Abstract
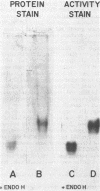
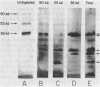
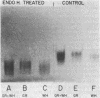
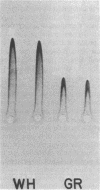
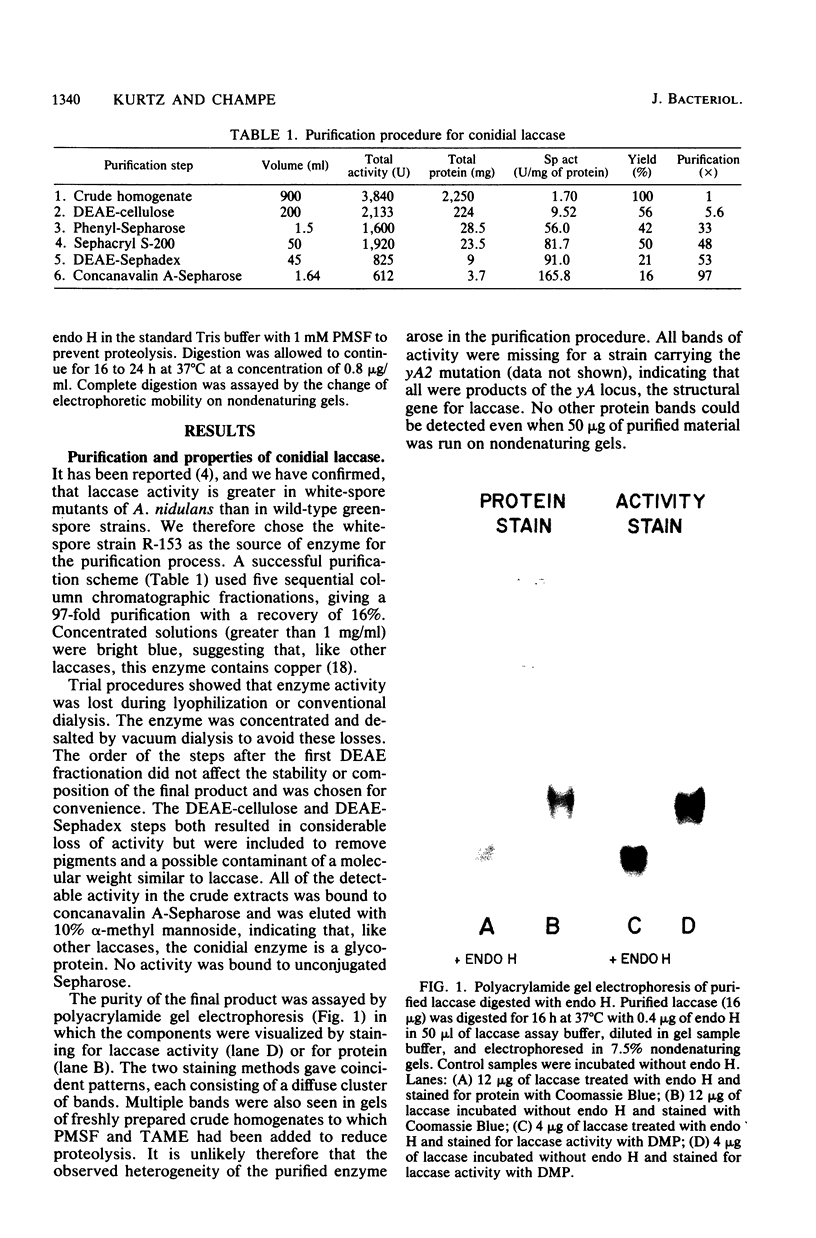
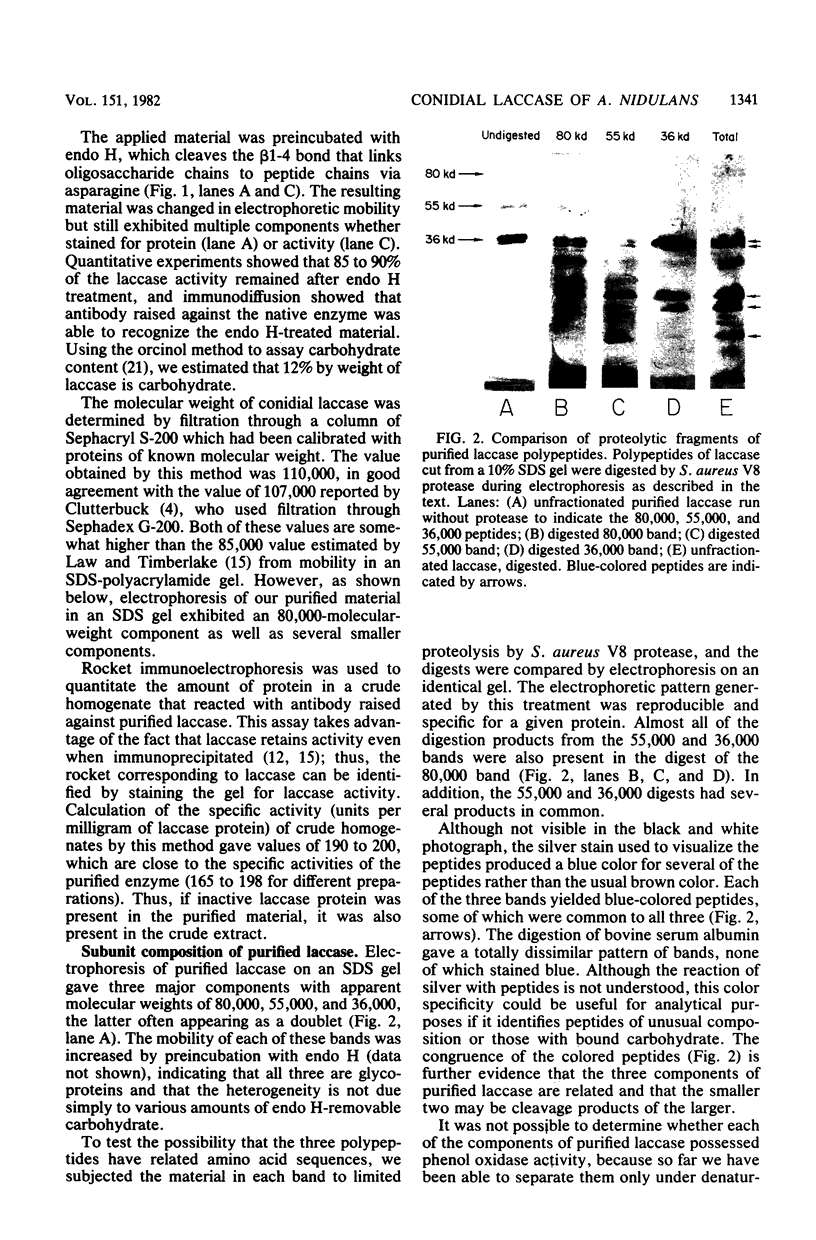
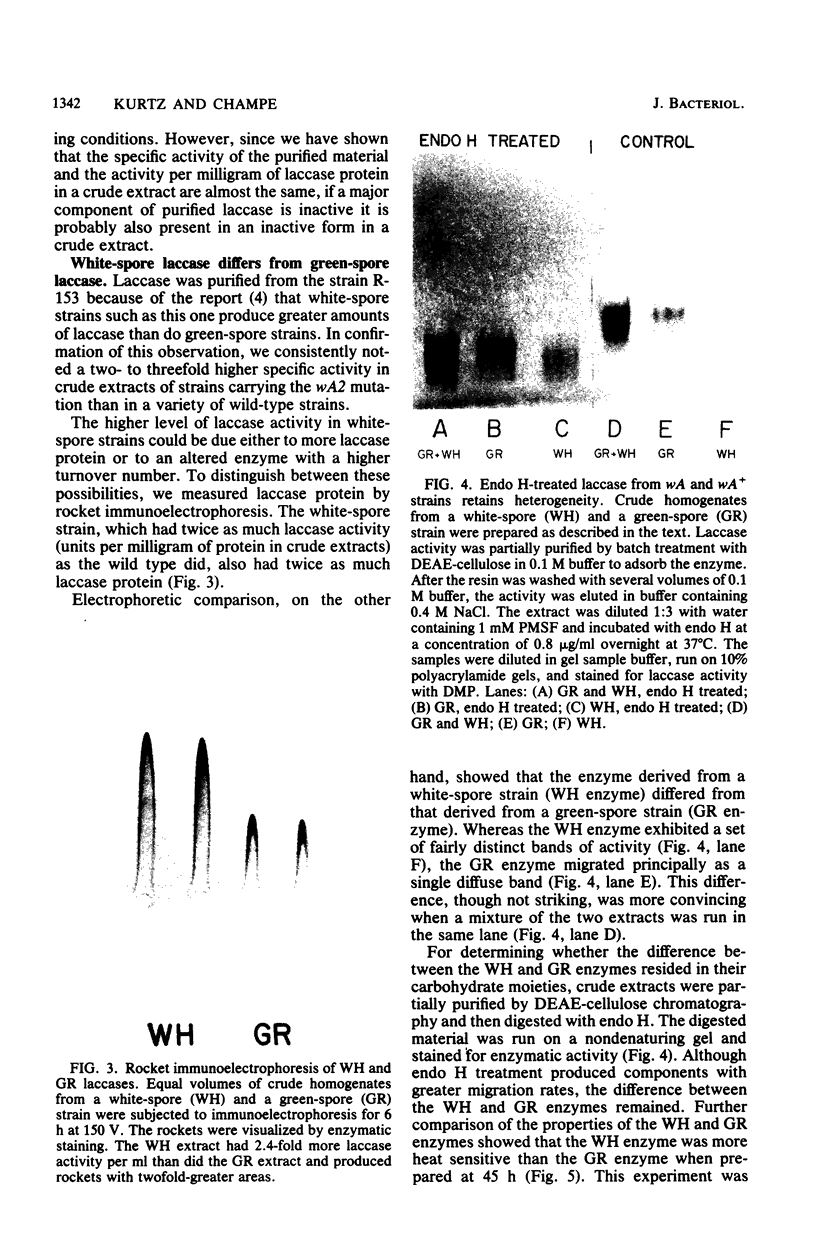
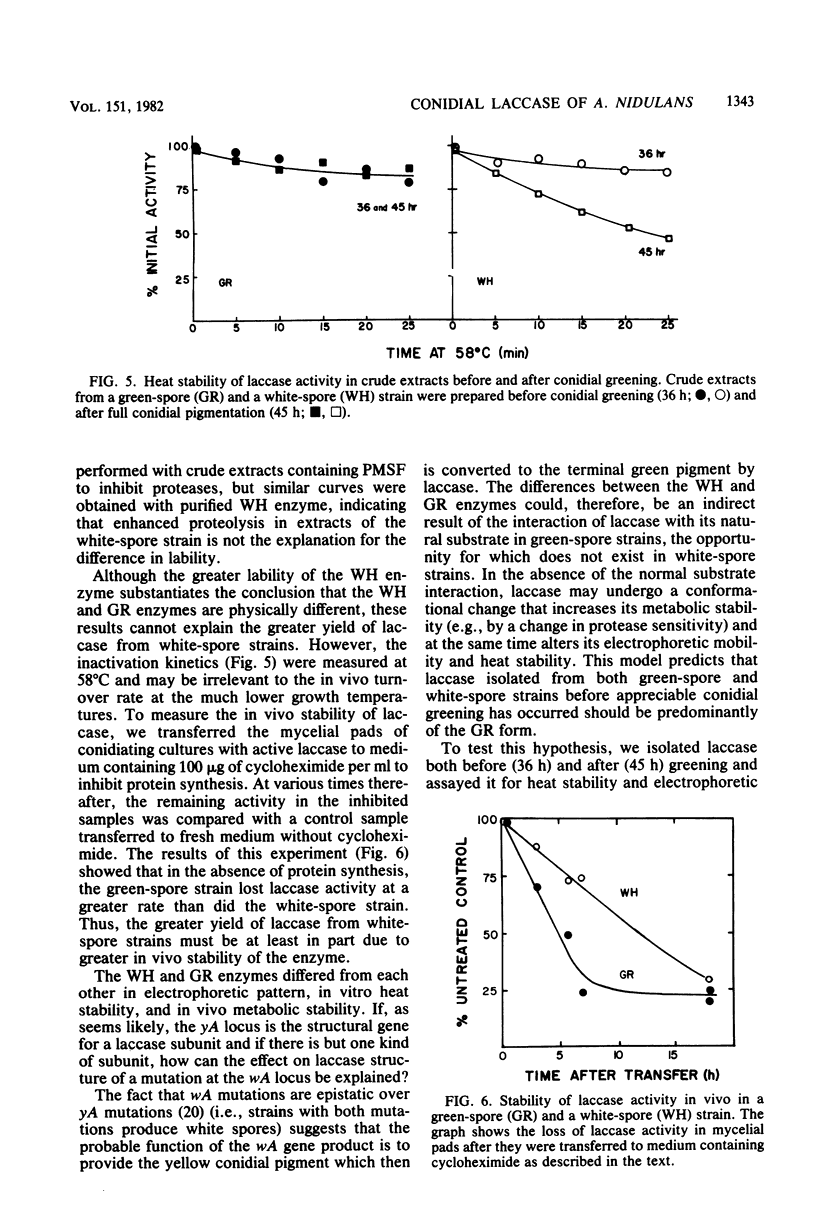
Conidial laccase of Aspergillus nidulans was purified by standard protein purification methods. Although the purified material showed a cluster of several protein bands on a nondenaturing gel, each of these protein bands had laccase activity. All bands of activity, however, were absent in a strain carrying a mutation in the structural gene for laccase. Concentrated solutions (greater than 1 mg/ml) were bright blue, suggesting that, like other laccases, this enzyme contains copper. The enzyme contained asparagine-linked carbohydrate (12% by weight) which could be removed by digestion with endo-beta-N-acetylglucosaminidase H. The molecular weight of native enzyme as determined by gel filtration was 110,000, but the largest component in a sodium dodecyl sulfate gel was 80,000. Several smaller components (55,000 and 36,000 molecular weight) were also visible. We present evidence which suggests that the smaller components are in vivo cleavage products tightly associated with enzymatically active molecules. Comparison of the laccase from a white-spore (wA) and a green-spore (wA+) strain showed, surprisingly, that the enzymes differed in electrophoretic pattern, in vitro heat stability, and in vivo metabolic stability. The difference was manifested for enzymes isolated from cultures after conidial pigmentation of the wA+ strain had occurred. If examined earlier, before pigmentation, the enzymes were indistinguishable. Since wA strains lack the precursor of the wild-type green pigment, i.e., the laccase substrate, we suggest that the transformation of the enzyme of the wA strain is due to its failure to interact with its normal substrate.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armitt S., McCullough W., Roberts C. F. Analysis of acetate non-utilizing (acu) mutants in Aspergillus nidulans. J Gen Microbiol. 1976 Feb;92(2):263–282. doi: 10.1099/00221287-92-2-263. [DOI] [PubMed] [Google Scholar]
- Bartsch E., Lerbs W., Luckner M. Phenol oxidase activity and pigment synthesis in conidiospores of Penicillium cyclopium. Z Allg Mikrobiol. 1979;19(2):75–82. doi: 10.1002/jobm.3630190202. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Clutterbuck A. J. Absence of laccase from yellow-spored mutants of Aspergillus nidulans. J Gen Microbiol. 1972 May;70(3):423–435. doi: 10.1099/00221287-70-3-423. [DOI] [PubMed] [Google Scholar]
- Cousins R. J. Synthesis and degradation of liver metallothionein. Experientia Suppl. 1979;34:293–301. doi: 10.1007/978-3-0348-6493-0_22. [DOI] [PubMed] [Google Scholar]
- Esser K., Minuth W. The phenoloxidases of the ascomycete Podospora anserina. Communication 4. Genetic regulation of the formation of laccase. Genetics. 1970 Mar-Apr;64(3):441–458. [PMC free article] [PubMed] [Google Scholar]
- Esser K. Phenol oxidases and morphogenesis in Podospora anserina. Genetics. 1968 Oct;60(2):281–288. doi: 10.1093/genetics/60.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehner S. C., Eriksson K. E. Induction of Neurospora crassa laccase with protein synthesis inhibitors. J Bacteriol. 1974 Oct;120(1):450–457. doi: 10.1128/jb.120.1.450-457.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOROWITZ N. H., FLING M., MACLEOD H., WATANABE Y. Structural and regulative genes controlling tyrosinase synthesis in Neurospora. Cold Spring Harb Symp Quant Biol. 1961;26:233–238. doi: 10.1101/sqb.1961.026.01.028. [DOI] [PubMed] [Google Scholar]
- Hurn B. A., Chantler S. M. Production of reagent antibodies. Methods Enzymol. 1980;70(A):104–142. doi: 10.1016/s0076-6879(80)70044-7. [DOI] [PubMed] [Google Scholar]
- Kurtz M. B., Champe S. P. Dominant spore color mutants of Aspergillus nidulans defective in germination and sexual development. J Bacteriol. 1981 Nov;148(2):629–638. doi: 10.1128/jb.148.2.629-638.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laurell C. B. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem. 1966 Apr;15(1):45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- Law D. J., Timberlake W. E. Developmental regulation of laccase levels in Aspergillus nidulans. J Bacteriol. 1980 Nov;144(2):509–517. doi: 10.1128/jb.144.2.509-517.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard T. J. Phenoloxidase activity and fruiting body formation Schizophyllum commune. J Bacteriol. 1971 Apr;106(1):162–167. doi: 10.1128/jb.106.1.162-167.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard T. J., Phillips L. E. Study of phenoloxidase activity during the reproductive cycle in Schizophyllum commune. J Bacteriol. 1973 Apr;114(1):7–10. doi: 10.1128/jb.114.1.7-10.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merril C. R., Dunau M. L., Goldman D. A rapid sensitive silver stain for polypeptides in polyacrylamide gels. Anal Biochem. 1981 Jan 1;110(1):201–207. doi: 10.1016/0003-2697(81)90136-6. [DOI] [PubMed] [Google Scholar]
- PONTECORVO G., ROPER J. A., HEMMONS L. M., MACDONALD K. D., BUFTON A. W. J. The genetics of Aspergillus nidulans. Adv Genet. 1953;5:141–238. doi: 10.1016/s0065-2660(08)60408-3. [DOI] [PubMed] [Google Scholar]
- Weeke B. Rocket immunoelectrophoresis. Scand J Immunol Suppl. 1973;1:37–46. doi: 10.1111/j.1365-3083.1973.tb03777.x. [DOI] [PubMed] [Google Scholar]