Abstract
Localization of mechanotransduction in sensory hair cells to hair bundles requires selective targeting of essential proteins to specific locations. Isoform 2 of the plasma-membrane Ca2+-ATPase (PMCA2), required for hearing and balance, is found exclusively in hair bundles. We determined the contribution of splicing at the two major splicing sites (A and C) to hair-cell targeting of PMCA2. When PMCA2 isoforms were immunoprecipitated from purified hair bundles of rat utricle, 2w was the only site A variant detected; moreover, immunocytochemistry for 2w in rat vestibular and cochlear tissues indicated that this splice form was located solely in bundles. To demonstrate the necessity of the 2w sequence, we transfected hair cells with PMCA2 containing different variants at splice sites A and C. Although native hair bundles exclusively use the 2a form at splice-site C, epitope-tagged PMCA2w/a and PMCA2w/b were both concentrated in bundles, indicating that site C is not involved in bundle targeting. In contrast, PMCA2z/a was excluded from bundles and was instead targeted to the basolateral plasma membrane. Bundle-specific targeting of PMCA2w/a tagged with green fluorescent protein (GFP) was diminished, suggesting that GFP interfered with splice-site A. Together, these data demonstrate that PMCA2w/a is the hair-bundle isoform of PMCA in rat hair cells and that 2w targets PMCA2 to bundles. The 2w sequence is thus the first targeting signal identified for a hair-bundle membrane protein; moreover, the striking distribution of inner-ear PMCA isoforms dictated by selective targeting suggests a critical functional role for segregated pathways of Ca2+ transport.
Keywords: hair cells, calcium, calcium pumps, stereocilia, apical targeting, alternative splicing, gene-gun
Introduction
Hair cells, the sensory cells of the inner ear, convert mechanical vibrations to electrical signals that the brain interprets as sound or movement. Transduction is initiated by deflection of the hair bundle, the mechanosensory organelle (Hudspeth, 1989). Bundle deflection directly gates the transduction channel, allowing a substantial influx of Ca2+ (Lumpkin et al., 1997; Ricci and Fettiplace, 1998), which is critical for bundle function (Lenzi and Roberts, 1994). Bundles not only are bathed in endolymph, a K+-rich extracellular fluid that cannot support Na+/Ca2+ exchange, but they also have no intracellular compartments that could sequester Ca2+ (Jacobs and Hudspeth, 1990). Instead, bundles rely on the plasma-membrane Ca2+-ATPase (PMCA) (Lumpkin and Hudspeth, 1998), a pump that is highly expressed in stereocilia (Yamoah et al., 1998; Dumont et al., 2001) and removes the majority of Ca2+ that enters through transduction channels (Lumpkin and Hudspeth, 1998).
There are four mammalian PMCA genes; they encode PMCA1 and PMCA4, which are ubiquitously expressed, as well as PMCA2 and PMCA3, of more restricted distribution (Stauffer et al., 1995). Additional protein diversity is generated via alternative RNA splicing at splice sites A and C (see Fig. 1A,B). The C splice site, which alters the affinity of PMCA for calmodulin (Enyedi et al., 1994) and determines the presence or absence of at least one phosphorylation site (Enyedi et al., 1997), has been closely scrutinized; in contrast, functional consequences of splicing within region A are poorly understood.
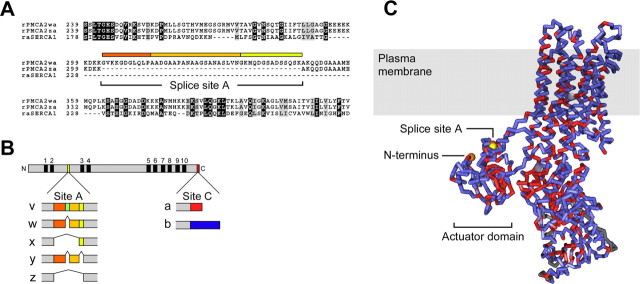
Figure 1.
Location of splice-site A in the amino acid sequence and predicted structure of PMCA2. A, Alignment of amino acid sequences of rabbit muscle SERCA with rat PMCA2 splice variants in the vicinity of splice-site A. SERCA lacks sequences corresponding to this splicing region. B, Scheme of the PMCA protein and diagram of splicing at sites A and C in frog and rat PMCA2. The PMCA is shown at the top as a gray bar; the 10 membrane-spanning domains are indicated by black boxes and numbered from 1 to 10. N, N terminus; C, C terminus. Colored boxes denote the positions where alternative splicing alters PMCA2 at sites A and C. Splicing at site A affects the first cytosolic loop between membrane-spanning domains 2 and 3; splicing at site C alters the C-terminal sequence of the pump protein. The colored boxes below the PMCA scheme indicate the amino acid insertions encoded by separate exons in different PMCA2 splice variants (labeled on the left). The colors of the boxes correspond to the colored bars above the sequence in A. Note that a unique sequence insertion in the 2v splice form of frog PMCA2 (indicated by an additional dark green box) interrupts the 2w sequence. This sequence insertion does not exist in rat (or human) PMCA2 and hence is not shown in A; frog PMCA2v is nevertheless targeted to hair bundles. C, Side view of the SERCA structure (1SU4). PMCA2 identities are red; nonidentical but aligned amino acids are blue. Gray areas do not align between the two sequences. The location of the actuator domain, N terminus (orange), and splice-site A (yellow) are indicated. The shaded box corresponds to the approximate location of the membrane bilayer. Note the proximity of the N terminus to splice-site A. The structure was generated with Cn3D version 4.1.
Although all four PMCA gene products and many splice variants are expressed in the mammalian cochlea (Crouch and Schulte, 1996; Furuta et al., 1998), only PMCA2 is known to be essential for hearing and balance (Noben-Trauth et al., 1997; Kozel et al., 1998; Street et al., 1998; Takahashi and Kitamura, 1999). PMCA2 with the 2a variant at splice-site C is the only PMCA expressed in frog and rat hair bundles (Dumont et al., 2001). Frog bundle PMCA2 uses 2v at splice-site A (Fig. 1B); because mammals do not express 2v, the variant used in rat bundles was unknown (Dumont et al., 2001). The 2w variant has been proposed to be the bundle isoform, given that 2w is closest in sequence to 2v and that PMCA2w traffics to the apical domain of Madin-Darby canine kidney (MDCK) cells (Chicka and Strehler, 2003). Although the latter data suggest that 2w can serve as an apical targeting signal in a heterologous system, this result does not necessarily indicate that the same targeting signal is used in vivo. Indeed, the additional amino acids present in 2v compared with 2w interrupt the contiguous 2w sequence (Fig. 1B), suggesting that rat PMCA2w/a might not target to the bundle.
Although little is known about how proteins are sorted to the hair bundle, PMCA is the best characterized membrane protein of the bundle. By determining the apical targeting sequence of PMCA, we can begin to deduce the signals required for assembling the transduction apparatus of the hair cell. We report here that splicing at site A dictates apical targeting in hair cells for PMCA2 and that the rodent hair-bundle isoform is PMCA2w/a.
Materials and Methods
Nomenclature.
We use the term “isoform” to refer to enzymes that catalyze the same reaction (e.g., PMCAs) but are produced by different genes. We adopt the naming system for full-length PMCA molecules used by Chicka and Strehler (2003) and furthermore label splice variants with the isoform number and letter designation. Accordingly, 2w refers to the “w” splice variant of PMCA2; PMCA2w/a refers to PMCA2 with variant 2w at splice-site A and variant 2a at splice-site C. In another example, PMCA2w refers to PMCA2 with 2w at splice-site A and either variant at splice-site C.
Generating antibodies against PMCA2 splice-site A variants.
Polyclonal rabbit antibodies were raised against human PMCA2 peptides with C-terminal cysteine residues (PMCA2w: GDGLQLPAADGAAASNAADSC, amino acids 307–326; PMCA2z: DDKKAKQQDGAAAMC, amino acids 300–312). Peptides were synthesized in the Mayo Clinic Protein Core facility (Rochester, MN) and used to immunize two rabbits each (Cocalico Biologicals, Reamstown, PA). Antibodies were affinity purified using positive and negative selection against peptides corresponding to rat PMCA2w (GDGLQLPAADGAAPANAAGSC), PMCA2x (KKGKMQ-GGGC), and PMCA2z (DKKAKQGGGC) using methods described previously (Dumont et al., 2001). Antibodies used here are listed in Table 1.
Table 1.
PMCA antibodies
| Name | Known specificity | Antigen |
|---|---|---|
| R2w | Rat PMCA2w | GDGLQLPAADGAAPANAAGSC |
| R2z | Rat PMCA2z | DKKAKQGGGC |
| F2a | Bullfrog PMCA2a, rat PMCA2a | CSSPTSASAAAAGQG (C terminus) |
| NR2 | Rat PMCA2 | TNSDFYSKNQRNESSC (N terminus) |
| 5F10 | PMCA (several species) | Human PMCA4 amino acids 724–783 |
Constructing PMCA2a variant expression vectors.
The coding region of PMCA2z/a was amplified with Phusion DNA polymerase (MJ Research, Waltham, MA) from pMT2-PMCA2z/a (gift from Dr. J. Penniston, Mayo Clinic, Rochester, MN), creating a 5′ XbaI site and a 3′ Hind III site. PMCA2z/a was subcloned into the XbaI and Hind III sites of pJPA5 (gift from Dr. J. Adelman, Oregon Health & Science University, Portland, OR), which contained an N-terminal C8 epitope tag [PRGPDRPEGIEE (Abacioglu et al., 1994)] downstream from the cytomegalovirus promoter. To create PMCA2w/a, we used site-directed mutagenesis to create unique PacI and NheI sites flanking splice-site A. Overlapping oligonucleotides were synthesized, annealed, and amplified to create the 2w splice sequence. After the products were introduced into the PacI and NheI sites of PMCA2z/a, the introduced restriction sites were then reverted to the native sequence, creating PMCA2w/a.
Splice-site analysis by reverse transcription-PCR.
Purified cochlear total RNA (RNeasy kit; Qiagen, Valencia, CA) from two adult rats was primed with random hexamers and reversed transcribed (ThermoScript RT-PCR System; Invitrogen, Carlsbad, CA) to synthesize first-strand cDNA. PMCA2 splice region A sequences were amplified by nested PCR. Primers for the initial PCR were (5′ to 3′) GGACGGATGGTGGTGACTG (R2A + 1) and AGATGGCTGTGGCGTTACC (R2A − 1); primers for the second PCR were GCTGTGGGTGTCAACTCTC (R2A + 2) and ACCACCAGCACCGTCACAC (R2A − 2). The amplification protocol comprised 4 min at 94°C, 30 cycles of 30 s at 94°C, 30 s at 55°C, 45 s at 72°C, and a final 10 min at 72°C. The DNA template for the second round of PCR consisted of 2.5 μl of first-round product. PCR products were directly cloned into pCR-TOPO vector (Invitrogen) and sequenced to confirm the splice-form identity.
SDS-PAGE.
Proteins were separated by SDS-PAGE and transferred to blotting membranes essentially as described previously (Dumont et al., 2001). Under these conditions, PMCA2 migrated at ∼170 kDa; treatment of samples with 50 mg/ml urea increased its migration to 140 kDa, which corresponds to the molecular mass of the protein (data not shown).
Immunoprecipitation.
Hair bundles were purified from postnatal day 7 (P7) to P10 Wistar rat utricles by using a modified twist-off technique (Gillespie and Hudspeth, 1991). Utricles were dissected in minimum essential medium (MEM; Invitrogen) supplemented with 25 mm HEPES at pH 7.5, adhered to coverslips with Cell-Tak (BD Biosciences, Bedford, MA), and embedded in 4% low-melting-point agarose at 37°C. After bundles were isolated mechanically (Gillespie and Hudspeth, 1991), they were superfused with MEM during excision. We estimated bundle recovery visually, in which one-ear equivalent corresponds to 100% of the bundles from one utricle (Gillespie and Hudspeth, 1991). Detergent-soluble proteins were extracted from bundles and maculae separately as described previously (Dumont et al., 2001), with slight modifications. The tissues were incubated in extraction buffer at 4°C for 30 min, gently flicking the tube every 10 min. Detergent-soluble proteins were isolated by centrifuging samples for 30 min at 12,000 × g. Extracts were incubated on ice for 4 h with 4 μg of affinity-purified anti-PMCA2 antibody. Immune complexes were precipitated by adding 15 μl of Protein-G Plus agarose (Santa Cruz Biotechnology, Santa Cruz, CA), incubating for 2 h at 4°C with gentle agitation, and centrifuging to sediment the antibody–agarose complex. After three 250 μl washes with extraction buffer, precipitated proteins were eluted from the agarose by incubating at 65°C for 20 min in an SDS-PAGE sample buffer that included 2% SDS and 100 mm DTT. Sequential immunoprecipitations were performed with R2z, R2w, and NR2. Proteins from the final supernatant were precipitated with 6 vol of acetone at −20°C and solubilized with SDS-PAGE sample buffer. Immunodetection of PMCA (Dumont et al., 2001) used pan-PMCA monoclonal antibody 5F10 (Affinity Bioreagents, Golden, CO) diluted to 1:5000, followed by a 1:20,000 dilution of peroxidase-conjugated donkey anti-mouse IgG (Jackson ImmunoResearch, West Grove, PA).
For immunoprecipitation from COS-7 cells, cells were grown to ∼90% confluency in 6-well plates (Fisher Scientific, Pittsburgh, PA) using DMEM (Invitrogen) supplemented with 10% fetal bovine serum, 10 U/ml penicillin, and 10 μg/ml streptomycin. Cells were transfected with 1.5 μg of pJPA5-PMCA2a variant DNA using 6 μl of Fugene 6 (Roche Applied Science, Indianapolis, IN). After 48 h, the cells were washed twice in cold PBS and lysed as described for tissue. Detergent-soluble proteins were isolated by centrifuging samples for 30 min at 12,000 × g. Extracts were incubated overnight at 4°C with 2 μg of affinity-purified R2w, R2z, mouse monoclonal anti-C8 (gift from Dr. T. Strassmaier, Oregon Health & Science University, Portland, OR), or F2a, an antibody specific for the 2a splice form of PMCA2 (Dumont et al., 2001). Immune complexes were precipitated, eluted, and detected as described above.
Immunofluorescence.
COS-7 cells were grown to ∼90% confluency on a coverslip in 24-well plates. Cells were transfected with 0.5 μg of pJPA5-PMCA2a variant DNA using the TransIT-COS Transfection kit (Mirus, Madison, WI). After 48 h, the cells were washed with PBS and fixed in 3% formaldehyde in PBS for 20 min. After another wash in PBS, the cells were permeabilized for 15 min with 0.2% saponin in blocking solution, which contained 3% normal donkey serum and 10 mg/ml bovine albumin serum (BSA) in PBS, and treated for 40 min with blocking solution without detergent. Cells were incubated for 2 h with primary antibody diluted in blocking solution (10 μg/ml R2w or 2.5 μg/ml NR2), washed in PBS, and incubated with 7.5 μg/ml Cy3-conjugated donkey anti-rabbit IgG (Invitrogen, Eugene, OR) in blocking solution for 2 h. Cells were washed in PBS, and the coverslips were mounted with Vectashield (Vector Laboratories, Burlingame, CA) and viewed with a Plan Apo 60× (numerical aperture, 1.40) oil lens on a Nikon Eclipse E800 upright microscope equipped with a Photometrics CoolSnap CCD camera and MetaMorph Imaging system (version 6.1r4; Molecular Devices, Sunnyvale, CA).
Auditory and vestibular organs from P21–P23 rats were dissected in MEM supplemented with 25 mm HEPES, pH 7.5. Tissues were fixed, permeabilized, and blocked as described for COS cells. Tissues were incubated overnight with primary antibody (1:500 5F10 ascites and 10 μg/ml R2w) diluted in blocking solution, washed in PBS, and incubated with secondary antibodies (7.5 μg/ml Cy3-conjugated donkey anti-rabbit IgG and Cy5-conjugated donkey anti-mouse IgG; Invitrogen) and 0.25 μm FITC-phalloidin (Sigma, St. Louis, MO) in the blocking solution for 2 h. In some experiments, the R2w antibody was incubated with 50 μg/ml antigenic peptide for 1 h before application to tissue. Tissues were washed in PBS, mounted with Vectashield, and viewed with a Plan Apo 60× (numerical aperture, 1.40) oil lens on a Nikon TE 300 inverted microscope with a Bio-Rad (Hercules, CA) 1024 confocal imaging system. Acquired images were processed with ImageJ (version 1.32j) and Photoshop (version 7.0; Adobe Systems, San Jose, CA).
Culturing and transfecting sensory epithelia.
Inner-ear sensory epithelia were harvested from P2–P3 rat pups in DMEM/F-12 (Invitrogen). The cochlea was dissected away from the modiolus, Reissner's membrane was opened, and the tectorial membrane was removed. The organ of Corti was cut into two to three pieces. The saccule, utricle, and ampullae were also harvested, and the overlying otoconia and membranes were removed. The sensory epithelia were cultured in 24-well plates on coverslips coated with 100–200 μg/ml rat tail type I collagen (Sigma) and bathed in DMEM/F-12 supplemented with 10% FBS and 15 μg/ml penicillin; cultures were maintained at 37°C and 5% CO2. Before transfection, tissues were cultured for 24 h to allow fibroblast outgrowth; this step allows firm attachment of the epithelia to the collagen substrate.
A Helios gene gun (Bio-Rad) was used to transfect inner-ear cultures (Schneider et al., 2002; Belyantseva et al., 2003, 2005; Rzadzinska et al., 2004). Gene-gun delivery of plasmids is the only established method that allows successful introduction of foreign genes into hair cells without using viral vectors (Holt, 2002) or making transgenic mice (Gao et al., 2004). Bullets were prepared by precipitating plasmid DNA onto 1-μm-diameter gold microcarriers at a ratio of 2 μg of DNA per 1 mg of gold particles; when transfecting two plasmids, each was precipitated at 1 μg/mg gold. The inner wall of Tefzel tubing was coated with the DNA-coated gold microcarriers and cut into individual bullets containing ∼1 μg of DNA. Cartridges were loaded into the bullet chamber of the gene gun, and a blast of helium gas was used to strip the inner wall of the tubing and bombard the culture with the gold microcarriers. For each culture, a Bio-Rad diffusion screen was placed ∼2–4 mm from coverslip-bound cultures; the gene-gun barrel (tip, 3–4 cm distant from the bullet exit) was placed directly against the diffusion screen, and one cartridge of DNA microcarriers was shot using a pressure of 140 psi. The gold microcarriers usually contained two plasmids, a fusion of green fluorescent protein and β-actin (GFP-actin; Clontech, Palo Alto, CA), and one of the C8-tagged PMCA2a expression constructs. In some experiments, a single plasmid encoding GFP-PMCA2w/a was used for transfection.
Cultures were fixed 24 h after transfection using 4% formaldehyde in PBS for 25 min, permeabilized in 0.2% saponin for 15 min, and incubated for 1 h in blocking solution (3% donkey or goat serum and 10 mg/ml BSA). Tissues were incubated overnight in 2.5 μg/ml mouse monoclonal anti-C8 in blocking solution, washed in PBS, and incubated with a secondary antibody (5 μg/ml of either Cy3- or Rhodamine Red-X-conjugated donkey anti-mouse IgG) and 0.25 μm Alexa 633 or 660 phalloidin (Invitrogen). Tissues were again washed in PBS, mounted in Vectashield, and viewed by confocal microscopy as described for immunofluorescence.
Images were analyzed using ImageJ version 1.34. For quantitation of bundles or basolateral membrane, a region of interest was selected from an individual confocal section, and the mean pixel intensity was determined with the Measure tool. A similar region from an untransfected cell was measured to determine background pixel intensity. A hair-bundle region of interest encompassed the entire bundle visible in a single confocal section. For the basolateral membrane, we chose a section between the cuticular plate and nucleus; a narrow region of interest along the brightest section of the membrane was chosen. Because the density of membrane present in a single confocal section will be different in bundles and basolateral membranes, ratios are most valuable for comparison between transfection conditions, rather than for directly determining the relative PMCA concentration in the two compartments.
Results
Generation of antibodies specific to PMCA2 splice A variants
We generated specific polyclonal antibodies to characterize splice-site A variants of PMCA2. The peptide sequence used for raising 2z-specific antibodies is found within all PMCA2 splice forms, albeit split across the splicing inserts. We therefore performed negative affinity selection with peptides spanning the splice junctions in PMCA2x and PMCA2w, which abut the 2z sequence, then positive selection using a PMCA2z peptide. Using this procedure, antibodies specific to the 2z variant (R2z) that were functional for immunoprecipitation (Fig. 2) were successfully purified. Using negative selection against PMCA2z and PMCA2x peptides and positive selection with a PMCA2w peptide, we also successfully purified antibodies specific to PMCA2w (R2w) that were useful for immunoprecipitation, immunoblotting, and immunocytochemistry (Fig. 2 and data not shown).
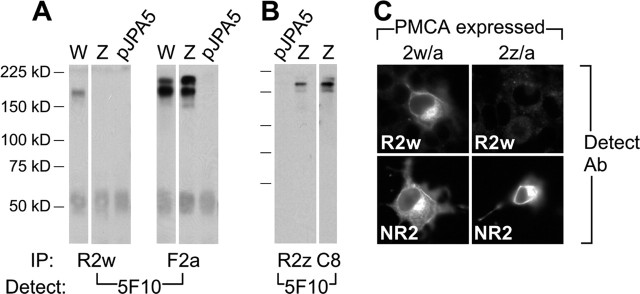
Figure 2.
R2w and R2z specifically recognize PMCA2w/a and PMCA2z/a in transfected COS-7 cells. A, B, Immunoprecipitation from COS-7 cells. A, Lysates from cells expressing 2w or 2z variants of PMCA2a or negative control plasmid (pJPA5) were incubated with R2w or F2a. The pan-PMCA antibody 5F10 was used to detect immunoprecipitated PMCA; in hair bundles, 5F10 precipitates bands of 150–170 kDa (Yamoah et al., 1998). R2w precipitated a band of ∼170 kDa only from cells expressing PMCA2w/a. F2a precipitated 170 kDa bands from cells expressing PMCA2w/a or PMCA2z/a. B, Lysates from cells expressing C8 epitope-tagged PMCA2z or pJPA5 were incubated with R2z or anti-C8. 5F10 detection was used as in A. R2z and anti-C8 precipitated a band of ∼170 kDa from transfected cells. C, Immunocytochemistry in COS-7 cells. R2w detects cells expressing PMCA2w/a but not PMCA2z/a. NR2 demonstrated that each PMCA2 variant was expressed in transfected cells. IP, Immunoprecipitation; Detect, detection; Ab, antibody.
The PMCA2a-specific antibody F2a immunoprecipitated a protein of ∼170 kDa from COS-7 cells expressing PMCA2w/a or PMCA2z/a (Fig. 2A). Demonstrating its specificity, R2w immunoprecipitated an ∼170 kDa band only in cells expressing PMCA2w/a. Consistent with the immunoprecipitation result, by immunocytochemistry, R2w only detected the 2w variant of PMCA2 when expressed in COS-7 cells (Fig. 2C).
Immunoprecipitation of PMCA2w from rat inner ear
We exploited our ability to purify hair bundles using the twist-off method (Gillespie and Hudspeth, 1991) to identify the PMCA2 isoform of rat utricle bundles by immunoprecipitation. Using splice-site-selective antibodies and a sequential immunoprecipitation procedure (Fig. 3A), we precipitated PMCAs from detergent extracts of purified bundles or residual maculae, the utricular tissue remaining after bundle isolation. We then detected precipitated PMCA with the pan-PMCA antibody 5F10.
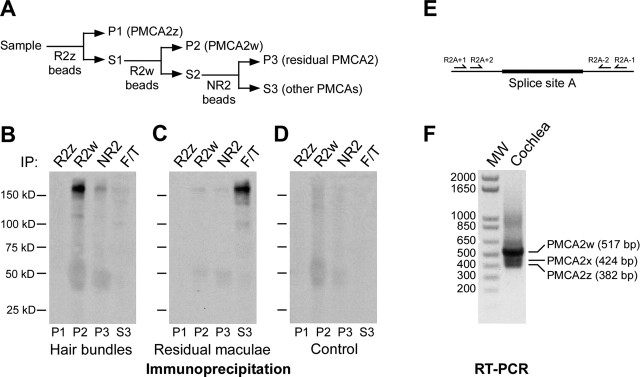
Figure 3.
PMCA2w is the predominant PMCA2 isoform of rat inner ear. A, Sequential immunoprecipitation (IP) scheme. P, Immunoprecipitate; S, supernatant. B–D, Sequential immunoprecipitations with PMCA2 antibodies. B, 5F10 detection of PMCA isoforms sequentially immunoprecipitated with PMCA2-selective antibodies from 36 ear equivalents of purified rat hair bundles. Nearly all bundle PMCA (∼170 kDa) were precipitated with R2w. F/T, PMCA not precipitated by any of the antibodies. C, Sequential immunoprecipitation from six ear equivalents of residual maculae. D, Sequential immunoprecipitation negative control; agarose from the bundle isolation procedure was used as the starting material. E, Location of primers used for nested PCR relative to splice-site A. F, Ethidium bromide-stained gel (inverted grayscale image) separating rat cochlear RT-PCR products. The sizes of products expected for PMCA2 splice forms and the sizes (in base pairs) of molecular weight markers (MW) are indicated.
R2w immunoprecipitated nearly all PMCA from a hair-bundle protein extract. R2z did not precipitate any PMCA2; moreover, after R2w treatment, only a small additional amount of PMCA2 was precipitated by the pan-PMCA2 antibody NR2. Consistent with PMCA2 being the only bundle isoform (Dumont et al., 2001), no PMCA remained in the final immunoprecipitation supernatant after R2z, R2w, and NR2 precipitation (Fig. 3B). In contrast, nearly all of the PMCA from the residual macula appeared in the final supernatant, indicating that there was little PMCA2 present. PMCA1x/b is the predominant isoform in the basolateral membranes of hair cells (Dumont et al., 2001) and is likely the isoform detected by 5F10 in the final supernatant (Fig. 3C). Faint PMCA bands appearing in the R2w and NR2 lanes of the residual macula likely were derived from hair bundles, because a small number of bundles remained in the residual macula preparation, or from PMCA2 present in the pericuticular necklace (see below). These immunoprecipitation experiments establish definitively that 2w is the only PMCA2 splice A form used by utricular hair bundles and that little PMCA2 is located outside of bundles.
2w is the predominant PMCA2 splice form in the cochlea
To confirm 2w is the major splice form of bundles, we performed reverse transcription (RT)-PCR experiments with rat cochlear tissue, where PMCA2 is localized to bundles (Dumont et al., 2001). Using nested primers that span splice-site A, we found that bands corresponding to the size of the 2w splice form were much more abundant than those corresponding to 2x and 2z (Fig. 3E,F). Sequencing of cloned PCR products confirmed that the 517, 424, and 382 bp bands corresponded to the 2w, 2x, and 2z splice forms. PMCA2w is thus the predominant splice-site A variant in rat cochlea.
Localization of PMCA2w in the rat inner ear
Consistent with the immunoprecipitation results, PMCA2w was prominent in hair bundles of P21–P23 rat auditory and vestibular organs imaged with immunocytochemistry (Fig. 4). Bundles of outer hair cells were labeled well by R2w (Fig. 4A,D). The concentration of R2w immunoreactivity in hair bundles of outer hair cells was visualized particularly well in an x–z section of a cochlear whole mount (Fig. 4M–Q). In contrast with the strong bundle labeling, PMCA2w immunoreactivity was nearly absent in the lateral membranes of outer hair cells (Fig. 4O, arrows). Inner hair cells also had little PMCA2w, except for a ring at their apical surfaces (Fig. 4M–Q and data not shown); these results were consistent with previous experiments that demonstrated that PMCA2a was nearly completely absent from inner hair cells (Dumont et al., 2001). The pan-PMCA antibody 5F10 labeled bundles of outer hair cells, as it did the cell bodies of the inner hair cells (Fig. 4B,F,P); the soma labeling was previously shown to be PMCA1x/b (Dumont et al., 2001). In contrast, 5F10 labeled cell bodies of outer hair cells very weakly and bundles of inner hair cells not at all (Fig. 4M–Q), consistent with our previous experiments (Dumont et al., 2001). The antigenic peptide abolished all R2w immunoreactivity (data not shown).
Figure 4.
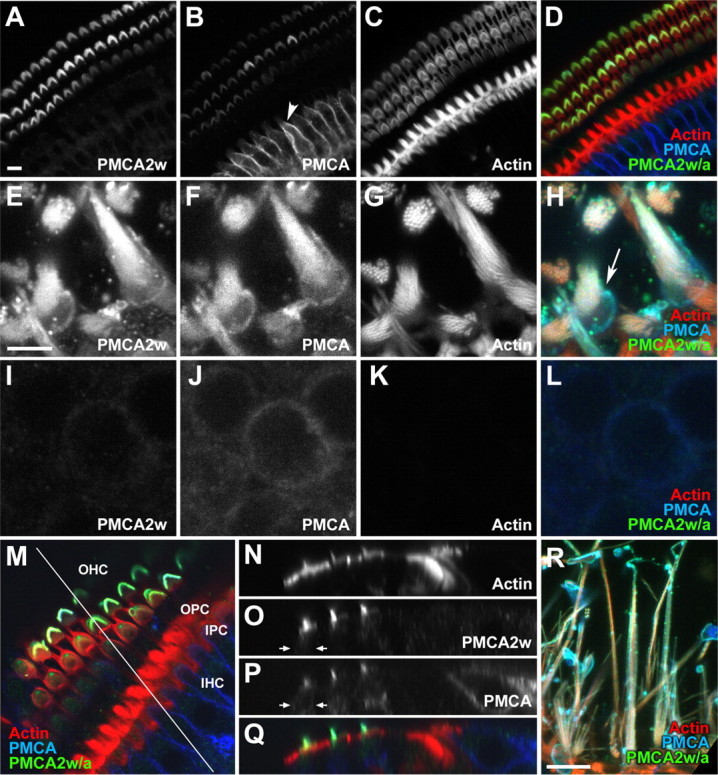
Localization of PMCA2w in rat hair bundles by immunofluorescence. A–D, Confocal cross sections through the P21 rat organ of Corti using R2w to detect PMCA2w (A; D, green), 5F10 to detect all PMCA isoforms (B; D, blue), and phalloidin to detect actin (C; D, red). Note the strong 5F10 immunoreactivity in basolateral membranes of inner hair cells (B, arrowhead). E–H, Utricular hair bundles were strongly labeled by R2w, as was the pericuticular necklace (H, arrow); the z-projection of two adjacent slices is shown. I–L, No R2w labeling of the lateral membranes of utricular hair cells; the z-projection of two adjacent slices is shown. M, En face view of a cochlea whole mount, with a line indicating the location of the x–z section for N–Q. OHC, Outer hair cells; OPC, outer pillar cells; IPC, inner pillar cells; IHC, inner hair cells. N–Q, The x–z section of cochlear whole mount shown in M. The color scheme in Q is the same as in M. PMCA2w immunoreactivity is strong in bundles of outer hair cells but absent from the cell bodies of outer hair cells (O, P, arrows), inner hair cells, and other cells of the organ of Corti. R, Lateral view of rat ampulla labeled with R2w. Scale bars: (in A) A–D, R, 10 μm; (in E) E–L, 5 μm; M, 91 × 91 μm; N–Q, 110 × 21 μm.
As in the auditory system, hair bundles in the utricle, a vestibular organ, were strongly labeled with R2w (Fig. 4E–H); we also observed R2w immunoreactivity surrounding the cuticular plate (Fig. 4H, arrow). There was almost no R2w labeling in lateral membranes of utricular hair cells (Fig. 4I–L). In the ampulla, another vestibular organ, hair bundles were also labeled well by R2w; 5F10 labeled both bundles and cell bodies (Fig. 4R and data not shown). These experiments confirm the identification of hair-bundle PMCA2 as the 2w splice form.
Expression of PMCA splice A variants in rat inner ear
Although these experiments, in combination with those of Dumont et al. (2001), established that the hair-bundle isoform is PMCA2w/a, it remained unclear whether alternative splicing at splice-site A was necessary for bundle targeting. To determine directly the role of 2w, we generated expression constructs of splice-site A variants 2w and 2z, using either of the two possible splice-site C variants (2a or 2b). We used gene-gun transfection (Rzadzinska et al., 2004) to introduce PMCA2 splice forms, N-terminally tagged with the C8 epitope, into hair cells. To facilitate locating the transfected cells, we cotransfected cells with GFP-actin, which was shown previously to incorporate into stereocilia actin cores (Schneider et al., 2002).
Consistent with the immunocytochemistry results, C8-PMCA2w/a was highly enriched in hair bundles and nearly absent from basolateral membranes of transfected rat auditory and vestibular hair cells (Fig. 5A,B). In one example, GFP-actin, which targeted to the basolateral membrane as well as the stereocilia, clearly outlined the soma of a transfected outer hair cell (Fig. 5A, arrows); in contrast, C8 antibody labeling was strong only in the bundle and absent in the soma. C8-PMCA2w/a was located throughout bundles, even when GFP-actin was concentrated at stereocilia tips. Although anti-C8 background labeling was relatively high at the apical surface, immunoreactivity was much higher in stereocilia of transfected cells. Moreover, C8 labeling in these cells was relatively uniform, unlike the particulate background labeling. Some specific C8 labeling in transfected hair cells was intracellular; C8-PMCA2w/a was often enriched in the region surrounding and just below the cuticular plate. This labeling could reflect either newly synthesized PMCA2 molecules destined for the bundle or molecules endocytosed from the apical membrane.
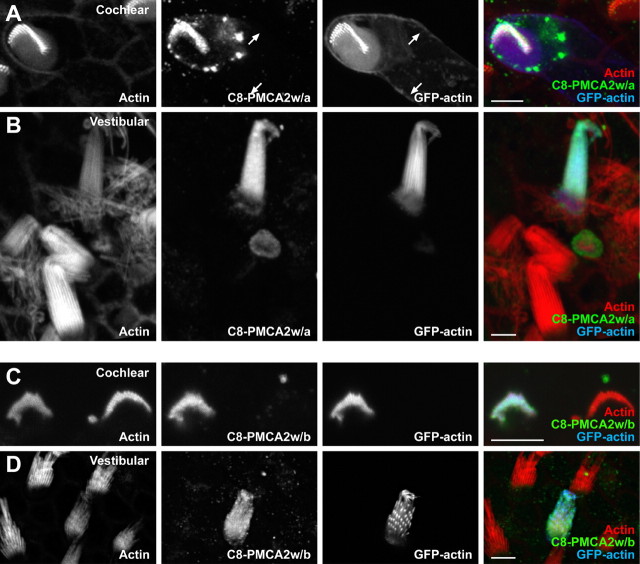
Figure 5.
Targeting of PMCA2w/a and PMCA2w/b transfected in rat hair cells. A, Bundle-specific PMCA2w/a expression in outer hair cells labeled with anti-C8 for epitope-tagged PMCA2w/a (green), GFP-actin (blue), and phalloidin to detect actin (red). Arrows indicate cortical actin from the GFP-actin signal in a transfected cell; note the absence of the C8-PMCA2w/a signal in the corresponding panel. The white color of the hair bundle in the last panel indicates overlap of PMCA2w/a, GFP-actin, and phalloidin signals. B, Hair-bundle targeting of PMCA2w/a in a utricular hair cell. C, Hair-bundle targeting of PMCA2w/b in a cochlear outer hair cell. D, Hair-bundle targeting of PMCA2w/b in a utricular hair cell. Scale bars, 5 μm.
To better illustrate the distribution of expressed PMCA2 isoforms, we quantified fluorescence intensity in hair bundles and basolateral membranes. In vestibular hair cells transfected with C8-PMCA2w/a, C8 labeling in hair bundles was nearly 20-fold greater than in basolateral membranes; in outer hair cells, the enrichment was >40-fold (Table 2). Because the differences between vestibular and cochlear bundles were not significant at the p < 0.05 level, we pooled the two sets of results for other comparisons.
Table 2.
Hair-bundle enrichment of PMCA2 splice forms
| Isoform | Bundle/basolateral membrane fluorescence (mean ± SEM) | Number of cells analyzed | Significance relative to PMCA2w/a |
|---|---|---|---|
| C8-PMCA2w/a | 31 ± 6 | 27 | |
| C8-PMCA2z/a | 0.26 ± 0.08 | 8 | 0.01 |
| C8-PMCA2w/b | 9 ± 2 | 3 | 0.19 |
| C8-PMCA2w-AAA/a | 14 ± 6 | 4 | 0.37 |
| GFP-PMCA2w/a | 3.0 ± 0.6 | 16 | 0.001 |
The fluorescence intensity of hair bundles and the basolateral membrane of transfected hair cells was compared with the intensity in nearby, untransfected cells. There were no statistically significant differences between auditory and vestibular hair cells. Conditions were compared statistically using the two-tailed t test, assuming equal variance.
Although C8-tagged PMCA2w with the 2b variant at splice-site C was also concentrated in hair bundles of transfected rat hair cells (Fig. 5C,D), we noted a somewhat higher level of basolateral membrane staining in some cells (data not shown), although the difference in intensity was not statistically significant (Table 2). The 2w sequence includes a tripeptide (VNG) also found in the excitatory amino acid transporter 3 apical targeting domain (Cheng et al., 2002). Mutation of VNG to AAA did not affect hair-bundle targeting of PMCA2w/a (Table 2 and data not shown), however, suggesting that other amino acids in 2w are necessary for apical localization.
PMCA2 with the 2z variant at site A was concentrated in basolateral plasma membranes of transfected cochlear and vestibular hair cells and was essentially excluded from the hair bundle (Fig. 6, Table 2). In a few cells, we detected weak expression of PMCA2z/a in the hair bundle, but only in the presence of high PMCA2-C8 expression in the soma (data not shown). The ∼100-fold reduced bundle/basolateral membrane fluorescence ratio was significantly different from that of PMCA2w/a (p < 0.02) (Table 2).
Figure 6.
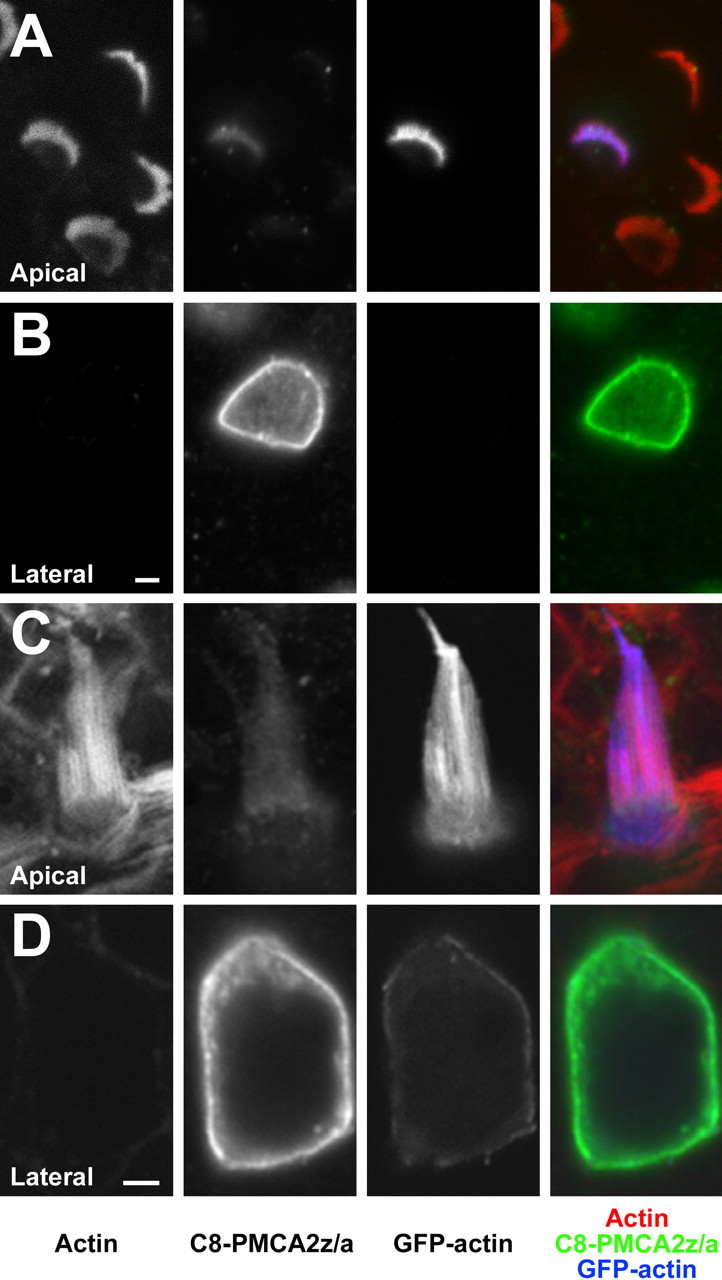
Targeting of PMCA2z/a in transfected rat hair cells. A, B, PMCA2z/a-C8 expression in the membrane of a cochlear outer hair cell at the level of the bundle (A) and near the nucleus (B). The magenta color of the hair bundle indicates lack of PMCA2z/a; the strong expression of PMCA2z/a on the basolateral membrane is apparent in B (green). C, D, PMCA2z/a-C8 on the membrane of a utricular hair cell, visualized at the level of the bundle (C) and near the nucleus (D). Green, Epitope-tagged PMCA2w/a; blue, GFP-actin; red, phalloidin to detect actin. Scale bars, 2 μm.
To ensure that the differential localization we saw did not arise from overexpression of transfected PMCA, we measured the bundle concentration of PMCA2a using the antibody F2a. In cells cotransfected with C8-PMCA2w/a and GFP-actin, the F2a signal in bundles of transfected hair cells was 1.0 ± 0.1-fold that of neighboring untransfected hair cells, indicating that recombinant PMCA2 represented only a small fraction of the total PMCA. In contrast, transfection of C8-PMCA2w/a alone produced PMCA2a levels that were 2.1 ± 0.1-fold higher than control, suggesting that GFP-actin expression suppressed PMCA2 translation under our usual conditions. Targeting of C8-PMCA2w/a in cells transfected without GFP-actin was identical to that in cells with it.
N-terminal GFP alters bundle targeting of PMCA2w/a
To allow direct visualization of PMCA in live hair cells, we transfected cells with GFP-tagged PMCA2w/a. In MDCK cells, the apical-to-basolateral distribution of GFP-tagged PMCA2 isoforms was similar to that of isoforms with much smaller epitope tags (Chicka and Strehler, 2003). Surprisingly, we found that N-terminal GFP tagging of PMCA2w/a altered the targeting of this PMCA2 variant; in cochlea and vestibular hair cells, the GFP signal was nearly as strong in the basolateral membrane as it was in the hair-bundle membrane (Fig. 7). The bundle/basolateral membrane fluorescence ratio was diminished >10-fold by GFP, statistically significant at p < 0.01 (Table 2). Examination of the alignment of PMCA2 with the structure of the sarcoplasmic-endoplasmic reticulum Ca2+-ATPase (SERCA) (Fig. 1C) indicated that the N terminus is very close to where splice-site A variants should be found, however, suggesting that the GFP tag may sterically interfere with the targeting sequence.
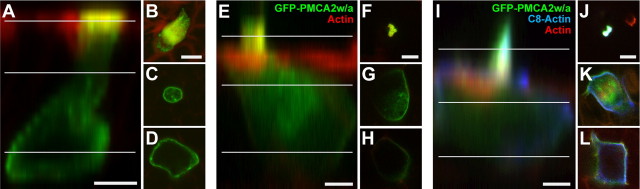
Figure 7.
Expression of GFP-PMCA2w/a in rat hair cells. Representative hair cells transfected with GFP-PMCA2w/a alone (A–H) or with GFP-PMCA2w/a and C8-actin (I–L). A–D, Vestibular hair cell transfected with GFP-PMCA2w/a (green) and labeled with phalloidin to detect actin (red). A, x–z projection. B–D, Individual sections at levels indicated in A. E–H, Cochlear outer hair cell transfected as in A–D. I–L, Cochlear outer hair cell transfected with GFP-PMCA2w/a (green) and C8-actin (blue); actin was detected with phalloidin (red). Scale bars: A, E, I, 5 μm; (in B, F, J) B–D, F–H, J–L, 5 μm.
Discussion
We show here that the 2w variant of splice-site A is required for targeting PMCA2 to the hair bundle. Because bundle PMCA2 uses 2a at splice-site C (Dumont et al., 2001), these experiments establish that the rodent hair-bundle calcium pump is PMCA2w/a. Moreover, 2w is not simply correlated with bundle targeting but instead is necessary; this sequence thus is the first identified that controls targeting of a membrane protein to bundles. Our experiments also highlight the degree to which the auditory and vestibular systems segregate their Ca2+ pumps to allow highly localized Ca2+ pumping.
The mammalian hair-bundle Ca2+ pump is PMCA2w/a
Of two locations within PMCA2 subjected to alternative splicing, most attention has been paid to the C-terminal site; because PMCA 2b variants interact with PDZ (postsynaptic density-95/Discs large/zona occludens-1) domains (Kim et al., 1998; DeMarco et al., 2002), which assist in targeting of several membrane proteins (Fanning and Anderson, 1999; Hung and Sheng, 2002), an initial expectation was that splicing at site C would regulate hair-bundle targeting. Unexpectedly, Chicka and Strehler (2003) demonstrated that 2w, but not 2z, drives PMCA2 to the apical region of MDCK cells, indicating that site A controls membrane targeting in these cells. Consistent with 2w dictating apical targeting, the predominant form of PMCA2 in apical membranes of mammary epithelia is PMCA2w/b, which transports Ca2+ into milk (Reinhardt and Horst, 1999; Reinhardt et al., 2000). Nevertheless, although 2w is important for apical membrane targeting in these examples, signals active in one cell type are not necessarily dominant in another cell (Muth and Caplan, 2003).
Our experiments demonstrated directly that 2w dictates hair-bundle targeting in rat hair cells. Not only was 2w the only splice A variant present in bundle PMCA2 (Fig. 3), but native PMCA2w also was found solely in bundles of mature auditory and vestibular epithelia (Fig. 4). Moreover, when transfected into P2 hair cells, epitope-tagged PMCA2w/a and PMCA2w/b were both targeted nearly exclusively to bundles (Fig. 5, Table 2). In contrast, PMCA2 with 2z was present nearly entirely on the basolateral membrane. Site C apparently does not contribute to targeting; 2a and 2b variants both sort to apical surfaces of MDCK cells (Chicka and Strehler, 2003), mammary epithelia (Reinhardt et al., 2000), and hair cells (Fig. 5). Our two complementary approaches thus together indicate that the site A identity controls localization of PMCA2 within hair cells, with 2w dictating bundle targeting.
Apical targeting mediated by 2w
Apical targeting in polarized epithelial cells follows either a direct pathway from the trans-Golgi network to the apical surface (Keller and Simons, 1997) or a transcytotic pathway, where apical proteins are first delivered to the basolateral membrane (Mostov, 1993); both pathways can be used in the same cell type (Polishchuk et al., 2004). Given the intracellular location of the apical targeting domain of PMCA2, several known apical signals are unlikely to be relevant, including glycosylphosphatidylinositol anchors (Lisanti et al., 1990) or N-linked glycosylation (Scheiffele et al., 1995). Apical targeting of PMCA2 instead relies on a short peptide sequence, similar to other apical membrane proteins with targeting sequences near transmembrane domains or at the C terminus (Dunbar et al., 2000; Jolimay et al., 2000; Cheng et al., 2002; Caplan, 2003; Inukai et al., 2004). We detected a small amount of PMCA2w/a in the basolateral membrane of hair cells in our gene-gun experiments, consistent with either a transcytotic pathway for apical targeting or a direct pathway with reduced fidelity. Recombinant PMCA2 was present at levels lower than native PMCA2, suggesting that this incomplete localization to the bundle reflected the native distribution. Likewise, epitope-tagged PMCA2 with the 2z variant appeared on stereocilia membranes to a limited degree. This imperfect targeting likely arose from our use of immature hair cells for transfection; indeed, native PMCA2 was detectable on basolateral membranes of P2 hair cells (data not shown). Exclusive localization of 2w to hair bundles of older animals (Fig. 4) presumably reflects more efficient targeting in mature cells.
Alignment of PMCA2 with the structure of SERCA, a related Ca2+ pump, illustrates the proximity of splice-site A to the plasma membrane and to a phospholipid regulatory site (Niggli et al., 1981). PMCA2w has 69 extra amino acids relative to SERCA at site A, more than enough to reach directly to the plasma membrane. Many apically targeted proteins are sorted because of interaction with specific lipid domains (van Meer and Simons, 1988; Simons and Ikonen, 1997), and the proximity of site A to the membrane suggests that PMCA2 sorting may require interaction with membrane components.
Because hair cells must localize key molecules for mechanotransduction to the hair bundle, mechanisms used for targeting PMCA2 may also be used for other membrane proteins, such as the channels (including the transduction channel), ion transporters, and cell-adhesion molecules. We have yet to identify a sequence in known bundle membrane proteins resembling 2w, however, indicating that either that exact sequence identity is not important or that multiple signals operate in hair bundles.
Segregation of PMCA isoforms in the cochlea
Why does the organ of Corti differentially segregate PMCA isoforms so markedly in inner and outer hair cells? Inner hair cells have a high PMCA1x/b density on their basolateral membranes and almost no PMCA on their apical surfaces (Dumont et al., 2001). Moreover, outer hair cells express little PMCA on their basolateral surfaces but instead concentrate PMCA2w/a on stereocilia membranes (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). Likewise, mobile Ca2+ buffers in the organ of Corti contribute ∼6 mm of Ca2+ binding sites to outer hair cells but only ∼0.5 mm to inner hair cells (Hackney et al., 2005). Although Ca2+ extrusion via Na+/Ca2+ exchangers may occur across the basolateral membrane (Ikeda et al., 1992; Chabbert et al., 1995), this mechanism has a relatively low affinity for Ca2+ (Blaustein and Lederer, 1999); accordingly, the high concentration of Ca2+ buffers in outer hair cells will starve exchangers for Ca2+. PMCA2 is clearly essential for proper cochlear function, because animals homozygous for mutant PMCA2 have poor or no auditory function and cochlear degeneration (Kozel et al., 1998; Street et al., 1998; Dodson and Charalabapoulou, 2001).
Because of these factors, we suggest that PMCA2w/a pumps nearly all Ca2+ that enters outer hair cells into the endolymph, regardless of whether the Ca2+ permeates transduction channels or basolateral Ca2+ channels (Yamoah et al., 1998; LeMasurier and Gillespie, 2005), with the net flux depending on the contribution of basolateral Ca2+ channels to Ca2+ dynamics (Kros, 1996). Transcellular Ca2+ flux is well established in transport epithelia and, like in the organ of Corti, is produced by asymmetric distribution of Ca2+ channels, buffers, and pumps (Hoenderop et al., 2005). Recent evidence supports the concept of a transcellular Ca2+ flux within the cochlea (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). The endolymph concentration of Ca2+ is unusually low (∼7 μm compared with 23 μm in control) in mice with a mutation in PMCA2 (Wood et al., 2004), much closer to the equilibrium concentration of ∼2 μm calculated from the Nernst equation. Because the only cochlear PMCA2 exposed to endolymph is that of the stereocilia (Dumont et al., 2001; Wood et al., 2004), PMCA2-dependent elevated endolymph Ca2+ reports net extrusion by outer hair cells.
The Ca2+ concentration in the subtectorial space could be substantial; for example, x-ray elemental microanalysis of the tectorial membrane adjacent to the stereocilia detected a Ca2+ concentration more than fivefold greater than that of the middle part (Anniko et al., 1984). Although the rate of diffusion of Ca2+ through the tectorial membrane is unknown, it does bind Ca2+ (Shah et al., 1995), suggesting that the x-ray technique detects a Ca2+ gradient emerging from hair cells. Ca2+ leaving the outer hair cells may enter inner hair cells (supplemental Fig. 1, available at www.jneurosci.org as supplemental material); the dearth of PMCA on inner hair cell stereocilia will prevent re-extrusion of Ca2+, which instead should be pumped into the perilymph by basolateral PMCA1x/b. The differential entry and exit of Ca2+ from outer and inner hair cells may lead to a local Ca2+ circuit (supplemental Fig. 1, available at www.jneurosci.org as supplemental material); whether this local circuit is a major conduit for Ca2+ in the cochlea depends on how fast Ca2+ exits the subtectorial space.
Although subtectorial Ca2+ elevation remains hypothetical, it makes physiological sense. If outer hair cells are to use Ca2+-dependent fast adaptation to power amplification (Kennedy et al., 2005; LeMasurier and Gillespie, 2005), the concentration of Ca2+ must be sufficiently high to allow fast adaptation to occur quickly enough. Future experiments must aim at measuring the static concentration of Ca2+ in the subtectorial space and the dynamics of Ca2+ entry and exit in outer hair cells under physiological conditions.
Footnotes
This work was supported by National Institutes of Health Grants R01 DC002368, R01 DC004571, P30 DC005893 (P.G.G.), and R01 GM28835 (E.E.S.).
References
- Abacioglu YH, Fouts TR, Laman JD, Claassen E, Pincus SH, Moore JP, Roby CA, Kamin-Lewis R, Lewis GK (1994). Epitope mapping and topology of baculovirus-expressed HIV-1 gp160 determined with a panel of murine monoclonal antibodies. AIDS Res Hum Retroviruses 10:371–381. [DOI] [PubMed] [Google Scholar]
- Anniko M, Lim D, Wroblewski R (1984). Elemental composition of individual cells and tissues in the cochlea. Acta Otolaryngol 98:439–453. [DOI] [PubMed] [Google Scholar]
- Belyantseva IA, Boger ET, Friedman TB (2003). Myosin XVa localizes to the tips of inner ear sensory cell stereocilia and is essential for staircase formation of the hair bundle. Proc Natl Acad Sci USA 100:13958–13963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyantseva IA, Boger ET, Naz S, Frolenkov GI, Sellers JR, Ahmed ZM, Griffith AJ, Friedman TB (2005). Myosin-XVa is required for tip localization of whirlin and differential elongation of hair-cell stereocilia. Nat Cell Biol 7:148–156. [DOI] [PubMed] [Google Scholar]
- Blaustein MP, Lederer WJ (1999). Sodium/calcium exchange: its physiological implications. Physiol Rev 79:763–854. [DOI] [PubMed] [Google Scholar]
- Caplan MJ (2003). How megalin finds its way: identification of a novel apical sorting motif. Focus on “Identification of an apical sorting determinant in the cytoplasmic tail of megalin.”. Am J Physiol 284:C1101–C1104. [DOI] [PubMed] [Google Scholar]
- Chabbert C, Canitrot Y, Sans A, Lehouelleur J (1995). Calcium homeostasis in guinea pig type-I vestibular hair cell: possible involvement of an Na+-Ca2+ exchanger. Hear Res 89:101–108. [DOI] [PubMed] [Google Scholar]
- Cheng C, Glover G, Banker G, Amara SG (2002). A novel sorting motif in the glutamate transporter excitatory amino acid transporter 3 directs its targeting in Madin-Darby canine kidney cells and hippocampal neurons. J Neurosci 22:10643–10652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chicka MC, Strehler EE (2003). Alternative splicing of the first intracellular loop of plasma membrane Ca2+-ATPase isoform 2 alters its membrane targeting. J Biol Chem 278:18464–18470. [DOI] [PubMed] [Google Scholar]
- Crouch JJ, Schulte BA (1996). Identification and cloning of site C splice variants of plasma membrane Ca-ATPase in the gerbil cochlea. Hear Res 101:55–61. [DOI] [PubMed] [Google Scholar]
- DeMarco SJ, Chicka MC, Strehler EE (2002). Plasma membrane Ca2+-ATPase isoform 2b interacts preferentially with Na+/H+ exchanger regulatory factor 2 in apical plasma membranes. J Biol Chem 277:10506–10511. [DOI] [PubMed] [Google Scholar]
- Dodson HC, Charalabapoulou M (2001). PMCA2 mutation causes structural changes in the auditory system in deafwaddler mice. J Neurocytol 30:281–292. [DOI] [PubMed] [Google Scholar]
- Dumont RA, Lins U, Filoteo AG, Penniston JT, Kachar B, Gillespie PG (2001). Plasma membrane Ca2+-ATPase isoform 2a is the PMCA of hair bundles. J Neurosci 21:5066–5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar LA, Aronson P, Caplan MJ (2000). A transmembrane segment determines the steady-state localization of an ion-transporting adenosine triphosphatase. J Cell Biol 148:769–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enyedi A, Verma AK, Heim R, Adamo HP, Filoteo AG, Strehler EE, Penniston JT (1994). The Ca2+ affinity of the plasma membrane Ca2+ pump is controlled by alternative splicing. J Biol Chem 269:41–43. [PubMed] [Google Scholar]
- Enyedi A, Elwess NL, Filoteo AG, Verma AK, Paszty K, Penniston JT (1997). Protein kinase C phosphorylates the “a” forms of plasma membrane Ca2+ pump isoforms 2 and 3 and prevents binding of calmodulin. J Biol Chem 272:27525–27528. [DOI] [PubMed] [Google Scholar]
- Fanning AS, Anderson JM (1999). Protein modules as organizers of membrane structure. Curr Opin Cell Biol 11:432–439. [DOI] [PubMed] [Google Scholar]
- Furuta H, Luo L, Hepler K, Ryan AF (1998). Evidence for differential regulation of calcium by outer versus inner hair cells: plasma membrane Ca-ATPase gene expression. Hear Res 123:10–26. [DOI] [PubMed] [Google Scholar]
- Gao J, Wu X, Zuo J (2004). Targeting hearing genes in mice. Brain Res Mol Brain Res 132:192–207. [DOI] [PubMed] [Google Scholar]
- Gillespie PG, Hudspeth AJ (1991). High-purity isolation of bullfrog hair bundles and subcellular and topological localization of constituent proteins. J Cell Biol 112:625–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney CM, Mahendrasingam S, Penn A, Fettiplace R (2005). The concentrations of calcium buffering proteins in mammalian cochlear hair cells. J Neurosci 25:7867–7875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoenderop JG, Nilius B, Bindels RJ (2005). Calcium absorption across epithelia. Physiol Rev 85:373–422. [DOI] [PubMed] [Google Scholar]
- Holt JR (2002). Viral-mediated gene transfer to study the molecular physiology of the mammalian inner ear. Audiol Neurootol 7:157–160. [DOI] [PubMed] [Google Scholar]
- Hudspeth AJ (1989). How the ear's works work. Nature 341:397–404. [DOI] [PubMed] [Google Scholar]
- Hung AY, Sheng M (2002). PDZ domains: structural modules for protein complex assembly. J Biol Chem 277:5699–5702. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Saito Y, Nishiyama A, Takasaka T (1992). Na+-Ca2+ exchange in the isolated cochlear outer hair cells of the guinea-pig studied by fluorescence image microscopy. Pflügers Arch 420:493–499. [DOI] [PubMed] [Google Scholar]
- Inukai K, Shewan AM, Pascoe WS, Katayama S, James DE, Oka Y (2004). Carboxy terminus of glucose transporter 3 contains an apical membrane targeting domain. Mol Endocrinol 18:339–349. [DOI] [PubMed] [Google Scholar]
- Jacobs RA, Hudspeth AJ (1990). Ultrastructural correlates of mechanoelectrical transduction in hair cells of the bullfrog's internal ear. Cold Spring Harb Symp Quant Biol 55:547–561. [DOI] [PubMed] [Google Scholar]
- Jolimay N, Franck L, Langlois X, Hamon M, Darmon M (2000). Dominant role of the cytosolic C-terminal domain of the rat 5-HT1B receptor in axonal-apical targeting. J Neurosci 20:9111–9118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller P, Simons K (1997). Post-Golgi biosynthetic trafficking. J Cell Sci 110:3001–3009. [DOI] [PubMed] [Google Scholar]
- Kennedy HJ, Crawford AC, Fettiplace R (2005). Force generation by mammalian hair bundles supports a role in cochlear amplification. Nature 433:880–883. [DOI] [PubMed] [Google Scholar]
- Kim E, DeMarco SJ, Marfatia SM, Chishti AH, Sheng M, Strehler EE (1998). Plasma membrane Ca2+ ATPase isoform 4b binds to membrane-associated guanylate kinase (MAGUK) proteins via their PDZ (PSD-95/Dlg/ZO-1) domains. J Biol Chem 273:1591–1595. [DOI] [PubMed] [Google Scholar]
- Kozel PJ, Friedman RA, Erway LC, Yamoah EN, Liu LH, Riddle T, Duffy JJ, Doetschman T, Miller ML, Cardell EL, Shull GE (1998). Balance and hearing deficits in mice with a null mutation in the gene encoding plasma membrane Ca2+-ATPase isoform 2. J Biol Chem 273:18693–18696. [DOI] [PubMed] [Google Scholar]
- Kros CJ (1996). In: Hair cell physiology (Dallos P, Popper AN, Fay RR, eds) pp. 318–385. New York: Springer.
- LeMasurier M, Gillespie PG (2005). Hair-cell mechanotransduction and cochlear amplification. Neuron 48:403–415. [DOI] [PubMed] [Google Scholar]
- Lenzi D, Roberts WM (1994). Calcium signalling in hair cells: multiple roles in a compact cell. Curr Opin Neurobiol 4:496–502. [DOI] [PubMed] [Google Scholar]
- Lisanti MP, Le Bivic A, Saltiel AR, Rodriguez-Boulan E (1990). Preferred apical distribution of glycosyl-phosphatidylinositol (GPI) anchored proteins: a highly conserved feature of the polarized epithelial cell phenotype. J Membr Biol 113:155–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumpkin EA, Hudspeth AJ (1998). Regulation of free Ca2+ concentration in hair-cell stereocilia. J Neurosci 18:6300–6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumpkin EA, Marquis RE, Hudspeth AJ (1997). The selectivity of the hair cell's mechanoelectrical-transduction channel promotes Ca2+ flux at low Ca2+ concentrations. Proc Natl Acad Sci USA 94:10997–11002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostov K (1993). Protein traffic in polarized epithelial cells: the polymeric immunoglobulin receptor as a model system. J Cell Sci Suppl 17:21–26. [DOI] [PubMed] [Google Scholar]
- Muth TR, Caplan MJ (2003). Transport protein trafficking in polarized cells. Annu Rev Cell Dev Biol 19:333–366. [DOI] [PubMed] [Google Scholar]
- Niggli V, Adunyah ES, Carafoli E (1981). Acidic phospholipids, unsaturated fatty acids, and limited proteolysis mimic the effect of calmodulin on the purified erythrocyte Ca2+-ATPase. J Biol Chem 256:8588–8592. [PubMed] [Google Scholar]
- Noben-Trauth K, Zheng QY, Johnson KR, Nishina PM (1997). mdfw: a deafness susceptibility locus that interacts with deaf waddler (dfw). Genomics 44:266–272. [DOI] [PubMed] [Google Scholar]
- Polishchuk R, Di Pentima A, Lippincott-Schwartz J (2004). Delivery of raft-associated, GPI-anchored proteins to the apical surface of polarized MDCK cells by a transcytotic pathway. Nat Cell Biol 6:297–307. [DOI] [PubMed] [Google Scholar]
- Reinhardt TA, Horst RL (1999). Ca2+-ATPases and their expression in the mammary gland of pregnant and lactating rats. Am J Physiol 276:C796–C802. [DOI] [PubMed] [Google Scholar]
- Reinhardt TA, Filoteo AG, Penniston JT, Horst RL (2000). Ca2+-ATPase protein expression in mammary tissue. Am J Physiol 279:C1595–C1602. [DOI] [PubMed] [Google Scholar]
- Ricci AJ, Fettiplace R (1998). Calcium permeation of the turtle hair cell mechanotransducer channel and its relation to the composition of endolymph. J Physiol (Lond) 506:159–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzadzinska AK, Schneider ME, Davies C, Riordan GP, Kachar B (2004). An actin molecular treadmill and myosins maintain stereocilia functional architecture and self-renewal. J Cell Biol 164:887–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiffele P, Peranen J, Simons K (1995). N-glycans as apical sorting signals in epithelial cells. Nature 378:96–98. [DOI] [PubMed] [Google Scholar]
- Schneider ME, Belyantseva IA, Azevedo RB, Kachar B (2002). Rapid renewal of auditory hair bundles. Nature 418:837–838. [DOI] [PubMed] [Google Scholar]
- Shah DM, Freeman DM, Weiss TF (1995). The osmotic response of the isolated, unfixed mouse tectorial membrane to isosmotic solutions: effect of Na+, K+, and Ca2+ concentration. Hear Res 87:187–207. [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E (1997). Functional rafts in cell membranes. Nature 387:569–572. [DOI] [PubMed] [Google Scholar]
- Stauffer TP, Guerini D, Carafoli E (1995). Tissue distribution of the four gene products of the plasma membrane Ca2+ pump. J Biol Chem 270:12184–12190. [DOI] [PubMed] [Google Scholar]
- Street VA, McKee-Johnson JW, Fonseca RC, Tempel BL, Noben-Trauth K (1998). Mutations in a plasma membrane Ca2+-ATPase gene cause deafness in deafwaddler mice. Nat Genet 19:390–394. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Kitamura K (1999). A point mutation in a plasma membrane Ca2+-ATPase gene causes deafness in Wriggle Mouse Sagami. Biochem Biophys Res Commun 261:773–778. [DOI] [PubMed] [Google Scholar]
- van Meer G, Simons K (1988). Lipid polarity and sorting in epithelial cells. J Cell Biochem 36:51–58. [DOI] [PubMed] [Google Scholar]
- Wood JD, Muchinsky SJ, Filoteo AG, Penniston JT, Tempel BL (2004). Low endolymph calcium concentrations in deafwaddler2J mice suggest that PMCA2 contributes to endolymph calcium maintenance. J Assoc Res Otolaryngol 5:99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamoah EN, Lumpkin EA, Dumont RA, Smith PJ, Hudspeth AJ, Gillespie PG (1998). Plasma membrane Ca2+-ATPase extrudes Ca2+ from hair cell stereocilia. J Neurosci 18:610–624. [DOI] [PMC free article] [PubMed] [Google Scholar]