Abstract
Functional disruption of either MC3R or MC4R results in obesity, implicating both in the control of energy homeostasis. The ligands for these receptors are derived from the prohormone POMC, which is post-translationally processed to produce a set of melanocortin peptides with a range of activities at the MC3R and MC4R. The relative importance of each of these peptides (α-MSH, γ3 MSH, γ2 MSH, γ lipotropin (γ-LPH) and, in man but not in rodents, β-MSH) in the maintenance of energy homeostasis is, as yet, unclear.
To investigate this further, equimolar amounts (2nmols) of each peptide were centrally administered to freely feeding, corticosterone supplemented, Pomc null (Pomc-/-) mice. Following a single dose at the onset of the dark cycle, α-MSH had the most potent anorexigenic effect, reducing food intake to 35% of sham treated animals. β-MSH, γ-LPH, γ3-and γ2- MSH all reduced food intake but to a lesser degree.
The effects of peptide administration over three days were also assessed. Only α-MSH significantly reduced body weight, affecting both fat and lean mass. Other peptides had no significant effect on body weight. Pair-feeding of sham-treated mice to those treated with α-MSH resulted in identical changes in total weight, fat and lean mass indicating that the effects of α-MSH were primarily due to reduced food intake rather than increased energy expenditure.
Although other melanocortins can reduce food intake in the short term, only α-MSH can reduce the excess fat and lean mass found in Pomc-/- mice, mediated largely through an effect on food intake.
Introduction
An intact melanocortin system is critical for normal energy homeostasis (1). Extensive data from human genetic and murine studies indicate that disrupted function of centrally expressed melanocortin receptors MC3R and MC4R results in obesity (2). Humans and mice with MC4R deficiency are characterized by an increase in fat and lean body mass, hyperphagia and severe hyperinsulinemia from a young age(3, 4). Homozygous null Mc3r mice exhibit a significant increase in fat mass (5, 6) and there is evidence that diminished MC3R expression may be a predisposing factor for excessive body weight gain in children (7).
The agonists for these two receptors are derived from the pro-hormone, pro-opiomelancortin (POMC). The POMC gene is actively transcribed in several tissues, including corticotrophs and melanotrophs within the pituitary, neurons originating in the hypothalamic arcuate nucleus, the brainstem and cells in the dermis and lymphoid system (8).
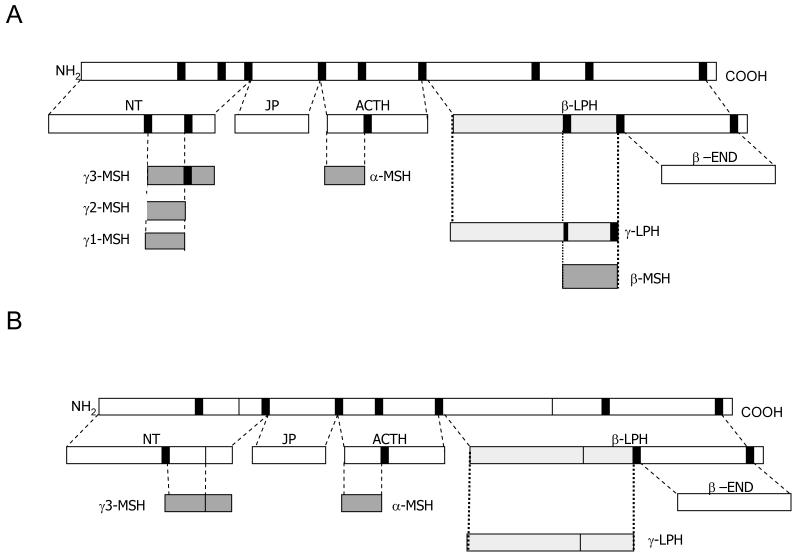
POMC itself is functionally inert but undergoes extensive, tissue-specific post-translation modification to generate a range of smaller, biologically active peptides (8) (Fig. 1). These include ACTH and α-, ß-, and γ-melanocyte stimulating hormone (MSH), collectively known as the melanocortins. The precise repertoire of POMC-derived products from any particular tissue is largely dependent on the range of processing enzymes expressed in that tissue. Thus, in humans, pituitary corticotrophs express prohormone convertase 1 (PC1), but not PC2, resulting in the production of N-terminal peptide, joining peptide, ACTH, and ß-lipotropin (β-LPH). In contrast, the expression of both PC1 and PC2 within the hypothalamus leads to the production of α-, ß-, and γ3-MSH, but not ACTH (8). γ3-MSH then undergoes further cleavage at a dibasic site to form the shorter peptide γ2-MSH. Cleavage of the carboxyl terminal alanine from γ2-MSH generates γ1-MSH.
Figure 1.
POMC processing in human (A) and mice (B). POMC is a large precursor peptide processed into smaller biologically active fragments by cleavage at dibasic cleavage sites (solid line). An important species difference between rodent and human is that only human have a dibasic site within γ-LPH to allow further processing of this larger peptide into the smaller melanocortin peptide, β-MSH. NT= N-terminal fragment, JP= joining peptide, β-LPH= β-lipotropin, β-END= β-endorphin.
A similar pattern of processing also occurs in mice, although there are important differences. Due to a lack of a dibasic site, β-LPH is processed no further than the intermediate γ-LPH, being unable to be fully processed into the smaller peptide β-MSH (9)(Fig. 1). Further, in contrast to humans, murine γ3-MSH lacks the Arg-Arg cleavage site required for formation of γ2-MSH.
Although impairment of the synthesis (10, 11) and processing (12, 13) of POMC also results in an obese phenotype, a precise understanding of the molecular physiology of POMC remains elusive. There is still uncertainty regarding the relative importance of particular POMC-derived melanocortin ligands in the processes that control energy balance and in particular whether α-, β- and γ-MSH (which are produced in equimolar amounts from the precursor peptide (14)) have unique, overlapping or redundant roles. It is clear from in vitro data that the three peptides have differing affinities to melanocortin receptors (15). Although α-MSH has equal affinity for MC3R and MC4R, β-MSH has the highest affinity for MC4R (16, 17) while γ3-MSH preferentially binds to MC3R (18). These differences between the in vitro characteristics of the melanocortins are illustrated by a recent study characterizing human MC4R polymorphisms that demonstrated β-MSH remained active at a number of receptors which were not stimulated by other melanocortins (19).
Several in vivo studies have compared the response of centrally administered melanocortins on food intake (20-22). α-, β- and γ-MSH appear to have differential effects upon short term feeding behaviour, both in terms of magnitude and time of onset of effect. However, none of these studies have been carried out in the total absence of endogenous melanocortins and none have directly compared the ability of the melanocortins to bring about changes in weight.
In this study we have used a mouse model lacking all endogenous POMC peptides (Pomc-/-) to further investigate the effects of the centrally derived melanocortin peptides upon food intake and body weight. By giving equimolar amounts of peptide icv to corticosterone-supplemented Pomc-/- mice, we wished to determine whether α-, β-, γ-MSH might have differential effects on the hyperphagia and excess fat and lean mass found in this mouse model. In addition we have also taken into account the species difference in POMC processing and investigated the effects of γ-LPH, the direct precursor of β-MSH, as well as using both γ3-MSH and γ2-MSH.
Methods
Pomc-/- mice
Pomc null mice were generated on a 129/SvEv background and genotypes were determined by PCR of DNA from ear tissue using a method previously described (11). All mice were maintained under controlled temperature (22 C) and light (12 h light from 7:00 a.m. -7:00 p.m.) and had ad libitum access to water and standard chow (4.5% fat chow; Special Diet Services, Witham, UK). Twelve week old male mice were used in all the studies and were individually caged throughout the duration of the experiment. All protocols were in accordance with the United Kingdom Home Office.
Intracerebroventricular (icv) studies
On day 0, mice underwent stereotaxic surgery to place an indwelling guide cannula into the lateral ventricle. Mice were anaesthetized with a mix of inhaled isoflurane and oxygen and a 26 gauge steel guide cannula (internal diameter 0.24 mm, outer diameter 0.46 mm, length 2mm (Semat International, Herts, UK) was implanted into the right lateral ventricle using the following co-ordinates: 1.0 mm lateral from bregma, 0.5 mm posterior to bregma. The guide cannula was secured to the skull using quick-drying cyanoacrylate glue and a dental cement (Associated Dental Products, Wiltshire, UK) and a dummy cannula was inserted. All animals received analgesia (Rimadyl, 5 mg/kg, Pfizer Animal Health, Kent,UK) and antibiotic (Teramycin LA, 60 mg/kg, Pfizer Animal Health, Kent, UK) before being returned to their home cage.
In addition, on day 0 drinking water was replaced by corticosterone-supplemented drinking water (as described below) which remained in place for the duration of the study. Our previous studies (23) have shown that without corticosterone supplementation, Pomc-/- mice are affected by a rapid fall off in body weight and food intake in the post-operative period. As the aim of the study was to directly compare the anorexigenic potencies of melanocortin peptides, we elected for continuous corticosterone supplementation.
On day 7, food intake and body weight were measured. If either parameter did not match pre-surgery values, mice were excluded from the study. On day 8, one hour before the onset of the dark cycle mice received either 2nmoles of peptide (α-MSH, γ-LPH, β-MSH, γ3-MSH or γ2-MSH) or PBS (sham) in a total volume of 2μl. This was administered using a Hamilton syringe over 2 minutes. Food intake was measured over at 2, 4, 12 and 24 hours.
On day 9 and 10, peptide was administered in an identical way to day 8 with food intake and body weight measured at 0800 each day.
On the morning of day 11 mice underwent a DEXA scan to assess body morphology and were sacrificed to collect blood and tissue. In addition, accurate cannula placement was confirmed by infusing 2μl of methylene blue dye into the indwelling cannula. Animals failing to show diffusion of the dye throughout the ventricular system were excluded from subsequent analysis.
Corticosterone replacement
Corticosterone replacement was given as supplemented drinking water at final concentration of 25μg/ml. Corticosterone was purchased from Sigma-Aldrich, Poole, UK. Corticosterone concentrations achieved in all 6 treatment groups were identical (sham vs α-MSH vs γ-LPH vs β-MSH vs γ3-MSH vs γ2-MSH: 50.9±23.0 vs 43.3±16.5 vs 45.4±18.0 vs 33.4±4.2 vs 42.4±23.6 vs 42.4±23.6, all p=n.s. vs sham).
Peptides
α-MSH (Ac-SYSMEHFRWGKPV-NH2, the acetylated form of α-MSH), β-MSH (H-DEGPYRMEHFRWGSPPKD-OH), γ3 MSH (HYVMGHFRWDRFGRRNSSSSGSSGAGQ-OH) and γ2-MSH (H-YVMGHFRWDRFG-OH) were all purchased from Bachem, St Helens, England.
γ-LPH (H-ELEGERPLGLEQVLESDAEKDDGPYRVEHFRWSNPPKD-OH) was custom synthesized by Pepscan Systems, Lelystad, The Netherlands. All peptides were dissolved in sterile phosphate buffered saline (PBS) with 2nmol of each peptide delivered in a volume of 2μl at each occasion.
Body Composition
Fat and lean body mass were determined by using dual-energy x-ray absorptiometry (Lunar PIXImus2 mouse densitometer, General Electric Medical Systems) as described by the manufacturer.
Blood glucose
Glucose analysis was done on tail vein blood using Lifescan One-Touch Ultra Glucometer (Lifescan, High Wycombe, U.K.).
Hormone assays
Plasma corticosterone (Immunodiagnostic, Tyne and Wear, U.K.) and insulin (Crystal Chem, Chicago, IL) were determined using commercially available kits according to the manufacturers’ protocols.
Statistics
All data are reported as means ± SE. All data sets were analyzed for statistical significance by using Student’s t test, except for the data presented in Figure 3, where significance was sought using 2-way ANOVA. The PRISM software package (GraphPad, San Diego, CA) was used for all analyses. Results were considered statistically significant at p< 0.05.
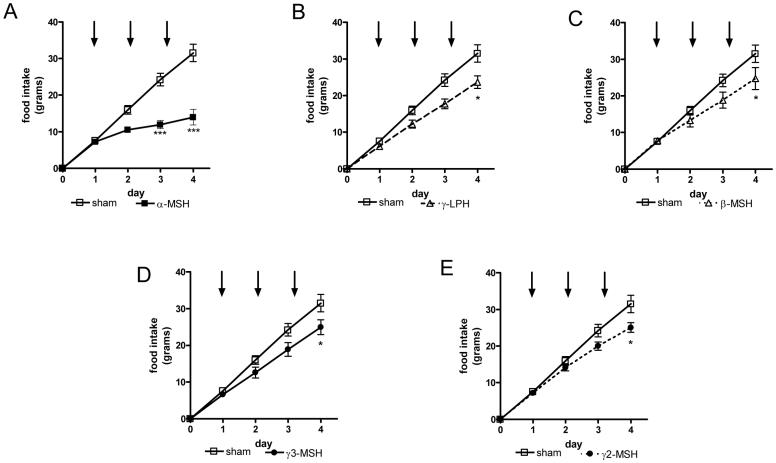
Figure 3.
The effects of repeated icv administration (once daily for three days, black arrows) of POMC-derived peptide on cumulative food intake; (A) α-MSH, (B) γ-LPH, (C) β-MSH, (D) γ3-MSH and (E) γ2-MSH. Again, 2 nmols of each peptide were administered to ad libitum fed mice one hour before the onset of the dark cycle (n=6-8 per group, * p<0.05, **p<0.01, ***p<0.001 vs sham)
Results
All centrally derived melanocortin peptides can reduce food intake
To determine the relative anorexigenic potency of each of the centrally derived melanocortins, we administered 2nmols of each peptide into the lateral ventricle of Pomc-/- mice who were also receiving corticosterone supplemented drinking water. All peptides were given 1 hour before the onset of the dark cycle with food intake over the subsequent 24 hour period recorded.
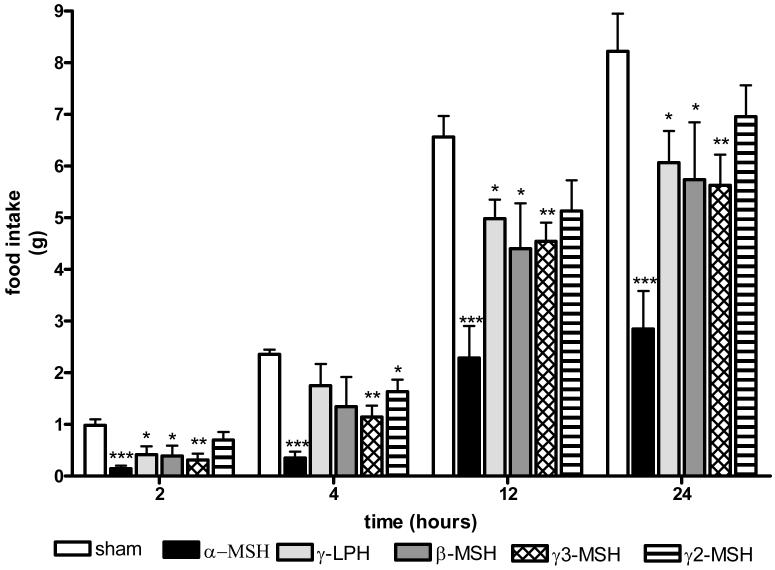
At all time points α-MSH caused the biggest reduction in food intake, with the amount consumed at 24 hours only a third of the amount eaten by sham treated animals ( sham vs α-MSH, 8.22±0.73 vs 2.85±0.74 g, p<0.001) (Fig. 2). γ3-MSH caused the second largest reduction in food intake, able to reduce food intake at all time points measured across the 24 hour period. Interestingly, γ-LPH and β-MSH had similar anorexigenic actions, although both were less effective than α- or γ3-MSH in reducing food intake. Finally, γ2-MSH was the least effective, only bringing about a significant reduction in food intake at 4 hours (sham vs γ2-MSH, 2.35±0.10 vs 1.63±0.24 g, p<0.05) (Fig. 1).
Figure 2.
The effects of a single intracerebroventricular (icv) dose of peptide on food intake. 2 nmols of each peptide were administered to ad libitum fed mice one hour before the onset of the dark cycle with food intake measured over the subsequent 24 hours at the time indicated (n=6-8 per group, * p<0.05, **p<0.01, ***p<0.001 vs sham)
Only α-MSH can reduce body weight in Pomc-/- mice
Although all the peptides given as a single dose were able to reduce food intake, none caused a significant reduction in body weight. We therefore wished to determine if repeated central administration of melanocortin peptides might be able to impact upon the excess fat and lean tissue mass we have previously reported in corticosterone-treated Pomc-/- mice (24) and in particular if different peptides might have differential effects upon this phenotype.
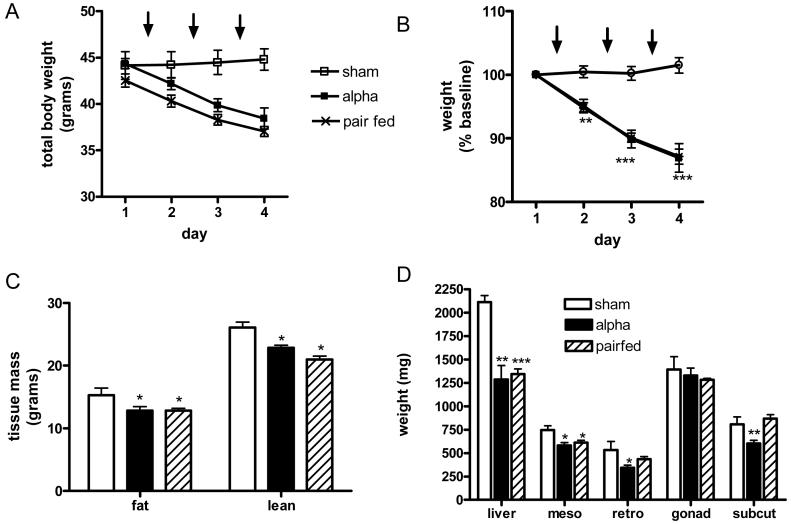
We therefore extended the study by administering equimolar amounts of each peptide for another two days, again with each dose given just prior to the onset of the dark cycle. Once again, α-MSH had the most potent effect on food intake, with cumulative food intake reduced to less than half that seen in sham animals (sham vs α-MSH, 31.50 ±2.38 vs 13.99±2.20 g, p<0.001) (Fig.3A). The other administered peptides also reduced cumulative food intake but their effects were all of a similar magnitude ( γ-LPH vs β-MSH vs γ3-MSH vs γ2-MSH, 23.63±1.72 vs 24.72±3.00 vs 24.72±3.00 vs 24.92±2.00 g, respectively, all p<0.05 vs sham) (Fig.3 B-E).
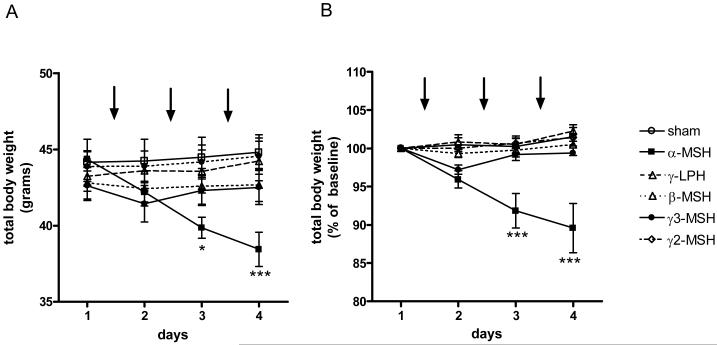
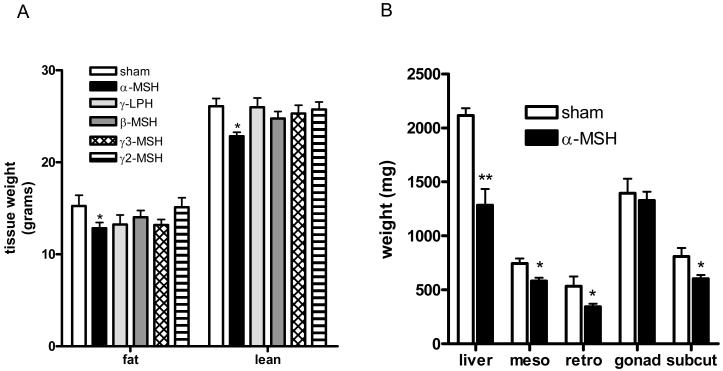
α-MSH was also the only administered peptide which brought about a significant reduction in weight. Thus, three doses of α-MSH were enough to cause a 6 gram weight loss in total body weight (Fig.4A), representing a reduction of 13% from baseline weight (Fig. 4B). Analysis of body composition by DEXA on the day following the third injection demonstrated this loss to be a combination of reduced fat and lean mass ( sham vs α-MSH; fat mass, 15.26±1.15 vs 12.82±0.63 g, p<0.05; lean mass, 26.08±0.86 vs 22.83±0.43 g, p<0.05) (Fig. 5A). Analysis of anatomical depots showed the fat loss to be from mesenteric (sham vs α-MSH, 745±46 vs 582±31 mg, p<0.01), retroperitoneal (534±90 vs 344±27 mg, p<0.05) and subcutaneous depots (809±78 vs 603±34, p<0.05) without any effect upon the gonadal fat pad (Fig. 5B). Whole liver mass was also significantly reduced following α- MSH treatment (2115±68 vs 1285±150, p<0.01) (Fig. 5B).
Figure 4.
The effects of repeated icv dosing on body weight shown as total body weight (A) and percentage of starting total body weight (B) over time. Arrows indicate timing of peptide administration (n=6-8 per group* p<0.05, ***p<0.001 vs sham).
Figure 5.
The effects of repeated icv administration (once daily for three days) of POMC-derived peptide on body composition. (A) Total lean and fat mass assessed by DEXA (B) Total liver mass and regional fat depot mass, meso=mesenteric fat, retro= retroperitoneal fat, gonad= gonadal fat, subcut= subcutaneous, (n=6-8 per group, * p<0.05, **p<0.01 vs sham).
Fat mass in γ3-MSH treated mice was reduced to 86% of that seen in sham treated animals but in absolute terms this failed to reach statistical significance (sham vs γ3-MSH, 15.26±1.1 vs 13.17±0.7 g, p=0.06). Further, although no difference was seen in mass of fat depots between sham and γ3-MSH treated mice, whole liver mass was significantly reduced with γ3-MSH treatment (sham vs γ3-MSH, 2115±68 vs 1776+94 mg, respectively, p<0.05).
Fed plasma glucose levels between the treatment groups were not significantly different (sham vs α-MSH vs γ-LPH vs β-MSH vs γ3-MSH vs γ2-MSH, 8.5±0.6 vs 6.6±1.1 vs 8.3±1.1vs 7.4±0.7 vs 8.7±1.4 vs 8.4±0.6 mmol, respectively).
However, in keeping with the marked reduction in food intake, α-MSH treated mice had a significantly lower plasma insulin compared to sham (4.6±1.0 vs 54.7±9.4 ng/ml, respectively, p<0.001). Insulin levels in the remaining treatment groups were also significantly reduced compared to sham, although again the magnitude of these effects were less than that of α-MSH ( γ-LPH vs β-MSH vs γ3-MSH vs γ2-MSH, 18.8±7.3 vs 22.4±7.8 vs 17.7±9.7 vs 30.8±7.7, respectively, all p<0.05 vs sham).
α-MSH brings about weight reduction in Pomc-/- mice primarily by reducing food intake
To being to determine if the weight loss seen following repeated icv dosing with α-MSH were due to reduced food intake alone, we undertook a pair feeding experiment. A further cohort of Pomc-/- mice received 3 sham injection icv as above with food intake matched to that consumed by α-MSH treated mice. The magnitude of the weight loss seen in these pair fed mice was near identical to that seen in α-MSH treated mice (Fig. 6 A&B) with a similar amount of fat and lean mass lost in pair-fed and α-MSH treated mice (Fig. 6C). There were differences in the amount lost from subcutaneous and retroperitoneal fat (α-MSH vs pair-fed: subcutaneous fat depot, 603±34 vs 868±42 mg, respectively, p<0.01; retroperitoneal, 344±27 vs 434±31 mg, respectively, p<0.05) but the other anatomical fat depots measured were indistinguishable. Plasma insulin levels seen following pair feeding were also no different from levels measured following α-MSH treatment (3.6±0.1 vs 4.6±1.0 ng/ml, respectively, n.s.).
Figure 6.
Pair-feeding Pomc-/- mice to the food intake seen following icv α-MSH. Arrows indicate timing of peptide administration. This results in near-identical body weight loss (A- total body weight, B- percentage of starting body weight) and similar body composition assessed by DEXA (C) or analysis of regional fat depots (D). (n=6 per group, * p<0.05, **p<0.01, ***p<0.001 vs sham).
Discussion
The current study is the first to have directly compared the effects upon food intake and body weight of melanocortins given centrally to a mouse model lacking all endogenously derived POMC products. α-MSH had the most potent anorexigenic effect, causing the biggest reduction in food intake. Further, α-MSH was the only melanocortin able to reduce the excess fat and lean mass found in Pomc-/- mice when given repeatedly over three days. On the basis of a pair-feeding experiment these effects were mediated largely through a reduction in food intake. Equimolar amounts of γ LPH, β-MSH, γ3-MSH and γ2-MSH were all able to reduce food intake but to a lesser degree than that seen with α-MSH and none caused a significant reduction in body weight.
Much of our current understanding of the importance of the melancortinergic system in energy homeostasis is from data derived using rodent models. Many previous studies have demonstrated that central administration of melanocortin agonists can significantly reduce food intake (25-28) even in agouti mice, another obese mouse model with disrupted melanocortinergic signaling(29). However, the majority of these studies have used potent pharmacological agents such as melanotan II (MTII)(30) rather than the endogenously produced peptides.
There are far fewer studies which have directly compared the central effects of specific POMC-derived melanocortin peptides on food intake, with each using different treatment and dosing regimens. Thus, Kask et al.(20) compared the effects of central α-MSH, β-MSH and γ1-MSH on food intake in ad libitum fed and 24 hr fasted rats. In both fed and fasted states α-MSH and β-MSH were equally effective in reducing food intake over 4 hrs, while γ1-MSH had no such effect. However, in contrast to our study, the larger precursor peptides of γ1-MSH and β-MSH (γ3-MSH and γ-LPH, respectively) were not used and equal μg not equimolar doses were given. The peptides were also administered in the early part of the light phase, a time when in the ad libitum fed rats in particular food intake is at its physiological nadir and no data about the impact upon body weight was presented. Abbott et al. (21)centrally administered equimolar amounts of α-, β- and γ2-MSH to rats fasted for 24 hours, with peptides given at the onset of the light cycle. α- and β-MSH both dose-dependently decreased food intake over 2 hrs with γ2-MSH unable to reduce food intake at any point measured. Intriguingly, this study also reported adverse locomotor activity with γ3-MSH and γ1-MSH, a finding which precluded these particular peptides being studied further. Finally, Millington et al. (22) compared the effects of centrally administered α-, β- and γ2-MSH on hypothalamic neuronal activation and on food intake in rats fasted for 48 hours. In contrast to the study by Abbott and colleagues, this study demonstrated that α- and γ2 MSH, but not β-MSH, suppressed food intake. Further, the two peptides shown to be biologically active brought about their effects over different time courses with α-MSH acting more rapidly than γ2 MSH. Each of the melanocortins used in this study also caused distinct anatomical patterns of activation within the hypothalamus, suggesting the individual peptides may have distinct biological roles (22).
Despite these studies there remains contention about the physiological heirachy of the melanocortin peptides. The results of the current study show α-MSH to be the most potent anorexigen, significantly reducing food intake over 24 hours after a single dose. Repeated administration of α-reduced both fat and lean mass but to an identical degree to that seen in pair-fed mice. We have previously reported that the phenotype of Pomc-/- mice results from increased food intake and a reduction in resting basal metabolic rate (11). The finding that α-MSH treated mice did not lose more weight that pair-fed mice indicates that, at least in the paradigm used in this study, administration of α-MSH did not significantly increase energy expenditure but simply ameliorated the hyperphagia.
These results are intriguing in the light of recent data clearly demonstrating functional divergence of the melanocortin pathway. Balthasar and colleagues engineered a lox P modified, null Mc4r allele which can be reactivated by Cre-recombinase (31). Using Sim1 cre transgenic mice, Mc4r expression was restored in the paraventricular hypothalamus and a subpopulation of the amygdala neurons. 60% of the obesity seen in total Mc4r null mice was prevented. This was entirely due to a normalisation in food intake with the reduced energy intake typical of Mc4r-/- mice unaffected by this targeted re-expression. Thus MC4R in the PVH and/or amgdala control food intake but MC4R elsewhere control energy expenditure. Given that we administered peptide into the lateral ventricle, it is likely that a higher concentration of peptide would have reached the MC4R in the PVN compared to more neuroanatomically distinct sites like the brainstem. This may, in part, explain why the predominant effect of α-MSH was on food intake.
Compared to α-MSH, the physiological roles of β-MSH and its direct precursor γ-LPH remain less well characterized. This may be understandable as rodents lack the N-terminal cleavage site necessary for the generation of β-MSH. Conclusions drawn from rodent data may therefore have resulted in an important role for β-MSH being overlooked. In fact, it has required human genetic studies to bring novel insights into the function of this peptide. Two recent reports have demonstrated that β-MSH is likely to be a physiologically relevant endogenous ligand at the MC4R (32, 33), thereby challenging the canonical view that α-MSH is the primary melanocortin ligand controlling energy homeostasis in humans. Like the previous rodent studies reviewed above, our data also suggest that β-MSH can reduce food intake but also show that POMC-derived peptide γ-LPH, which is more physiologically relevant to mice, can also significantly reduce food intake to a similar degree. Thus γ-LPH may have a function in modulating appetite and food intake in rodents.
Much of the data derived from rodent studies suggest that the primary role of the γ-MSH species may be in the regulation of the cardiovascular system and in particular integrating the response to dietary sodium excess (34). A high-sodium diet increases the pituitary content of γ-MSH and results in a doubling of plasma γ-MSH concentration. Renal MC3-Rs are also upregulated by a high salt diet and acting via these receptors, γ-MSH is thought to be able to bring about a marked natriuresis. However, there is as yet no mouse model which solely lacks γ-MSH, with some of the key conclusions regarding the role of this melanocortin drawn from a mouse model functionally lacking γ-MSH because of a lack of the prohormone converting enzyme, PC2 (35). This enzyme is involved in processing a range of other peptides, with the knock-on effect of these unprocessed hormones and the lack of smaller downstream peptides likely to impact upon the key physiological functions within the mouse. The data in the current study show both γ3-MSH and γ2-MSH can both reduce food intake. Although this is in accordance with many of the previous published studies(20-22), a recent report by Marks et al. demonstrated that an analogue of γ-MSH (D-Trp8-γ-MSH) with high affinity for MC3R can increase food intake(36). The explanation of this orexigenic action invoked a central mechanism in which the ligand bound to MC3R on arcuate POMC neurons. Acting as an inhibitory autoreceptor, increased activity at MC3R could therefore bring about a reduction in melanocortinergic tone and decrease food intake. The data presented in the current study are not able to directly address this putative mechanism of action of γ-MSH, as even if centrally administered peptide were acting upon MC3R on POMC neurons, the very nature of the Pomc-/- mouse means melanocortinergic tone is non-existent before any peptide is given. However it is noteworthy that the peptide analogue studied by Marks et al. (36) was given peripherally, not centrally, and as MC3R are found in many other tissues including adipose tissue and the stomach it is possible that the effects of D-Trp8-γ-MSH seen were from a site of action outside of the hypothalamus.
The current study also demonstrated that γ3-MSH significantly reduced liver mass and showed a trend to reducing total fat mass, indicating that this ligand may have a potential role in controlling fat deposition. This may be via stimulation of the sympathetic system (37, 38) although the nature of the receptor involved cannot be determined by the present study.
One limitation of a pharmacological study is that it can never wholly replicate a complex physiological scenario, with any results undoubtedly an underestimate of the true elegance and subtlety at work within the system. POMC is highly post-translationally modified and there is still much to understand with respect to the regulation of these processes. Indeed, some intriguing recent data have suggested that leptin can critically alter an important step in melancortin processing(39). By analysis of the protein content and enzymatic activity within the hypothalamus of wild type and leptin deficient ob/ob mice, Guo and colleagues demonstrated that leptin specifically and rapidly stimulated the generation of acetylated α-MSH (39). Their data suggested that this is the result of a leptin-dependent increase in the activity of an N-acetyltransferase in hypothalamic POMC neurons. The effects of leptin on energy balance via the central melanocortin pathway now appear to have an additional level of complexity by regulating acetylation of α-MSH. In addition, the fact that there are multiple POMC-derived peptides but only two centrally expressed melanocortin receptor subtype through which they can act, brings a further layer of complexity to the system. It will take carefully targeted genetic models to tease apart the system further and ascribe true physiological function to each peptide.
Although all the peptides were administered in equimolar amounts, in keeping with what is known about POMC processing in vivo (14), we have no data pertaining to the pharmacokinetics of peptides following administration. Such data would be valuable in further interpreting the results presented as it is conceivable that the ability of α-MSH to bring about the biggest change in food intake and body weight result, in part, from an increased resistance to degradation and clearance.
Finally, there are compelling data demonstrating that an intact central melanocortin system is required for normal glucose homeostasis, independent of its role in controlling food intake and body weight (40) and we have previously shown that ten days of glucocorticoid treatment rendered Pomc-/- mice markedly insulin resistant(24). The current study was not designed to compare the ability of centrally administered melanocortin peptides to potentially ameliorate this insulin resistant state. Such future studies may be informative in resolving unanswered issues surrounding the true physiological roles of the melancortin peptides.
In summary, although other centrally administered melanocortins can reduce food intake in the short term, only α-MSH is also able to reduce the excess fat and lean mass found in Pomc-/- mice, mediated largely mediated through its effects on food intake.
Footnotes
Disclosure summary
DISCLOSURE STATEMENT: The authors have nothing to disclose
References
- 1.Cone RD. The Central Melanocortin System and Energy Homeostasis. Trends Endocrinol Metab. 1999;10:211–216. doi: 10.1016/s1043-2760(99)00153-8. [DOI] [PubMed] [Google Scholar]
- 2.Coll AP, Farooqi IS, Challis BG, Yeo GS, O’Rahilly S. Proopiomelanocortin and energy balance: insights from human and murine genetics. J Clin Endocrinol Metab. 2004;89:2557–62. doi: 10.1210/jc.2004-0428. [DOI] [PubMed] [Google Scholar]
- 3.Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–41. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 4.Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O’Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med. 2003;348:1085–95. doi: 10.1056/NEJMoa022050. [DOI] [PubMed] [Google Scholar]
- 5.Butler AA, Kesterson RA, Khong K, Cullen MJ, Pelleymounter MA, Dekoning J, Baetscher M, Cone RD. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology. 2000;141:3518–21. doi: 10.1210/endo.141.9.7791. [DOI] [PubMed] [Google Scholar]
- 6.Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan XM, Yu H, Rosenblum CI, Vongs A, Feng Y, Cao L, Metzger JM, Strack AM, Camacho RE, Mellin TN, Nunes CN, Min W, Fisher J, Gopal-Truter S, MacIntyre DE, Chen HY, Van der Ploeg LH. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat Genet. 2000;26:97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- 7.Feng N, Young SF, Aguilera G, Puricelli E, Adler-Wailes DC, Sebring NG, Yanovski JA. Co-occurrence of two partially inactivating polymorphisms of MC3R is associated with pediatric-onset obesity. Diabetes. 2005;54:2663–7. doi: 10.2337/diabetes.54.9.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raffin-Sanson ML, de Keyzer Y, Bertagna X. Proopiomelanocortin, a polypeptide precursor with multiple functions: from physiology to pathological conditions. Eur J Endocrinol. 2003;149:79–90. doi: 10.1530/eje.0.1490079. [DOI] [PubMed] [Google Scholar]
- 9.Pritchard LE, Turnbull AV, White A. Pro-opiomelanocortin processing in the hypothalamus: impact on melanocortin signalling and obesity. J Endocrinol. 2002;172:411–21. doi: 10.1677/joe.0.1720411. [DOI] [PubMed] [Google Scholar]
- 10.Krude H, Biebermann H, Luck W, Horn R, Brabant G, Gruters A. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet. 1998;19:155–7. doi: 10.1038/509. [DOI] [PubMed] [Google Scholar]
- 11.Challis BG, Coll AP, Yeo GS, Pinnock SB, Dickson SL, Thresher RR, Dixon J, Zahn D, Rochford JJ, White A, Oliver RL, Millington G, Aparicio SA, Colledge WH, Russ AP, Carlton MB, O’Rahilly S. Mice lacking pro-opiomelanocortin are sensitive to high-fat feeding but respond normally to the acute anorectic effects of peptide-YY(3-36) Proc Natl Acad Sci U S A. 2004;101:4695–700. doi: 10.1073/pnas.0306931101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson RS, Creemers JW, Ohagi S, Raffin-Sanson ML, Sanders L, Montague CT, Hutton JC, O’Rahilly S. Obesity and impaired prohormone processing associated with mutations in the human prohormone convertase 1 gene. Nat Genet. 1997;16:303–6. doi: 10.1038/ng0797-303. [DOI] [PubMed] [Google Scholar]
- 13.Lloyd DJ, Bohan S, Gekakis N. Obesity, hyperphagia and increased metabolic efficiency in Pc1 mutant mice. Hum Mol Genet. 2006;15:1884–93. doi: 10.1093/hmg/ddl111. [DOI] [PubMed] [Google Scholar]
- 14.Bertagna X. Proopiomelanocortin-derived peptides. Endocrinol Metab Clin North Am. 1994;23:467–85. [PubMed] [Google Scholar]
- 15.Hadley ME, Hruby VJ, Jiang J, Sharma SD, Fink JL, Haskell-Luevano C, Bentley DL, al-Obeidi F, Sawyer TK. Melanocortin receptors: identification and characterization by melanotropic peptide agonists and antagonists. Pigment Cell Res. 1996;9:213–34. doi: 10.1111/j.1600-0749.1996.tb00111.x. [DOI] [PubMed] [Google Scholar]
- 16.Schioth HB, Muceniece R, Wikberg JE. Characterisation of the melanocortin 4 receptor by radioligand binding. Pharmacol Toxicol. 1996;79:161–5. doi: 10.1111/j.1600-0773.1996.tb00261.x. [DOI] [PubMed] [Google Scholar]
- 17.Harrold JA, Widdowson PS, Williams G. beta-MSH: a functional ligand that regulated energy homeostasis via hypothalamic MC4-R? Peptides. 2003;24:397–405. doi: 10.1016/s0196-9781(03)00054-8. [DOI] [PubMed] [Google Scholar]
- 18.Roselli-Rehfuss L, Mountjoy KG, Robbins LS, Mortrud MT, Low MJ, Tatro JB, Entwistle ML, Simerly RB, Cone RD. Identification of a receptor for gamma melanotropin and other proopiomelanocortin peptides in the hypothalamus and limbic system. Proc Natl Acad Sci U S A. 1993;90:8856–60. doi: 10.1073/pnas.90.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiang Z, Litherland SA, Sorensen NB, Proneth B, Wood MS, Shaw AM, Millard WJ, Haskell-Luevano C. Pharmacological Characterization of 40 Human Melanocortin-4 Receptor Polymorphisms with the Endogenous Proopiomelanocortin-Derived Agonists and the Agouti-Related Protein (AGRP) Antagonist. Biochemistry. 2006;45:7277–88. doi: 10.1021/bi0600300. [DOI] [PubMed] [Google Scholar]
- 20.Kask A, Rago L, Wikberg JE, Schioth HB. Differential effects of melanocortin peptides on ingestive behaviour in rats: evidence against the involvement of MC(3) receptor in the regulation of food intake. Neurosci Lett. 2000;283:1–4. doi: 10.1016/s0304-3940(00)00837-5. [DOI] [PubMed] [Google Scholar]
- 21.Abbott CR, Rossi M, Kim M, AlAhmed SH, Taylor GM, Ghatei MA, Smith DM, Bloom SR. Investigation of the melanocyte stimulating hormones on food intake. Lack Of evidence to support a role for the melanocortin-3-receptor. Brain Res. 2000;869:203–10. doi: 10.1016/s0006-8993(00)02386-6. [DOI] [PubMed] [Google Scholar]
- 22.Millington GW, Tung YC, Hewson AK, O’Rahilly S, Dickson SL. Differential effects of alpha-, beta- and gamma(2)-melanocyte-stimulating hormones on hypothalamic neuronal activation and feeding in the fasted rat. Neuroscience. 2001;108:437–45. doi: 10.1016/s0306-4522(01)00428-6. [DOI] [PubMed] [Google Scholar]
- 23.Coll AP, Fassnacht M, Klammer S, Hahner S, Schulte DM, Piper S, Tung YC, Challis BG, Weinstein Y, Allolio B, O’Rahilly S, Beuschlein F. Peripheral administration of the N-terminal pro-opiomelanocortin fragment 1-28 to Pomc-/- mice reduces food intake and weight but does not affect adrenal growth or corticosterone production. J Endocrinol. 2006;190:515–525. doi: 10.1677/joe.1.06749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coll AP, Challis BG, Lopez M, Piper S, Yeo GS, O’Rahilly S. Proopiomelanocortin-deficient mice are hypersensitive to the adverse metabolic effects of glucocorticoids. Diabetes. 2005;54:2269–76. doi: 10.2337/diabetes.54.8.2269. [DOI] [PubMed] [Google Scholar]
- 25.Grill HJ, Ginsberg AB, Seeley RJ, Kaplan JM. Brainstem application of melanocortin receptor ligands produces long-lasting effects on feeding and body weight. J Neurosci. 1998;18:10128–35. doi: 10.1523/JNEUROSCI.18-23-10128.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hwa JJ, Ghibaudi L, Gao J, Parker EM. Central melanocortin system modulates energy intake and expenditure of obese and lean Zucker rats. Am J Physiol Regul Integr Comp Physiol. 2001;281:R444–51. doi: 10.1152/ajpregu.2001.281.2.R444. [DOI] [PubMed] [Google Scholar]
- 27.Pierroz DD, Ziotopoulou M, Ungsunan L, Moschos S, Flier JS, Mantzoros CS. Effects of acute and chronic administration of the melanocortin agonist MTII in mice with diet-induced obesity. Diabetes. 2002;51:1337–45. doi: 10.2337/diabetes.51.5.1337. [DOI] [PubMed] [Google Scholar]
- 28.Li G, Zhang Y, Wilsey JT, Scarpace PJ. Unabated anorexic and enhanced thermogenic responses to melanotan II in diet-induced obese rats despite reduced melanocortin 3 and 4 receptor expression. J Endocrinol. 2004;182:123–32. doi: 10.1677/joe.0.1820123. [DOI] [PubMed] [Google Scholar]
- 29.Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–8. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 30.Hadley ME, Hruby VJ, Blanchard J, Dorr RT, Levine N, Dawson BV, al-Obeidi F, Sawyer TK. Discovery and development of novel melanogenic drugs. Melanotan-I and -II. Pharm Biotechnol. 1998;11:575–95. doi: 10.1007/0-306-47384-4_25. [DOI] [PubMed] [Google Scholar]
- 31.Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell BB. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 32.Lee YS, Challis BG, Thompson DA, Yeo GS, Keogh JM, Madonna ME, Wraight V, Sims M, Vatin V, Meyre D, Shield J, Burren C, Ibrahim Z, Cheetham T, Swift P, Blackwood A, Hung CC, Wareham NJ, Froguel P, Millhauser GL, O’Rahilly S, Farooqi IS. A POMC variant implicates beta-melanocyte-stimulating hormone in the control of human energy balance. Cell Metab. 2006;3:135–40. doi: 10.1016/j.cmet.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 33.Biebermann H, Castaneda TR, van Landeghem F, von Deimling A, Escher F, Brabant G, Hebebrand J, Hinney A, Tschop MH, Gruters A, Krude H. A role for beta-melanocyte-stimulating hormone in human body-weight regulation. Cell Metab. 2006;3:141–6. doi: 10.1016/j.cmet.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 34.Humphreys MH. Gamma-MSH, sodium metabolism, and salt-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol. 2004;286:R417–30. doi: 10.1152/ajpregu.00365.2003. [DOI] [PubMed] [Google Scholar]
- 35.Ni XP, Pearce D, Butler AA, Cone RD, Humphreys MH. Genetic disruption of gamma-melanocyte-stimulating hormone signaling leads to salt-sensitive hypertension in the mouse. J Clin Invest. 2003;111:1251–8. doi: 10.1172/JCI16993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marks DL, Hruby V, Brookhart G, Cone RD. The regulation of food intake by selective stimulation of the type 3 melanocortin receptor (MC3R) Peptides. 2006;27:259–64. doi: 10.1016/j.peptides.2005.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dunbar JC, Lu H. Leptin-induced increase in sympathetic nervous and cardiovascular tone is mediated by proopiomelanocortin (POMC) products. Brain Res Bull. 1999;50:215–21. doi: 10.1016/s0361-9230(99)00197-5. [DOI] [PubMed] [Google Scholar]
- 38.Song CK, Jackson RM, Harris RB, Richard D, Bartness TJ. Melanocortin-4 receptor mRNA is expressed in sympathetic nervous system outflow neurons to white adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1467–76. doi: 10.1152/ajpregu.00348.2005. [DOI] [PubMed] [Google Scholar]
- 39.Guo L, Munzberg H, Stuart RC, Nillni EA, Bjorbaek C. N-acetylation of hypothalamic alpha-melanocyte-stimulating hormone and regulation by leptin. Proc Natl Acad Sci U S A. 2004;101:11797–802. doi: 10.1073/pnas.0403165101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Obici S, Rossetti L. Minireview: nutrient sensing and the regulation of insulin action and energy balance. Endocrinology. 2003;144:5172–8. doi: 10.1210/en.2003-0999. [DOI] [PubMed] [Google Scholar]