Abstract
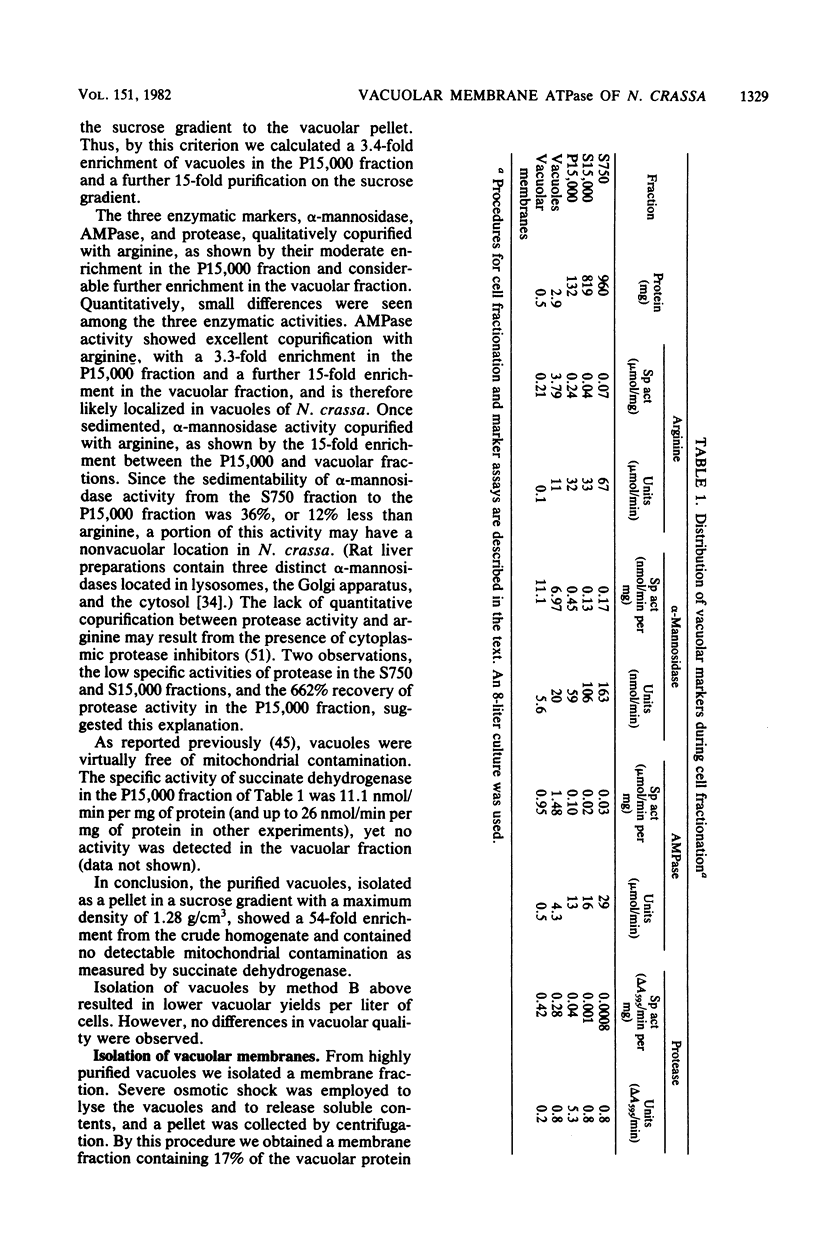
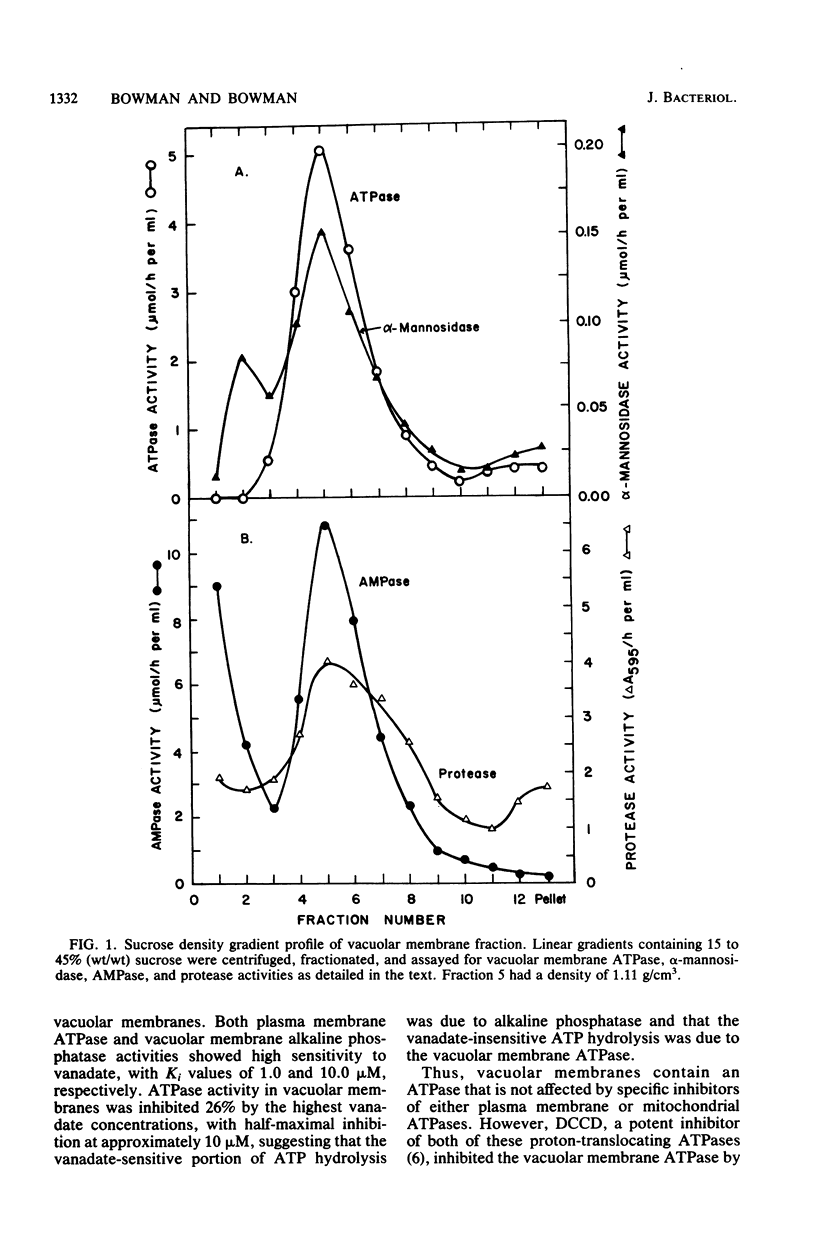
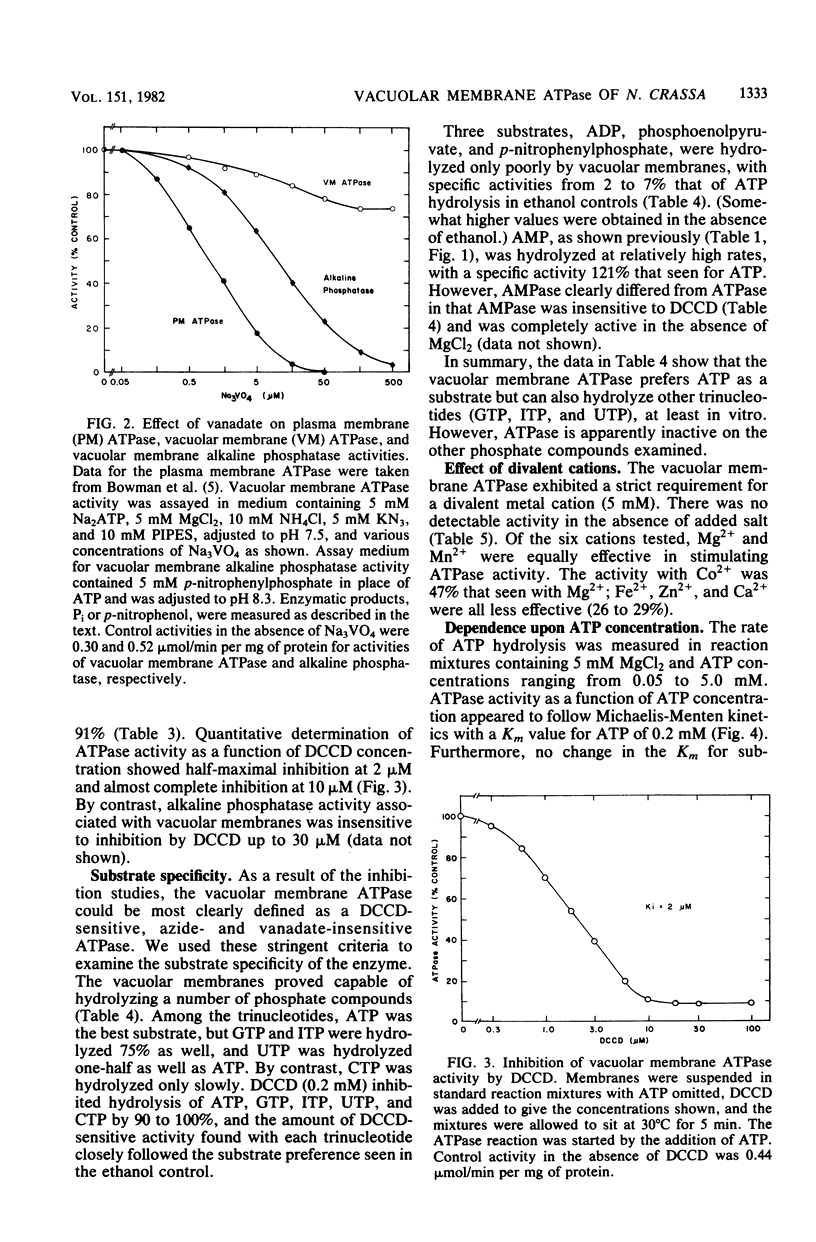
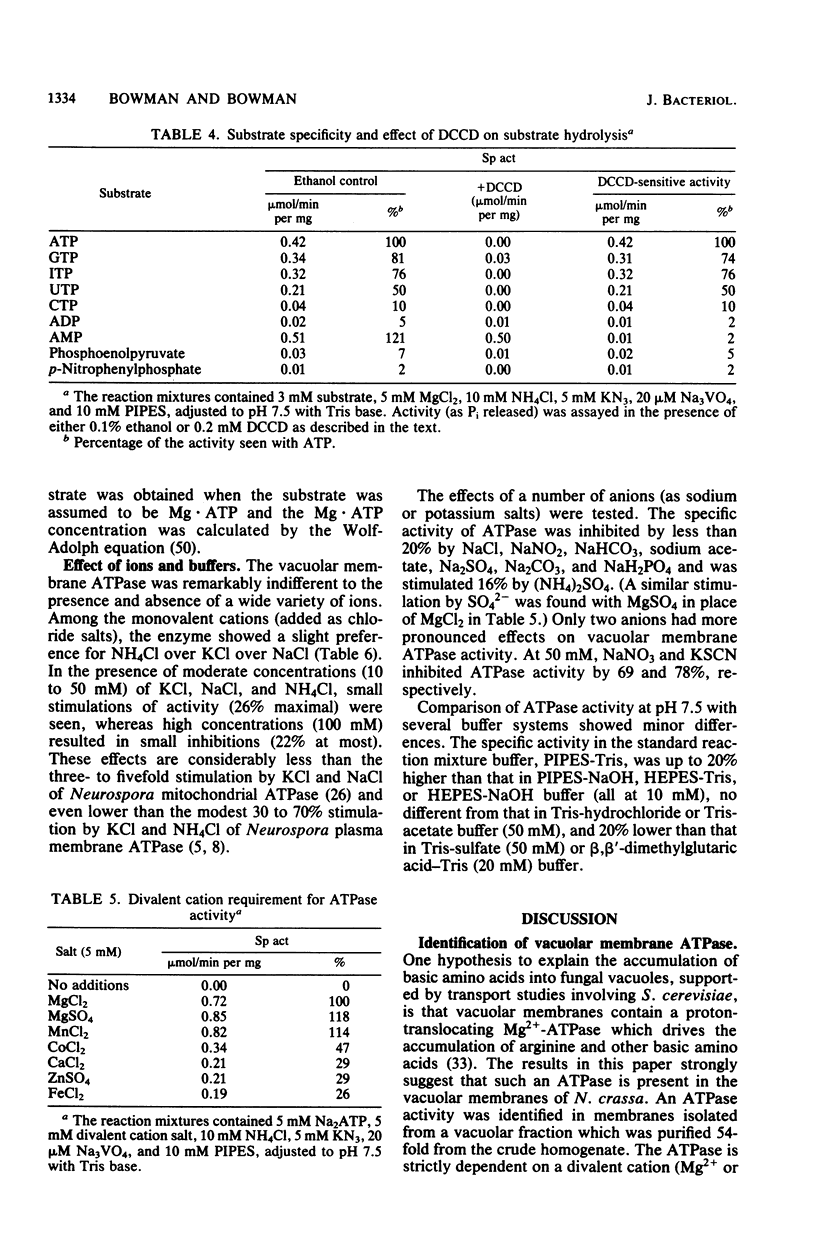
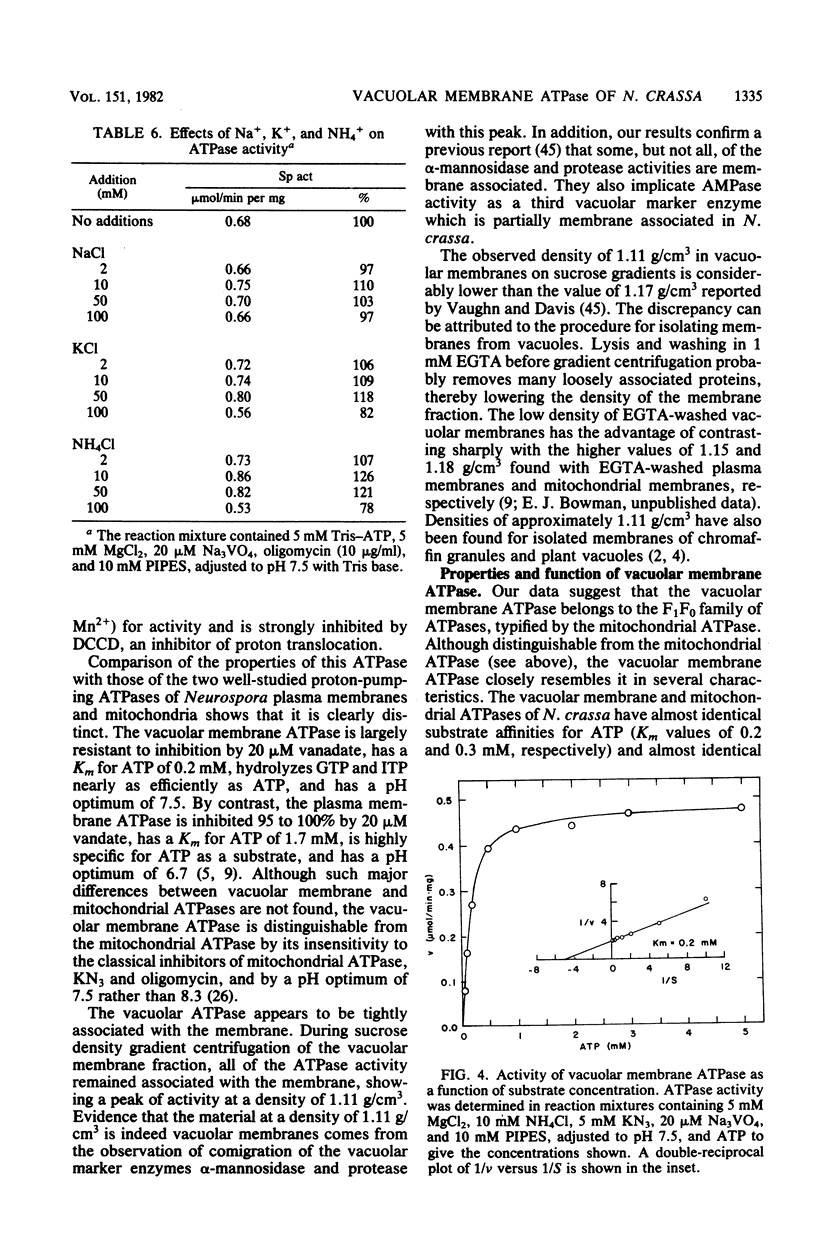
Using a vacuolar preparation virtually free of contamination by other organelles, we isolated vacuolar membranes and demonstrated that they contain an ATPase. Sucrose density gradient profiles of vacuolar membranes show a single peak of ATPase activity at a density of 1.11 g/cm3. Comparison of this enzyme with the two well-studied proton-pumping ATPases of Neurospora plasma membranes and mitochondria shows that it is clearly distinct. The vacuolar membrane ATPase is insensitive to the inhibitors oligomycin, azide, and vanadate, but sensitive to N,N'-dicyclohexylcarbodiimide (Ki = 2 microM). It has a pH optimum of 7.5, requires a divalent cation (Mg2+ or Mn2+) for activity, and is remarkably unaffected (+/- 20%) by a number of monovalent cations, anions, and buffers. In its substrate affinity (Km for ATP = 0.2 mM), substrate preference (ATP greater than GTP, ITP greater than UTP greater than CTP), and loss of activity with repeated 1 mM ethylene glycol-bis(beta-aminoethyl ether)-N,N,N',N'-tetraacetic acid washes, the vacuolar membrane ATPase resembles the F1F0 type of ATPase found in mitochondria and differs from the integral membrane type of ATPase in plasma membranes.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apps D. K., Pryde J. G., Sutton R., Phillips J. H. Inhibition of adenosine triphosphatase, 5-hydroxytryptamine transport and proton-translocation activities of resealed chromaffin-granule 'ghosts'. Biochem J. 1980 Aug 15;190(2):273–282. doi: 10.1042/bj1900273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps D. K., Reid G. A. Adenosine triphosphatase and adenosine diphosphate/adenosine triphosphate isotope-exchange activities of the chromaffin-granule membrane. Biochem J. 1977 Oct 1;167(1):297–300. doi: 10.1042/bj1670297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps D. K., Schatz G. An adenosine triphosphatase isolated from chromaffin-granulate membranes is closely similar to F1-adenosine triphosphatase of mitochondria. Eur J Biochem. 1979 Oct 15;100(2):411–419. doi: 10.1111/j.1432-1033.1979.tb04184.x. [DOI] [PubMed] [Google Scholar]
- Boller T., Kende H. Hydrolytic enzymes in the central vacuole of plant cells. Plant Physiol. 1979 Jun;63(6):1123–1132. doi: 10.1104/pp.63.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman B. J., Blasco F., Slayman C. W. Purification and characterization of the plasma membrane ATPase of Neurospora crassa. J Biol Chem. 1981 Dec 10;256(23):12343–12349. [PubMed] [Google Scholar]
- Bowman B. J., Mainzer S. E., Allen K. E., Slayman C. W. Effects of inhibitors on the plasma membrane and mitochondrial adenosine triphosphatases of Neurospora crassa. Biochim Biophys Acta. 1978 Sep 11;512(1):13–28. doi: 10.1016/0005-2736(78)90214-6. [DOI] [PubMed] [Google Scholar]
- Bowman B. J., Slayman C. W. Characterization of plasma membrane adenosine triphosphatase of Neurospora crassa. J Biol Chem. 1977 May 25;252(10):3357–3363. [PubMed] [Google Scholar]
- Bowman B. J., Slayman C. W. The effects of vanadate on the plasma membrane ATPase of Neurospora crassa. J Biol Chem. 1979 Apr 25;254(8):2928–2934. [PubMed] [Google Scholar]
- Bowman E. J., Bowman B. J., Slayman C. W. Isolation and characterization of plasma membranes from wild type Neurospora crassa. J Biol Chem. 1981 Dec 10;256(23):12336–12342. [PubMed] [Google Scholar]
- Burton E. G., Metzenberg R. L. Properties of repressible alkaline phosphates from wild type and a wall-less mutant of Neurospora crassa. J Biol Chem. 1974 Aug 10;249(15):4679–4688. [PubMed] [Google Scholar]
- Cramer C. L., Vaughn L. E., Davis R. H. Basic amino acids and inorganic polyphosphates in Neurospora crassa: independent regulation of vacuolar pools. J Bacteriol. 1980 Jun;142(3):945–952. doi: 10.1128/jb.142.3.945-952.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRYER R. L., TAMMES A. R., ROUTH J. I. The determination of phosphorus and phosphatase with N-phenyl-p-phenylenediamine. J Biol Chem. 1957 Mar;225(1):177–183. [PubMed] [Google Scholar]
- Davis R. H., Bowman B. J., Weiss R. L. Intracellular compartmentation and transport of metabolites. J Supramol Struct. 1978;9(4):473–488. doi: 10.1002/jss.400090403. [DOI] [PubMed] [Google Scholar]
- De Duve C., Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- Drainas C., Weiss R. L. Energetics of vacuolar compartmentation of arginine in Neurospora crassa. J Bacteriol. 1982 May;150(2):770–778. doi: 10.1128/jb.150.2.770-778.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingame R. H. The proton-translocating pumps of oxidative phosphorylation. Annu Rev Biochem. 1980;49:1079–1113. doi: 10.1146/annurev.bi.49.070180.005243. [DOI] [PubMed] [Google Scholar]
- HALVORSON H., ELLIAS L. The purification and properties of an alpha-glucosidase of Saccharomyces italicus Y1225. Biochim Biophys Acta. 1958 Oct;30(1):28–40. doi: 10.1016/0006-3002(58)90237-3. [DOI] [PubMed] [Google Scholar]
- HESS J., KITO E., MARTIN R. P., VAN PILSUM J. F. Determination of creatine, creatinine, arginine, guanidinoacetic acid, guanidine, and methylguanidine in biological fluids. J Biol Chem. 1956 Sep;222(1):225–235. [PubMed] [Google Scholar]
- Jackl G., Sebald W. Identification of two products of mitochondrial protein synthesis associated with mitochondrial adenosine triphosphatase from Neurospora crassa. Eur J Biochem. 1975 May;54(1):97–106. doi: 10.1111/j.1432-1033.1975.tb04118.x. [DOI] [PubMed] [Google Scholar]
- KIRSHNER N. Uptake of catecholamines by a particulate fraction of the adrenal medulla. J Biol Chem. 1962 Jul;237:2311–2317. [PubMed] [Google Scholar]
- Kakinuma Y., Ohsumi Y., Anraku Y. Properties of H+-translocating adenosine triphosphatase in vacuolar membranes of SAccharomyces cerevisiae. J Biol Chem. 1981 Nov 10;256(21):10859–10863. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Legerton T. L., Weiss R. L. Mobilization of sequestered metabolities into degradative reactions by nutritional stress in Neurospora. J Bacteriol. 1979 Jun;138(3):909–914. doi: 10.1128/jb.138.3.909-914.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainzer S. E., Slayman C. W. Mitochondrial adenosine triphosphatase of wild-type and poky Neurospora crassa. J Bacteriol. 1978 Feb;133(2):584–592. doi: 10.1128/jb.133.2.584-592.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin B., Marin-Lanza M., Komor E. The protonmotive potential difference across the vacuo-lysosomal membrane of Hevea brasiliensis (rubber tree) and its modification by a membrane-bound adenosine triphosphatase. Biochem J. 1981 Aug 15;198(2):365–372. doi: 10.1042/bj1980365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinoia E., Heck U., Boller T., Wiemken A., Matile P. Some properties of vacuoles isolated from Neurospora crassa slime variant. Arch Microbiol. 1979 Jan 16;120(1):31–34. doi: 10.1007/BF00413268. [DOI] [PubMed] [Google Scholar]
- Nishimura M., Beevers H. Hydrolases in vacuoles from castor bean endosperm. Plant Physiol. 1978 Jul;62(1):44–48. doi: 10.1104/pp.62.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsumi Y., Anraku Y. Active transport of basic amino acids driven by a proton motive force in vacuolar membrane vesicles of Saccharomyces cerevisiae. J Biol Chem. 1981 Mar 10;256(5):2079–2082. [PubMed] [Google Scholar]
- Opheim D. J., Touster O. Lysosomal alpha-D-mannosidase of rat liver. Purification and comparison with the golgi and cytosolic alpha-D-mannosidases. J Biol Chem. 1978 Feb 25;253(4):1017–1023. [PubMed] [Google Scholar]
- PENNINGTON R. J. Biochemistry of dystrophic muscle. Mitochondrial succinate-tetrazolium reductase and adenosine triphosphatase. Biochem J. 1961 Sep;80:649–654. doi: 10.1042/bj0800649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves J. P., Reames T. ATP stimulates amino acid accumulation by lysosomes incubated with amino acid methyl esters. Evidence for a lysosomal proton pump. J Biol Chem. 1981 Jun 25;256(12):6047–6053. [PubMed] [Google Scholar]
- Scarborough G. A. Properties of Neurospora crassa plasma membrane ATPase. Arch Biochem Biophys. 1977 Apr 30;180(2):384–393. doi: 10.1016/0003-9861(77)90052-2. [DOI] [PubMed] [Google Scholar]
- Schneider D. L. ATP-dependent acidification of intact and disrupted lysosomes. Evidence for an ATP-driven proton pump. J Biol Chem. 1981 Apr 25;256(8):3858–3864. [PubMed] [Google Scholar]
- Schneider D. L. Membranous localization and properties of ATPase of rat liver lysosomes. J Membr Biol. 1977 Jun 6;34(2-3):247–261. doi: 10.1007/BF01870302. [DOI] [PubMed] [Google Scholar]
- Seargeant L. E., Stinson R. A. Inhibition of human alkaline phosphatases by vanadate. Biochem J. 1979 Jul 1;181(1):247–250. doi: 10.1042/bj1810247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebald W., Graf T., Lukins H. B. The dicyclohexylcarbodiimide-binding protein of the mitochondrial ATPase complex from Neurospora crassa and Saccharomyces cerevisiae. Identification and isolation. Eur J Biochem. 1979 Feb 1;93(3):587–599. doi: 10.1111/j.1432-1033.1979.tb12859.x. [DOI] [PubMed] [Google Scholar]
- Vaughn L. E., Davis R. H. Purification of vacuoles from Neurospora crassa. Mol Cell Biol. 1981 Sep;1(9):797–806. doi: 10.1128/mcb.1.9.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. L. Compartmentation and control of arginine metabolism in Neurospora. J Bacteriol. 1976 Jun;126(3):1173–1179. doi: 10.1128/jb.126.3.1173-1179.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. L. Intracellular localization of ornithine and arginine pools in Neurospora. J Biol Chem. 1973 Aug 10;248(15):5409–5413. [PubMed] [Google Scholar]
- Wolf H. U., Adolph L. Untersuchungen über eine Mg2plus-(Ca2+)-aktivierte ATPase aus Rinderhirnmikrosomen. Eur J Biochem. 1969 Mar;8(1):68–74. doi: 10.1111/j.1432-1033.1969.tb00496.x. [DOI] [PubMed] [Google Scholar]
- Yu P. H., Kula M. R., Tsai H. Isolation of specific protease inhibitors from Neurospora crassa. Can J Biochem. 1976 Aug;54(8):699–703. doi: 10.1139/o76-100. [DOI] [PubMed] [Google Scholar]



