MJ, Song HK, Wehrli FW, Soslowsky LJ. A noncontact, nondestructive method for quantifying intratissue deformations and strains. J Biomech Engin. 2002 Apr;124(2):253–258. doi: 10.1115/1.1449917. [DOI] [PubMed] [Google Scholar]
- 10.Carpenter JE, Thomopoulos S, Soslowsky LJ. Animal models of tendon and ligament injuries for tissue engineering applications. [Review] [117 refs] Clin Orthop Rel Res. 1999 Oct;367(Suppl):S296–S311. doi: 10.1097/00003086-199910001-00029. [DOI] [PubMed] [Google Scholar]
- 11.Cofield RH, Parvizi J, Hoffmeyer PJ, Lanzer WL, Ilstrup DM, Rowland CM. Surgical repair of chronic rotator cuff tears. A prospective long-term study. J Bone Jt Surg [Am] 2001 Jan;83-A(1):71–77. doi: 10.2106/00004623-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Coleman SH, Fealy S, Ehteshami JR, MacGillivray JD, Altchek DW, Warren RF, et al. Chronic rotator cuff injury and repair model in sheep. J Bone Jt Surg [Am] 2003 Dec;85-A(12):2391–2402. doi: 10.2106/00004623-200312000-00018. [DOI] [PubMed] [Google Scholar]
- 13.Dejardin LM, Arnoczky SP, Ewers BJ, Haut RC, Clarke RB. Tissue-engineered rotator cuff tendon using porcine small intestine submucosa. Histologic and mechanical evaluation in dogs. Am J Sports Med. 2001 Mar;29(2):175–184. doi: 10.1177/03635465010290021001. [DOI] [PubMed] [Google Scholar]
- 14.Derwin K, Androjna C, Spencer E, Safran O, Bauer TW, Hunt T, et al. Porcine small intestine submucosa as a flexor tendon graft. Clin Orthop Relat Res. 2004 Jun;(423):245–252. doi: 10.1097/01.blo.0000131235.91264.d7. [DOI] [PubMed] [Google Scholar]
- 15.Fabis J, Danilewicz M, Omulecka A. Rabbit supraspinatus tendon detachment: effects of size and time after tenotomy on morphometric changes in the muscle. Acta Orthop Scand. 2001 Jun;72(3):282–286. doi: 10.1080/00016470152846637. [DOI] [PubMed] [Google Scholar]
- 16.Fealy S, Rodeo SA, MacGillivray JD, Nixon AJ, Adler RS, Warren RF. Biomechanical evaluation of the relation between number of suture anchors and strength of the bone-tendon interface in a goat rotator cuff model. Arthroscopy. 2006 Jun;22(6):595–602. doi: 10.1016/j.arthro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Funakoshi T, Majima T, Iwasaki N, Yamane S, Masuko T, Minami A, et al. Novel chitosan-based hyaluronan hybrid polymer fibers as a scaffold in ligament tissue engineering. J Biomed Mater Res A. 2005 Sep 1;74(3):338–346. doi: 10.1002/jbm.a.30237. [DOI] [PubMed] [Google Scholar]
- 18.Funakoshi T, Majima T, Suenaga N, Iwasaki N, Yamane S, Minami A. Rotator cuff regeneration using chitin fabric as an acellular matrix. J Shoulder Elbow Surg. 2006 Jan;15(1):112–118. doi: 10.1016/j.jse.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 19.Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Jt Surg [Am] 2004 Feb;86-A(2):219–224. doi: 10.2106/00004623-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Gazielly DF, Gleyze P, Montagnon C. Functional and anatomical results after rotator cuff repair. Clin Orthop Rel Res. 1994 Jul;304:43–53. [PubMed] [Google Scholar]
- 21.Gerber C, Meyer DC, Schneeberger AG, Hoppeler H, Von Rechenberg B. Effect of tendon release and delayed repair on the structure of the muscles of the rotator cuff: an experimental study in sheep. J Bone Jt Surg [Am] 2004 Sep;86-A(9):1973–1982. doi: 10.2106/00004623-200409000-00016. [DOI] [PubMed] [Google Scholar]
- 22.Gerber C, Schneeberger AG, Beck M, Schlegel U. Mechanical strength of repairs of the rotator cuff. Journal of Bone & Joint Surgery - British Volume. 1994 May;76-B(3):371–380. [PubMed] [Google Scholar]
- 23.Gerber C, Schneeberger AG, Perren SM, Nyffeler RW. Experimental rotator cuff repair. A preliminary study. J Bone Jt Surg [Am] 1999 Sep;81(9):1281–1290. doi: 10.2106/00004623-199909000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Gimbel JA, Mehta S, Van Kleunen JP, Williams GR, Soslowsky LJ. The tension required at repair to reappose the supraspinatus tendon to bone rapidly increases after injury. Clin Orthop Rel Res. 2004 Sep;426:258–265. doi: 10.1097/01.blo.0000136831.17696.80. [DOI] [PubMed] [Google Scholar]
- 25.Gimbel JA, Van Kleunen JP, Mehta S, Perry SM, Williams GR, Soslowsky LJ. Supraspinatus tendon organizational and mechanical properties in a chronic rotator cuff tear animal model. J Biomech. 2004 May;37(5):739–749. doi: 10.1016/j.jbiomech.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 26.Goutallier D, Postel JM, Gleyze P, Leguilloux P, Van Driessche S. Influence of cuff muscle fatty degeneration on anatomic and functional outcomes after simple suture of full-thickness tears. Journal of Shoulder & Elbow Surgery. 2003 Nov;12(6):550–554. doi: 10.1016/s1058-2746(03)00211-8. [DOI] [PubMed] [Google Scholar]
- 27.Hamada K, Tomonaga A, Gotoh M, Yamakawa H, Fukuda H. Intrinsic healing capacity and tearing process of torn supraspinatus tendons: in situ hybridization study of alpha 1 (I) procollagen mRNA. J Orthop Res. 1997 Jan;15(1):24–32. doi: 10.1002/jor.1100150105. [DOI] [PubMed] [Google Scholar]
- 28.Harryman DT, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA., III Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Jt Surg [Am] 1991 Aug;73-A(7):982–989. [PubMed] [Google Scholar]
- 29.Hersche O, Gerber C. Passive tension in the supraspinatus musculotendinous unit after long-standing rupture of its tendon: a preliminary report. Journal of Shoulder & Elbow Surgery. 1998 Jul;7(4):393–396. doi: 10.1016/s1058-2746(98)90030-1. [DOI] [PubMed] [Google Scholar]
- 30.Iannotti JP. Full Thickness Rotator Cuff Tears: Factors Affecting Surgical Outcome. J Am Acad Orthop Surg. 1994 Feb;:87–95. doi: 10.5435/00124635-199403000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Inoue N, Ikeda K, Aro HT, Frassica FJ, Sim FH, Chao EY. Biologic tendon fixation to metallic implant augmented with autogenous cancellous bone graft and bone marrow in a canine model. J Orthop Res. 2002 Sep;20(5):957–966. doi: 10.1016/S0736-0266(02)00037-2. [DOI] [PubMed] [Google Scholar]
- 32.Kimura A, Aoki M, Fukushima S, Ishii S, Yamakoshi K. Reconstruction of a defect of the rotator cuff with polytetrafluoroethylene felt graft. Recovery of tensile strength and histocompatibility in an animal model. J Bone Joint Surg [Br] 2003 Mar;85(2):282–287. doi: 10.1302/0301-620x.85b2.12823. [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi M, Itoi E, Minagawa H, Miyakoshi N, Takahashi S, Tuoheti Y, et al. Expression of growth factors in the early phase of supraspinatus tendon healing in rabbits. J Shoulder Elbow Surg. 2006 May;15(3):371–377. doi: 10.1016/j.jse.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 34.Koike Y, Trudel G, Curran D, Uhthoff HK. Delay of supraspinatus repair by up to 12 weeks does not impair enthesis formation: a quantitative histologic study in rabbits. J Orthop Res. 2006 Feb;24(2):202–210. doi: 10.1002/jor.20031. [DOI] [PubMed] [Google Scholar]
- 35.Koike Y, Trudel G, Uhthoff HK. Formation of a new enthesis after attachment of the supraspinatus tendon: A quantitative histologic study in rabbits. J Orthop Res. 2005 Nov;23(6):1433–1440. doi: 10.1016/j.orthres.2005.02.015.1100230628. [DOI] [PubMed] [Google Scholar]
- 36.Mantone JK, Burkhead WZ, Jr, Noonan J., Jr Nonoperative treatment of rotator cuff tears. [Review] [56 refs] Orthopedic Clinics of North America. 2000 Apr;31(2):295–311. doi: 10.1016/s0030-5898(05)70149-8. [DOI] [PubMed] [Google Scholar]
- 37.Matsumoto F, Uhthoff HK, Trudel G, Loehr JF. Delayed tendon reattachment does not reverse atrophy and fat accumulation of the supraspinatus--an experimental study in rabbits. J Orthop Res. 2002 Mar;20(2):357–363. doi: 10.1016/S0736-0266(01)00093-6. [DOI] [PubMed] [Google Scholar]
- 38.Meyer DC, Hoppeler H, von RB, Gerber C. A pathomechanical concept explains muscle loss and fatty muscular changes following surgical tendon release. J Orthop Res. 2004 Sep;22(5):1004–1007. doi: 10.1016/j.orthres.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 39.Meyer DC, Lajtai G, von RB, Pfirrmann CW, Gerber C. Tendon retracts more than muscle in experimental chronic tears of the rotator cuff. J Bone Joint Surg Br. 2006 Nov;88(11):1533–1538. doi: 10.1302/0301-620X.88B11.17791. [DOI] [PubMed] [Google Scholar]
- 40.Meyer DC, Pirkl C, Pfirrmann CW, Zanetti M, Gerber C. Asymmetric atrophy of the supraspinatus muscle following tendon tear. Journal of Orthopaedic Research. 2005 Mar;23(2):254–258. doi: 10.1016/j.orthres.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 41.Oguma H, Murakami G, Takahashi-Iwanaga H, Aoki M, Ishii S. Early anchoring collagen fibers at the bone-tendon interface are conducted by woven bone formation: light microscope and scanning electron microscope observation using a canine model. J Orthop Res. 2001 Sep;19(5):873–880. doi: 10.1016/S0736-0266(01)00021-3. [DOI] [PubMed] [Google Scholar]
- 42.Riley GP, Harrall RL, Constant CR, Chard MD, Cawston TE, Hazleman BL. Tendon degeneration and chronic shoulder pain: changes in the collagen composition of the human rotator cuff tendons in rotator cuff tendinitis. Ann Rheum Dis. 1994 Jun;53(6):359–366. doi: 10.1136/ard.53.6.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Romeo AA, Hang DW, Bach BR, Jr, Shott S. Repair of full thickness rotator cuff tears. Gender, age, and other factors affecting outcome. Clin Orthop Rel Res. 1999 Oct;367:243–255. [PubMed] [Google Scholar]
- 44.Safran O, Derwin KA, Powell K, Iannotti JP. Changes in rotator cuff muscle volume, fat content, and passive mechanics after chronic detachment in a canine model. J Bone Joint Surg Am. 2005 Dec;87(12):2662–2670. doi: 10.2106/JBJS.D.02421. [DOI] [PubMed] [Google Scholar]
- 45.Sano H, Kumagai J, Sawai T. Experimental fascial autografting for the supraspinatus tendon defect: remodeling process of the grafted fascia and the insertion into bone. Journal of Shoulder & Elbow Surgery. 2002 Mar;11(2):166–173. doi: 10.1067/mse.2002.120808. [DOI] [PubMed] [Google Scholar]
- 46.Schlegel TF, Hawkins RJ, Lewis CW, Motta T, Turner AS. The effects of augmentation with Swine small intestine submucosa on tendon healing under tension: histologic and mechanical evaluations in sheep. Am J Sports Med. 2006 Feb;34(2):275–280. doi: 10.1177/0363546505279912. [DOI] [PubMed] [Google Scholar]
- 47.Seiler JG. Autogenous flexor-tendon grafts. A biomechanical and morphological study in dogs. J Bone Joint Surg [Am] 1993 Jul;75(7):1004–1014. doi: 10.2106/00004623-199307000-00006. [DOI] [PubMed] [Google Scholar]
- 48.Setton LA, Mow VC, Muller FJ, Pita JC, Howell DS. Mechanical behavior and biochemical composition of canine knee cartilage following periods of joint disuse and disuse with remobilization. Osteoarthritis Cartilage. 1997 Jan;5(1):1–16. doi: 10.1016/s1063-4584(97)80027-1. [DOI] [PubMed] [Google Scholar]
- 49.Sidaway BK, McLaughlin RM, Elder SH, Boyle CR, Silverman EB. Role of the tendons of the biceps brachii and infraspinatus muscles and the medial glenohumeral ligament in the maintenance of passive shoulder joint stability in dogs. American Journal of Veterinary Research. 2004 Sep;65(9):1216–1222. doi: 10.2460/ajvr.2004.65.1216. [DOI] [PubMed] [Google Scholar]
- 50.Soslowsky LJ, Carpenter JE, DeBano CM, Banerji I, Moalli MR. Development and use of an animal model for investigations on rotator cuff disease. Journal of Shoulder & Elbow Surgery. 1996 Sep;5(5):383–392. doi: 10.1016/s1058-2746(96)80070-x. [DOI] [PubMed] [Google Scholar]
- 51.Soslowsky LJ, Thomopoulos S, Esmail A, Flanagan CL, Iannotti JP, Williamson JD, III, et al. Rotator cuff tendinosis in an animal model: role of extrinsic and overuse factors. Ann Biomed Eng. 2002 Sep;30(8):1057–1063. doi: 10.1114/1.1509765. [DOI] [PubMed] [Google Scholar]
- 52.Soslowsky LJ, Thomopoulos S, Tun S, Flanagan CL, Keefer CC, Mastaw J, et al. Neer Award 1999. Overuse activity injures the supraspinatus tendon in an animal model: a histologic and biomechanical study. Journal of Shoulder & Elbow Surgery. 2000 Mar;9(2):79–84. [PubMed] [Google Scholar]
- 53.St Pierre P, Olson EJ, Elliott JJ, O'Hair KC, McKinney LA, Ryan J. Tendon-healing to cortical bone compared with healing to a cancellous trough. A biomechanical and histological evaluation in goats. J Bone Jt Surg [Am] 1995 Dec;77(12):1858–1866. doi: 10.2106/00004623-199512000-00010. [DOI] [PubMed] [Google Scholar]
- 54.Thomopoulos S, Hattersley G, Rosen V, Mertens M, Galatz L, Williams GR, et al. The localized expression of extracellular matrix components in healing tendon insertion sites: an in situ hybridization study. J Orthop Res. 2002 May;20(3):454–463. doi: 10.1016/S0736-0266(01)00144-9. [DOI] [PubMed] [Google Scholar]
- 55.Thomopoulos S, Williams GR, Soslowsky LJ. Tendon to bone healing: differences in biomechanical, structural, and compositional properties due to a range of activity levels. J Biomech Engin. 2003 Feb;125(1):106–113. doi: 10.1115/1.1536660. [DOI] [PubMed] [Google Scholar]
- 56.Tuzuner S, Balci N, Ozkaynak S. Results of zone II flexor tendon repair in children younger than age 6 years: botulinum toxin type A administration eased cooperation during the rehabilitation and improved outcome. J Pediatr Orthop. 2004 Nov;24(6):629–633. doi: 10.1097/01241398-200411000-00006. [DOI] [PubMed] [Google Scholar]
- 57.Uhthoff HK, Matsumoto F, Trudel G, Himori K. Early reattachment does not reverse atrophy and fat accumulation of the supraspinatus--an experimental study in rabbits. J Orthop Res. 2003 May;21(3):386–392. doi: 10.1016/S0736-0266(02)00208-5. [DOI] [PubMed] [Google Scholar]
- 58.Uhthoff HK, Trudel G, Himori K. Relevance of pathology and basic research to the surgeon treating rotator cuff disease. [Review] [39 refs] Journal of Orthopaedic Science. 2003;8(3):449–456. doi: 10.1007/s10776-002-0624-5. [DOI] [PubMed] [Google Scholar]
- 59.Vasseur PB, Moore D, Brown SA. Stability of the canine shoulder joint: an in vitro analysis. American Journal of Veterinary Research. 1982;43:352–355. [PubMed] [Google Scholar]


