Abstract
Adolescence is marked by profound psychological and neuroendocrine changes. Cognitive functions that depend on the prefrontal cortex and dopamine (DA), such as decision making, are acquired or refined during adolescence; yet, little is known about how neural circuits mature in the transition to adulthood. Here, we conducted electrophysiological recordings in rat brain slices, unveiling an enhancement of the excitability of interneurons, which are important for cortical network activity, by D1 and D2 DA receptors. The D2 effect was observed in slices from adult (postnatal day [PD] > 50) but not preadolescent (PD < 36) animals suggesting a possible neural substrate for the maturation of DA-dependent prefrontal cortical functions during or after adolescence and identifying a critical neural population that could be involved in the periadolescent onset of neuropsychiatric disorders, such as schizophrenia.
Keywords: D1, D2, electrophysiology, GABA, schizophrenia
Introduction
There has been a surge of interest in the neurobiological changes that may occur during late adolescence, after the pubertal hormonal changes (Spear 2000). Acquisition of adult behaviors may be paralleled by progressive changes in cortical areas, in particular in the prefrontal cortex (PFC) (Casey and others 2000). For example, imaging studies have shown changes in the human cortical mantle during adolescence (Giedd and others 1999). It has also been determined that the dopamine (DA) innervation of the PFC peaks during adolescence in rats and monkeys (Kalsbeek and others 1988; Rosenberg and Lewis 1994; Tarazi and Baldessarini 2000), and neurochemical changes elicited by psychostimulants are distinct at that stage (Andersen and others 2001). Furthermore, interactions between DA and N-methyl-D-aspartate (NMDA) receptors acquire mature electrophysiological characteristics in young adult rats (Tseng and O’Donnell 2005). Thus, not only the cortex is having morphological adjustments at this critical age but also the physiological properties change in the PFC, perhaps allowing the emergence of cortical network information processing mechanisms that can support more complex behavioral outcomes. Indeed, many behaviors in which the PFC is a key player (i.e., response selection, decision making, working memory) (Funahashi and Inoue 2000; Matsumoto and others 2003) do change with the transition to adulthood. As an example, error-related negativity, a PFC event-related potential assumed to reflect self-monitoring activity, completes its maturation late into adolescence (Segalowitz and Davies 2004). As PFC circuits responsible for those functions (e.g., the DA innervation of PFC pyramidal neurons and γ-aminobutyric acid (GABA) interneurons) are being unveiled (Seamans and Yang 2004), it is tempting to hypothesize that they mature during adolescence. Specifically, interactions between interneurons and pyramidal neurons, along with their control by DA, are likely to acquire different characteristics in the postadolescent brain. We decided to explore those interactions in brain slices obtained from pre- and postadolescent rats. Age groups are defined as preadolescent (postnatal day [PD] < 36), adolescent (PD36–50), or young adults (PD > 50), as the groups that best fit the various aspects of late maturation described in the excellent review by Linda Spear (2000).
Materials and Methods
All experimental procedures were performed according to the United States Public Health Service “Guide for Care and Use of Laboratory Animals” and approved by the Albany Medical College Institutional Animal Care and Use Committee. As previously described (Tseng and O’Donnell 2004, 2005), rats were anesthetized with chloral hydrate (400 mg/kg intraperitoneally) before being decapitated, and the brains were rapidly removed into ice-cold artificial cerebrospinal fluid (aCSF, pH 7.45, osmolarity 295 ± 5 mOsm) containing (in mM) 125 NaCl, 25 NaHCO3, 10 glucose, 3.5 KCl, 1.25 NaH2PO4, 0.5 CaCl2, and 3 MgCl2. Coronal brain slices (300-μm thick) including the medial PFC were cut in ice-cold aCSF using a Vibratome, transferred and incubated in warm (around 35°C) aCSF continuously oxygenated with 95%:5% O2:CO2 for at least 60 min before recording. In the recording aCSF, CaCl2 was increased to 2 mM, and MgCl2 was decreased to 1 mM. All recordings were conducted at 33–35°C in oxygenated aCSF delivered at 2 mL/min. Patch pipettes (7–10 MΩ) were filled with a 0.125% Neurobiotin solution containing (in mM) 115 K gluconate, 10 4-(2-hydroxyethyl)- 1-piperazineethanesulfonic acid, 2 MgCl2, 20 KCl, 2 MgATP, 2 Na2-ATP, and 0.3 GTP (pH 7.3, 280 ± 5 mOsm).
Deep-layer nonpyramidal neurons were identified under visual guidance with infrared (IR)-differential interference contrast videomicroscopy (Olympus BX-51-WI) with a × 40 water-immersion objective. The image was detected with an IR-sensitive charge coupled device camera (Dage-MTI) and displayed on a monitor. Whole-cell current clamp recordings were conducted with a computer-controlled amplifier (Multiclamp 700A, Axon Instruments, Foster City, CA) and acquired using Axoscope 8.1 (Axon) at a sampling rate of 10 kHz. The liquid junction potential was not corrected, and electrode potentials were adjusted to zero before recording. Input resistance (measured with hyperpolarizing current pulses), membrane potential, and cell excitability (measured as the number of evoked spikes and latency of the first spike evoked with a depolarizing current injection) were compared before and after drug treatment. DA D1 and D2 agonists (SKF38393 and quinpirole) and antagonists (SCH23390 and eticlopride) were mixed into oxygenated aCSF and applied into the recording solution at known concentrations. Both control and drug-containing aCSF were continuously oxygenated throughout the experiments. Following 15 min of baseline recording, a solution containing the drugs was perfused for 5–7 min, followed by washout. All data are expressed as mean ± standard deviation (SD). Drug effects were compared using student’s t-test or repeated measures analysis of variance (ANOVA), and the differences between experimental conditions were considered significant when P < 0.05.
The morphology of recorded neurons was analyzed by neurobiotin staining. After the recording session, slices with injected neurons were fixed overnight in 4% paraformaldehyde and then stored in phosphate-buffered saline (PBS). Slices were incubated in 1% Triton X-100 in PBS for 1–2 h, followed by 2 h in Vectastain Elite ABC reagent (Vector Laboratories, Burlingame, CA). Following rinses, slices were reacted with 3.3′-diaminobenzidine (DAB) and urea-hydrogen peroxide (Sigma FAST DAB set). Slices were rinsed, mounted in gelatine-coated slides, dried, cleared in xylene, coverslipped in permount, and examined on a microscope.
Results
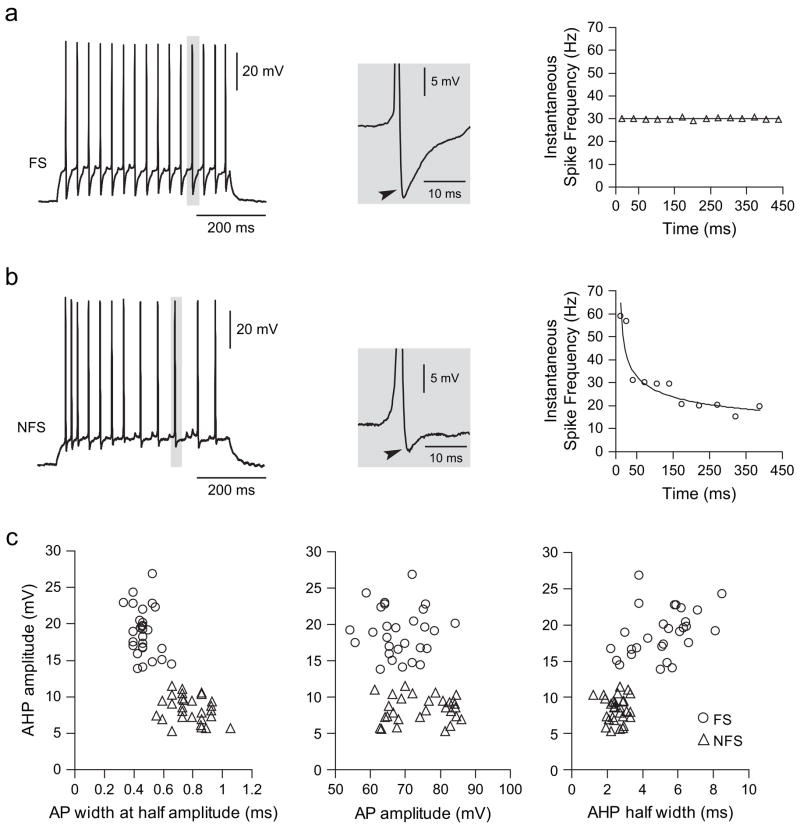
Whole-cell patch clamp recordings of PFC GABAergic inter-neurons were conducted in brain slices obtained from pre-pubertal (PD < 35) and postadolescent (PD > 50) rats, examining the effect of the D2 DA agonist quinpirole and the D1 agonist SKF38393 on cell excitability. All neurons included in the present study (n = 59) were located in layers V and VI of the medial PFC (prelimbic and infralimbic regions), and could be categorized into fast-spiking (FS, n = 29) or non-FS (NFS, n = 30) interneurons, based on their firing pattern, afterhyperpolarization (AHP), and spike-frequency adaptation characteristics (Cauli and others 1997; Gibson and others 1999) (Fig. 1). Both types of interneurons exhibited similar resting membrane potential (FS: −61.5 ± 3.4 mV; NFS: −61.8 ± 2.6 mV; mean ± SD) and input resistance (FS: 255.9 ± 83.9 MΩ; NFS: 270.0 ± 74.3 MΩ), and no differences were observed in these parameters among cells recorded from prepubertal (n = 27) and post-pubertal (n = 32) animals. FS interneurons exhibited more pronounced AHP (18.9 ± 3.3 mV) and significantly longer AHP half width (5.2 ± 1.6 ms) than NFS cells (AHP amplitude: 8.4 ± 1.8 mV; AHP half width: 2.5 ± 0.5 ms, P < 0.00001, student’s t-test; Fig. 1c). Similarly, action potentials were significantly smaller and faster in FS (68.5 ± 7.1 mV; 0.53 ± 0.08 ms at half amplitude) than in NFS (amplitude: 74.7 ± 8.4 mV; duration: 0.89 ± 0.14 ms, P < 0.005, student’s t-test). Some, but not all, FS neurons (5/14, 37.5%) recorded from prepubertal animals showed a stuttering firing response to current pulse injection (typically observed with lower suprathreshold current pulses), whereas none of the 15 FS interneurons from the postpubertal PFC exhibited this firing pattern. These proportions are significantly different (P = 0.0169, Fisher exact test).
Figure 1.
Interneurons could be classified as FS and NFS. Representative traces illustrating the firing pattern of FS (a) and NFS (b) interneurons recorded in the PFC of developmentally mature animals (PD > 50). Depolarizing current steps induced repetitive action potential firing in both types of interneurons (left panel). FS interneurons exhibit a pronounced fast AHP (arrowhead), whereas AHP in NFS interneurons was less pronounced. The most distinctive difference was the lack of spike-frequency adaptation in FS interneurons, revealed as a constant instantaneous firing rate throughout the current pulse (right panel). NFS interneurons instead exhibited increasingly long interspike intervals. (c) FS and NFS interneurons exhibited different basic electrophysiological characteristics as indicated by AHP amplitude, AHP half width, action potential duration (measured at half width), and amplitude.
All cells included in the present study were filled with neurobiotin and displayed nonpyramidal morphology, and both FS and NFS exhibited a range of dendritic arborization. Most neurons had small bi- or multipolar somata, with dendritic trees extending in different directions (Fig. 2).
Figure 2.
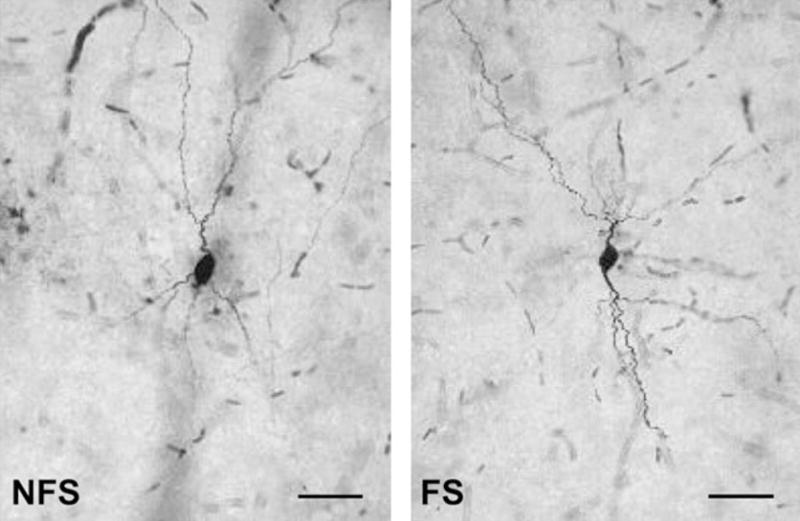
Neurobiotin staining of recorded GABAergic interneurons. Examples of layer V NFS and FS interneurons obtained in the medial PFC from postpubertal rats. Scale bar: 50 μm
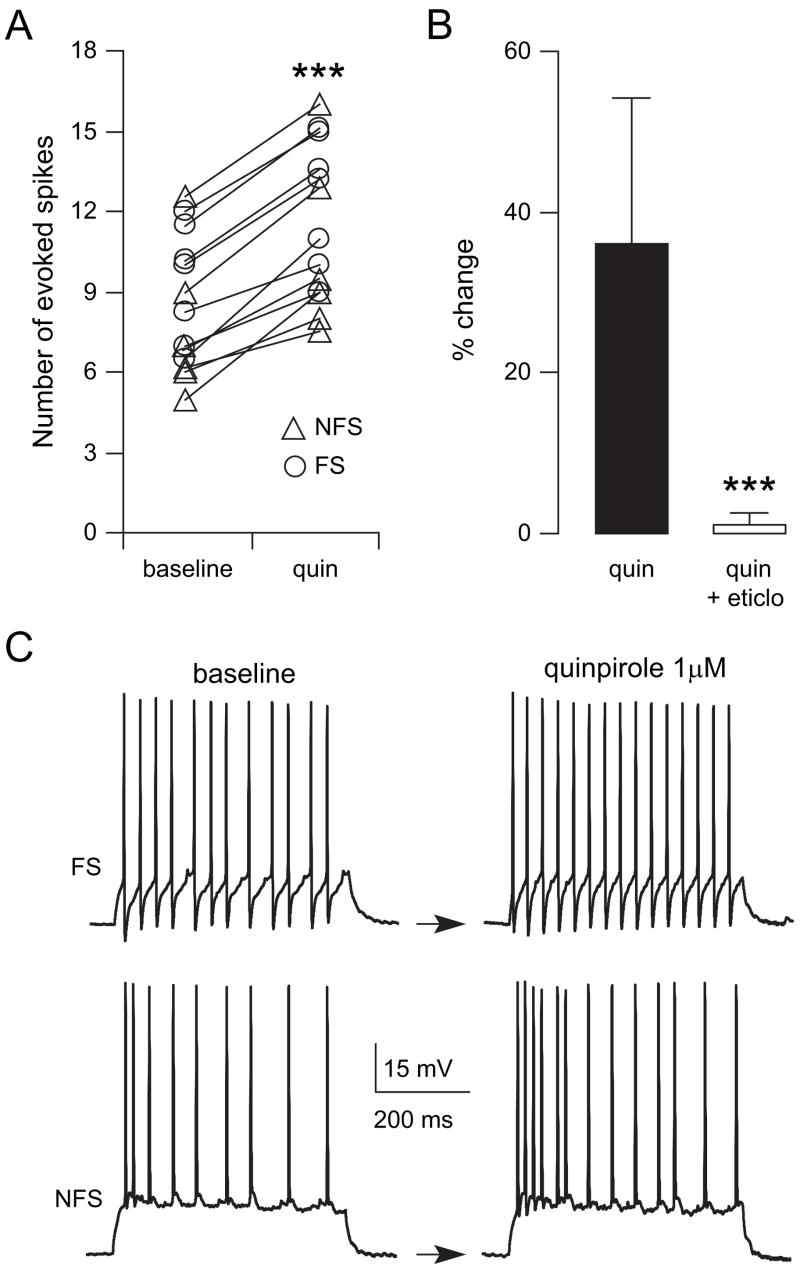
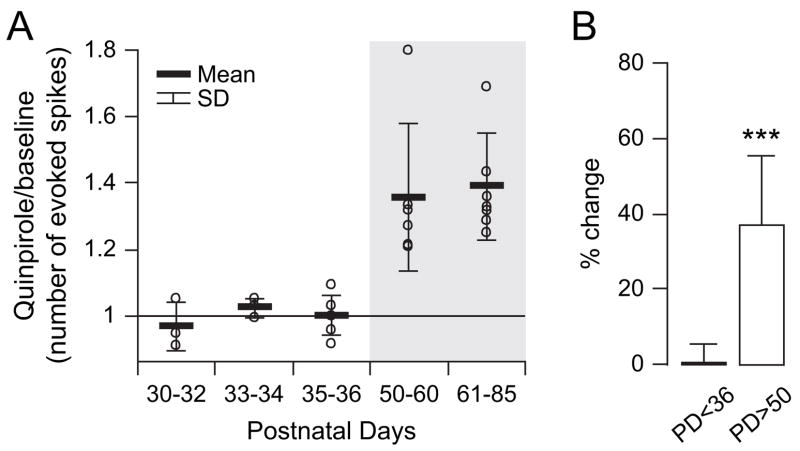
PFC GABA neurons receive DA innervation (Sesack and others 1995) and express both D1 and D2 receptors (Vincent and others 1993; Gaspar and others 1995). We explored whether DA modulation of this important neural population changed after adolescence. Bath application of an effective dose of the D2 agonist quinpirole (1 μM) (Tseng and O’Donnell 2004) increased excitability in all FS and NFS interneurons recorded in adult animals (Fig. 3). Because no apparent differences were observed between FS and NFS responses to quinpirole, as indicated by similar percentage of increases (NFS: 35.1 ± 10.6% vs. FS: 34.8 ± 15.5%, P = 0.97, student’s t-test), the data were pooled. Excitability was assessed by counting the number of action potentials and latency to the first spike evoked by a constant-amplitude intracellular current injection. Current amplitude was adjusted to initially evoke 5–10 spikes. Following 5–7 min of quinpirole (1 μM), the number of evoked spikes increased significantly from 8.6 ± 2.5 to 11 ± 2.9 (P < 0.0001, paired student’s t-test), an effect that concurred with an increase in input resistance from 274.7 ± 40.2 to 321.8 ± 45.8 MΩ (P < 0.0001) and a small depolarization from −62.5 ± 1.3 to −60.6 ± 1.9 mV (P < 0.007). No significant changes in the average amplitude of spontaneous depolarizing postsynaptic potentials (dPSP) were observed after quinpirole (from 2.14 ± 0.95 to 2.16 ± 0.86 mV, P = 0.98, paired t-test), suggesting that the excitatory effect may not result from an increased responsiveness to synaptic inputs. These effects were not observed in presence of the D2 antagonist eticlopride (20 μM, n = 5; Fig. 3), confirming that the excitatory action of quinpirole in the adult PFC is mediated by D2 receptors. Additional experiments were conducted in PFC slices obtained from prepubertal (PD30–36) animals. In contrast to what was observed in the adult PFC, bath application of 1 μM quinpirole failed to change significantly the number of evoked spikes in immature PFC interneurons (n = 12, Fig. 4). Even raising quinpirole concentration to 5 μM (n = 5, data not shown) failed to increase interneuron excitability in slices from prepubertal animals. Thus, activation of D2 receptors increases PFC interneuron excitability, but only after puberty.
Figure 3.
The D2 agonist quinpirole increases interneuron excitability in adult animals. (A) Scatter plot showing the effect of 1 μM quinpirole on FS (open circles) and NFS (triangles) interneuron excitability. The data shown are the number of evoked spikes to intracellular current pulse injection and were obtained in the PFC of adult (PD > 50) animals. Quinpirole increased the number of evoked spikes in all PFC interneurons (***P < 0.0001, paired student’s t-test). (B) Bar graph summarizing the effect of quinpirole and its blockade by 20 μM of the D2 antagonist eticlopride (indicated as percent change to baseline; ***P < 0.0001, repeated measures ANOVA). (C) Two representative traces of FS and NFS interneurons illustrating the number of evoked spikes before (baseline) and its increase after 5 min of bath application of quinpirole (1 μM) in both interneuron types.
Figure 4.
The excitatory effect of the D2 agonist is observed only in slices from adult animals. (A) Scatter plot showing the effect of quinpirole on PFC interneurons excitability from prepubertal to adult ages, expressed as the ratio between evoked firing after and before quinpirole administration. Open circles are individual neurons recorded from each group, and the data were grouped into age ranges. The excitatory effect of quinpirole (1 μM) was observed only in developmentally mature rats. (B) Bar graph (mean ± SD) summarizing the effect of quinpirole on prepubertal PFC interneurons (PD < 36) and those observed in the PFC of adult (PD > 50) animals (***P < 0.0001, repeated measures ANOVA).
We also investigated whether D1 modulation of PFC inter-neurons changes during adolescence. The effects of the D1 agonist SKF38393 on PFC interneuron excitability was tested in slices obtained from pre- and postpubertal animals. As previously shown (Gorelova and others 2002), bath application of an effective dose of SKF38393 (8 μM) selectively increased the excitability of FS interneurons (n = 6) without affecting the responses of NFS (n = 7) recorded in the PFC of prepubertal (PD < 36) animals (Fig. 5A). The number of evoked spikes in FS cells increased from 9.5 ± 1.4 to 12.4 ± 1.9 after 5 min of SKF38393 (8 μM) administration (P < 0.0001, paired student’s t-test), an effect that was not observed in presence of the D1 antagonist SCH23390 (10 μM; data not shown, n = 3). On the other hand, a postpubertal D1-mediated enhancement of excitability was observed in both FS and NFS interneurons (Fig. 5B). Bath application of 8 μM SKF38393 increased the number of evoked spikes in FS interneurons from 8.4 ± 2.5 to 11.6 ± 3.4 (n = 4; P < 0.02, paired student’s t-test). In NFS, the D1 agonist increased action potential firing from 9.1 ± 3.1 to 12.0 ± 4.0 (n = 6; P < 0.002, paired student’s t-test). In contrast to what was observed with quinpirole, this excitatory action concurred with significant increases of spontaneous dPSP average amplitude from 2.14 ± 0.41 (baseline) to 2.68 ± 0.66 mV (P = 0.0008, paired t-test), suggesting that the D1 effect on cell excitability may reflect an increased responsiveness to synaptic inputs. These changes were not observed in presence of the D1 antagonist SCH23390 (10 μM; data not shown, n = 6, 3 FS and 3 NFS), indicating that the excitatory action of SKF38393 on both FS and NFS PFC interneurons was mediated by D1 receptors. Thus, whereas FS interneurons are positively modulated by D1 receptors at early ages, NFS interneurons acquire the excitatory response to D1 receptors only after puberty (Fig. 5C).
Figure 5.
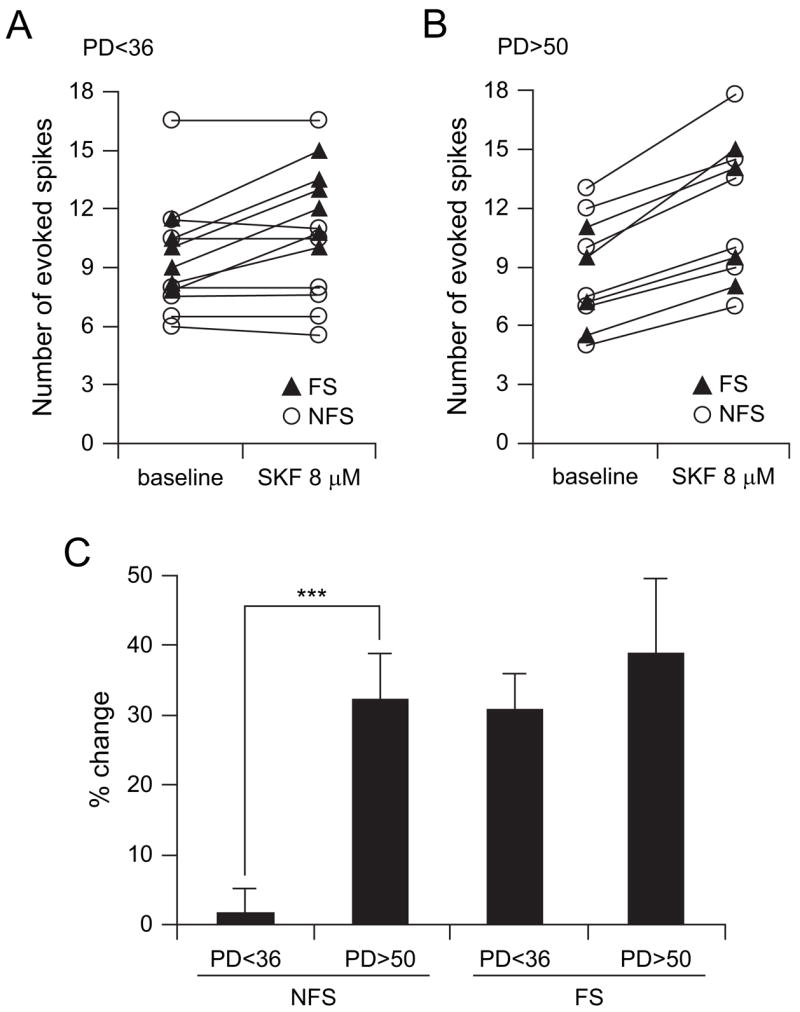
The D1 agonist SKF38393 increases interneuron excitability in the adult PFC. (A) Scatter plot showing the effect of 8 μM SKF38393 on FS (black triangles) and NFS (open circles) interneuron excitability, assessed with the number of spikes evoked by intracellular current pulse injection, in slices from prepubertal (PD < 36) animals. Bath application of 8 μM SKF38393 increased the number of evoked spikes in all FS (n = 6) interneurons, whereas not apparent changes were observed in NFS (n = 7). (B) Scatter plot illustrating changes in FS- and NFS-evoked spikes induced by the D1 agonist in slices from young adult animals (PD > 50). Both FS (n = 4) and NFS (n = 6) interneurons exhibited similar increases in neuronal excitability. (C) Bar graph (mean ± SD) summarizing the effect of SKF38393 on pre- and postpubertal PFC FS and NFS interneuron excitability, expressed as percent change from baseline. PFC NFS interneurons acquire a D1 modulation only after adolescence (***P < 0.0001, student’s t-test).
Discussion
Bath application of the D2 agonist quinpirole or the D1 agonist SKF38393 causes an increase in cell excitability in PFC interneurons recorded using the whole-cell technique. As cell excitability was determined by assessing the response to intracellular current injection, this is likely to reflect post-synaptic effects of the agonist and not a modulation of other inputs. FS interneurons were excited by the D1 agonist at both prepubertal and postadolescent ages. The D2 effect on all interneurons and the D1 modulation of NFS interneurons, on the other hand, were observed in slices obtained from adult but not preadolescent rats, indicating that the dopaminergic modulation of interneurons matures late during development.
Anatomical studies have shown that DA innervation of the medial PFC increases progressively until P50–60 (Verney and others 1982; Kalsbeek and others 1988; Benes and others 2000) and D2/D4 DA receptor expression reaches a stable adult level at P35 (Tarazi and others 1998; Tarazi and Baldessarini 2000). Similar developmental differences for electrophysiological D2 responses can also be found by closely examining the existing literature. Most previous electrophysiological studies have been conducted in slices from prepubertal rats (i.e., PD20–28) and showed little or no effect of D2 receptors on PFC interneuron excitability (Seamans and others 2001; Gorelova and others 2002), whereas a recent recording using a similar technique in slices from adult animals unveiled an excitatory effect of quinpirole in the PFC (Tseng and O’Donnell 2004). Further-more, in vivo microdialysis revealed a D2-mediated increase in extracellular GABA in the PFC of adult rats (Grobin and Deutch 1998), suggesting interneuron activation by D2 receptors. These data strongly support our finding that the excitatory effect of D2 receptors emerges after puberty. Further studies will be required to determine whether this excitatory effect reflects a direct D2 postsynaptic action on FS and NFS interneurons (Tseng and O’Donnell 2004) or activation of D2 autoreceptors and release of the cotransmitter neurotensin, as recently suggested (Petrie and others 2005).
The D1 modulation of PFC interneurons is also excitatory, as revealed by the increase in the number of action potentials evoked by intracellular current injection by 35% following application of a D1 agonist. This effect is consistent with previous studies in slices from prepubertal animals (Gorelova and others 2002). Furthermore, whereas FS interneurons exhibited the positive D1 modulation at both prepubertal and postadolescent age groups, NFS interneurons only showed an increase in excitability by the D1 agonist in slices from adult rats, suggesting that maturation of DA control of interneurons occurs at different times for different cell types.
A periadolescent maturation of cortical interneurons has important implications for the refinement of several brain functions. Interneurons have been repeatedly proposed to be essential for proper tuning of dynamical activation of cortical networks (Kawaguchi 2001; Zhu and others 2004), including the coordination of oscillatory activity (Fellous and others 2001; Shu and others 2003). Cortical networks include a variety of interneuron types (Cauli and others 1997; Kawaguchi and Kondo 2002; Lewis and others 2005; Kawaguchi and others 2006). In this study, we have focused on those presenting the well-described profile of FS interneurons, which correspond to parvalbumin-positive basket and chandelier cells (Kawaguchi and Kondo 2002). However, as the D2 modulation was also observed in other nonpyramidal cells that did not exhibit a FS profile (NFS), it is possible that many different interneuron types acquire this modulation around puberty. Although synaptic contacts between DA terminals and parvalbumin-positive cells have been observed (Sesack and others 1995), others have also found D2/D4 receptors in calbindin-positive interneurons (Le Moine and Gaspar 1998; Wedzony and others 2000). A more thorough study of well-defined interneuron types (using neurochemical markers) is necessary to identify changes in specific interneuron populations.
A similar impact of quinpirole on both cell populations, despite the preferential DA innervation of parvalbumin-positive neurons, does not necessarily reflect a common cellular/sub-cellular mechanism. Although speculative, it is possible that the effects of quinpirole on FS and NFS are mediated by different D2 receptor subtypes (D2 vs. D4). Our findings and those of others reveal that in prepubertal animals, only D1 (not D2) receptors exert an excitatory effect on interneurons (Gorelova and others 2002), but during adolescence, the net effect of DA is to drive PFC interneurons by both D1- and D2-dependent mechanisms. This would cause a powerful inhibition of PFC pyramidal neurons in the presence of phasic DA, as it was observed in vivo with burst ventral tegmental area (VTA) stimulation (Lewis and O’Donnell 2000). This is consistent with recent data showing that FS interneuron excitation matches the temporal course of pyramidal cell inhibition in response to chemical VTA stimulation in vivo, as revealed with FS pyramidal paired recordings (Tseng and others 2006). Although PFC D1 receptors have been clearly linked to working memory functions (Sawaguchi and Goldman-Rakic 1991; Wang and others 2004), the functional role of D2 receptors is not as clear. By providing a strong positive modulation of interneurons, they may be contributing to reducing noiselike activity and thereby enhancing the saliency of the activity of strongly activated pyramidal neurons. In this regard, the proposal that D2-modulated activity in the cortex is a corollary discharge providing feedback to the PFC (Wang and others 2004) is an interesting possibility.
A specific population of GABAergic interneurons, the FS electrophysiological profile, has a potentially important role in the pathophysiology of schizophrenia (Lewis and others 2005), a disorder with a clear prefrontal compromise and symptoms that emerge during late adolescence or early adulthood. Defective GABA transmission in the PFC and other cortical areas, stemming from developmental alterations but not expressed until after late adolescence, is among the prevailing ideas regarding schizophrenia pathophysiology (Benes and Berretta 2001; Lewis and others 2005). Thus, our finding that the DA control of PFC interneurons matures during adolescence may explain why developmental alterations result in a delayed onset of symptoms and may open avenues to revisit therapeutic and even preventive approaches for this devastating disorder.
Acknowledgments
We thank Drs S.D. Glick and A.A. Gruber for helpful comments on a previous version of the manuscript. This work was supported by a grant from the National Institute of Mental Health (MH57683).
Footnotes
Conflict of Interest: None declared.
References
- Andersen SL, LeBlanc CJ, Lyss PJ. Maturational increases in c-fos expression in the ascending dopamine systems. Synapse. 2001;41:345–350. doi: 10.1002/syn.1091. [DOI] [PubMed] [Google Scholar]
- Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsycho-pharmacology. 2001;25:1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- Benes FM, Taylor JB, Cunningham MC. Convergence and plasticity of monoaminergic systems in the medial prefrontal cortex during the postnatal period: implications for the development of psychopathology. Cereb Cortex. 2000;10:1014–1027. doi: 10.1093/cercor/10.10.1014. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biol Psychol. 2000;54:241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Cauli B, Audinat E, Lambolez B, Angulo MC, Ropert N, Tsuzuki K, Hestrin S, Rossier J. Molecular and physiological diversity of cortical nonpyramidal cells. J Neurosci. 1997;17:3894–3906. doi: 10.1523/JNEUROSCI.17-10-03894.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellous JM, Houweling AR, Modi RH, Rao RP, Tiesinga PH, Sejnowski TJ. Frequency dependence of spike timing reliability in cortical pyramidal cells and interneurons. J Neurophysiol. 2001;85:1782–1787. doi: 10.1152/jn.2001.85.4.1782. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Inoue M. Neuronal interactions related to working memory processes in the primate prefrontal cortex revealed by cross-correlation analysis. Cereb Cortex. 2000;10:535–551. doi: 10.1093/cercor/10.6.535. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Bloch B, Le Moine C. D1 and D2 receptor gene expression in the rat frontal cortex: cellular localization in different classes of efferent neurons. Eur J Neurosci. 1995;7:1050–1063. doi: 10.1111/j.1460-9568.1995.tb01092.x. [DOI] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gorelova N, Seamans JK, Yang CR. Mechanisms of dopamine activation of fast-spiking interneurons that exert inhibition in rat prefrontal cortex. J Neurophysiol. 2002;88:3150–3166. doi: 10.1152/jn.00335.2002. [DOI] [PubMed] [Google Scholar]
- Grobin AC, Deutch AY. Dopaminergic regulation of extracellular gamma-aminobutyric acid levels in the prefrontal cortex of the rat. J Pharmacol Exp Ther. 1998;285:350–357. [PubMed] [Google Scholar]
- Kalsbeek A, Voorn P, Buijs RM, Pool CW, Uylings HBM. Development of the dopaminergic innervation in the prefrontal cortex of the rat. J Comp Neurol. 1988;269:58–72. doi: 10.1002/cne.902690105. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y. Distinct firing patterns of neuronal subtypes in cortical synchronized activities. J Neurosci. 2001;21:7261–7272. doi: 10.1523/JNEUROSCI.21-18-07261.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Karube F, Kubota Y. Dendritic branch typing and spine expression patterns in cortical nonpyramidal cells. Cereb Cortex. 2006;16:696–711. doi: 10.1093/cercor/bhj015. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kondo S. Parvalbumin, somatostatin and cholecystokinin as chemical markers for specific GABAergic interneuron types in the rat frontal cortex. J Neurocytol. 2002;31:277–287. doi: 10.1023/a:1024126110356. [DOI] [PubMed] [Google Scholar]
- Le Moine C, Gaspar P. Subpopulations of cortical GABAergic interneurons differ by their expression of D1 and D2 dopamine receptor subtypes. Mol Brain Res. 1998;58:231–236. doi: 10.1016/s0169-328x(98)00118-1. [DOI] [PubMed] [Google Scholar]
- Lewis BL, O’Donnell P. Ventral tegmental area afferents to the prefrontal cortex maintain membrane potential ‘up’ states in pyramidal neurons via D1 dopamine receptors. Cereb Cortex. 2000;10:1168–1175. doi: 10.1093/cercor/10.12.1168. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Suzuki W, Tanaka K. Neuronal correlates of goal-based motor selection in the prefrontal cortex. Science. 2003;301:229–232. doi: 10.1126/science.1084204. [DOI] [PubMed] [Google Scholar]
- Petrie KA, Schmidt D, Bubser M, Fadel J, Carraway RE, Deutch AY. Neurotensin activates GABAergic interneurons in the prefrontal cortex. J Neurosci. 2005;25:1629–1636. doi: 10.1523/JNEUROSCI.3579-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg DR, Lewis DA. Changes in the dopaminergic innervation of monkey prefrontal cortex during late postnatal development: a tyrosine hydroxylase immunohistochemical study. Biol Psychiatry. 1994;36:272–277. doi: 10.1016/0006-3223(94)90610-6. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science. 1991;251:947–950. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Gorelova N, Durstewitz D, Yang CR. Bidirectional dopamine modulation of GABAergic inhibition in prefrontal cortical pyramidal neurons. J Neurosci. 2001;21:3628–3638. doi: 10.1523/JNEUROSCI.21-10-03628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Segalowitz SJ, Davies PL. Charting the maturation of the frontal lobe: an electrophysiological strategy. Brain Cogn. 2004;55:116–133. doi: 10.1016/S0278-2626(03)00283-5. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Snyder CL, Lewis DA. Axon terminals immunolabeled for dopamine or tyrosine hydroxylase synapse on GABA-immunoreactive dendrites in rat and monkey cortex. J Comp Neurol. 1995;363:264–280. doi: 10.1002/cne.903630208. [DOI] [PubMed] [Google Scholar]
- Shu Y, Hasenstaub A, McCormick DA. Turning on and off recurrent balanced cortical activity. Nature. 2003;423:288–293. doi: 10.1038/nature01616. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Baldessarini RJ. Comparative postnatal development of dopamine D1, D2 and D4 receptors in rat forebrain. Int J Dev Neurosci. 2000;18:29–37. doi: 10.1016/s0736-5748(99)00108-2. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Tomasini EC, Baldessarini RJ. Postnatal development of dopamine D4-like receptors in rat forebrain regions: comparison with D2-like receptors. Dev Brain Res. 1998;110:227–233. doi: 10.1016/s0165-3806(98)00111-4. [DOI] [PubMed] [Google Scholar]
- Tseng KY, Mallet N, Toreson KL, Le Moine C, Gonon F, O’Donnell P. Excitatory response of prefrontal cortical fast-spiking inter-neurons to ventral tegmental area stimulation in vivo. Synapse. 2006;59:412–417. doi: 10.1002/syn.20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, O’Donnell P. Dopamine-glutamate interactions controlling prefrontal cortical pyramidal cell excitability involve multiple signaling mechanisms. J Neurosci. 2004;24:5131–5139. doi: 10.1523/JNEUROSCI.1021-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, O’Donnell P. Post-pubertal emergence of prefrontal cortical up states induced by D1-NMDA co-activation. Cereb Cortex. 2005;15:49–57. doi: 10.1093/cercor/bhh107. [DOI] [PubMed] [Google Scholar]
- Verney C, Berger B, Adrein J, Vigny A, Gay M. Development of the dopaminergic innervation of the rat cerebral cortex. A light microscope immunocytochemical study using anti-tyrosine hydrox-ylase antibodies. Brain Res. 1982;281:41–52. doi: 10.1016/0165-3806(82)90111-0. [DOI] [PubMed] [Google Scholar]
- Vincent SL, Khan Y, Benes FM. Cellular distribution of dopamine D1 and D2 receptors in rat medial prefrontal cortex. J Neurosci. 1993;13:2551–2564. doi: 10.1523/JNEUROSCI.13-06-02551.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Vijayraghavan S, Goldman-Rakic PS. Selective D2 receptor actions on the functional circuitry of working memory. Science. 2004;303:853–856. doi: 10.1126/science.1091162. [DOI] [PubMed] [Google Scholar]
- Wedzony K, Chocyk A, Mackowiak M, Fijal K, Czyrak A. Cortical localization of dopamine D4 receptors in the rat brain—immunocytochemical study. J Physiol Pharmacol. 2000;51:205–221. [PubMed] [Google Scholar]
- Zhu Y, Stornetta RL, Zhu JJ. Chandelier cells control excessive cortical excitation: characteristics of whisker-evoked synaptic responses of layer 2/3 nonpyramidal and pyramidal neurons. J Neurosci. 2004;24:5101–5108. doi: 10.1523/JNEUROSCI.0544-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]