Abstract
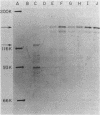
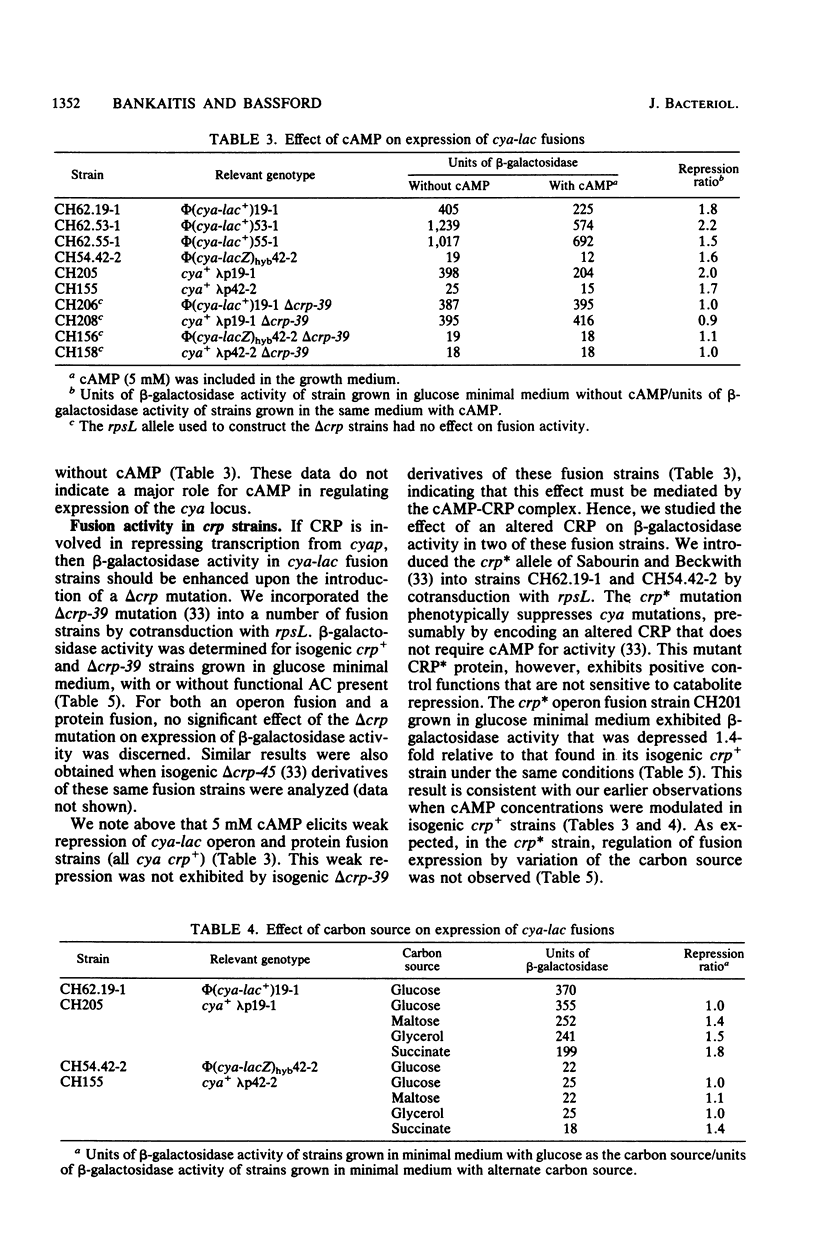
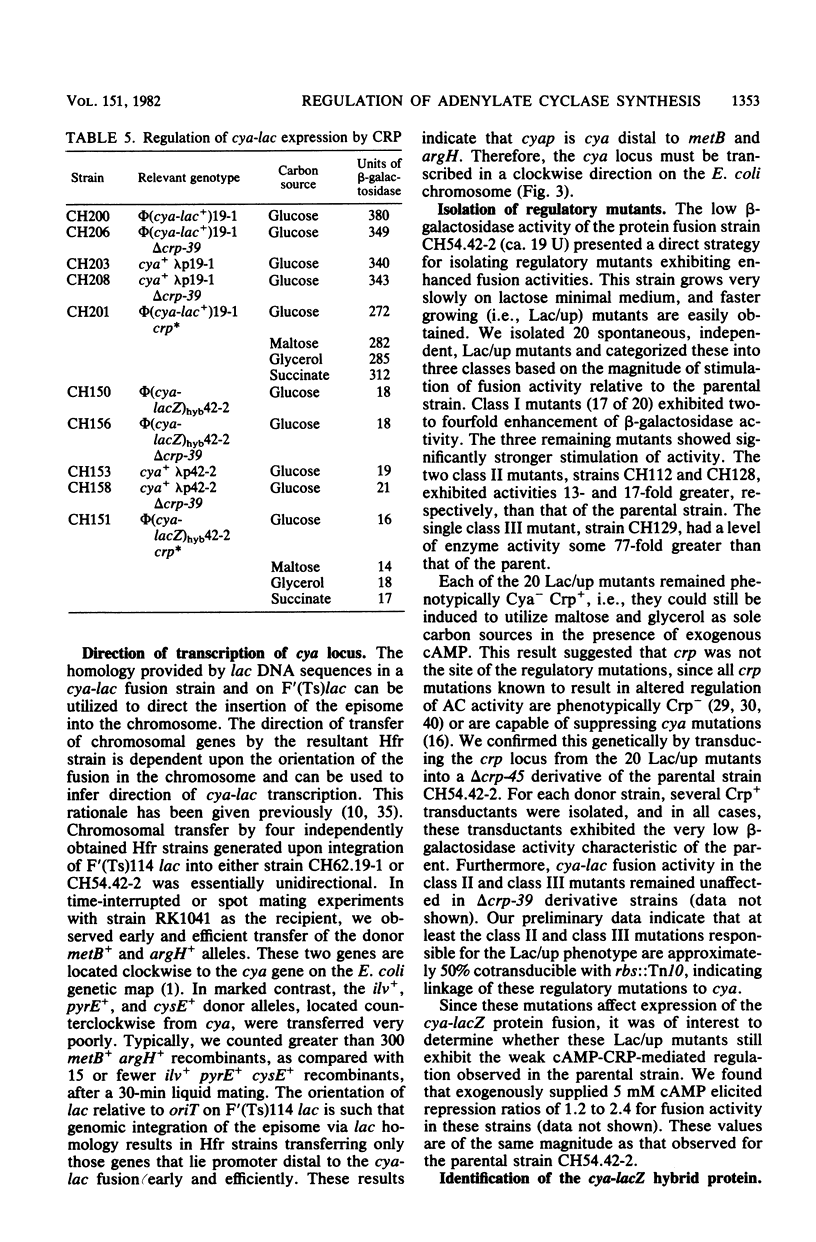
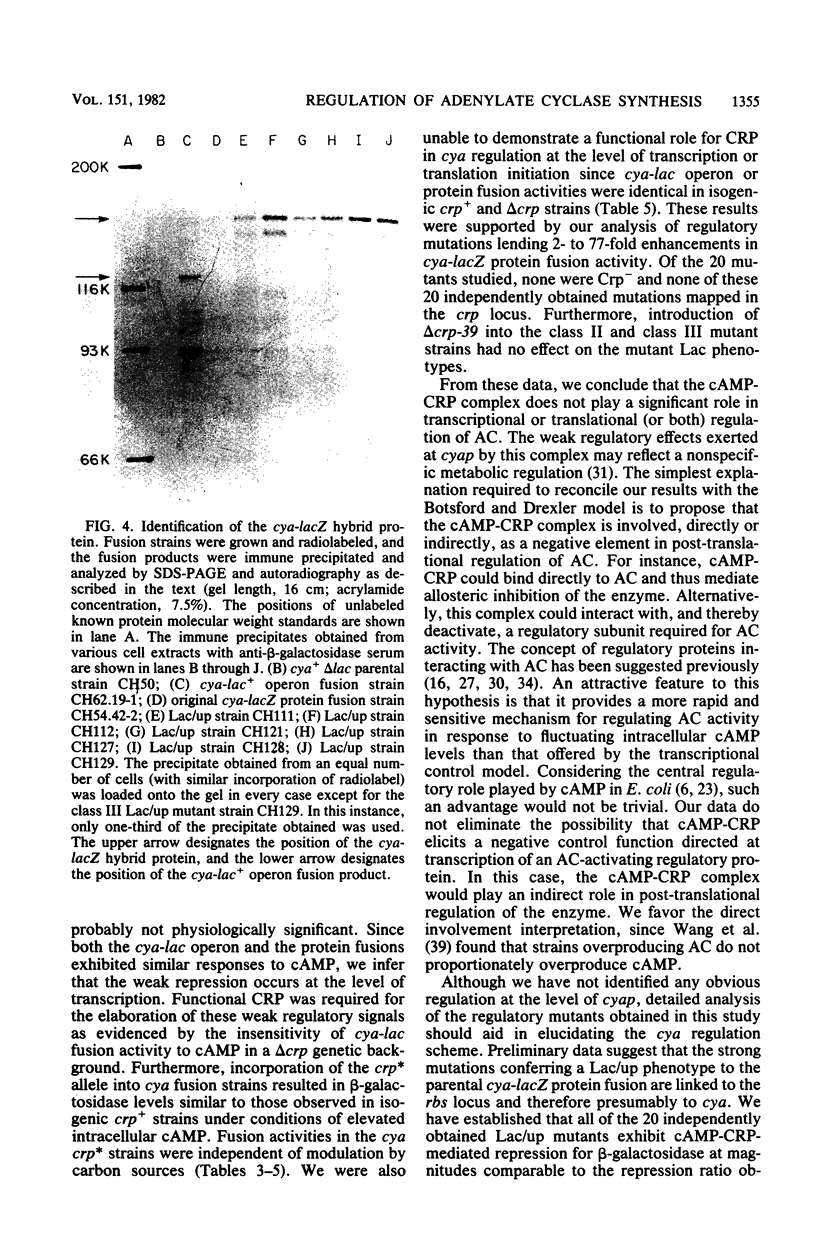
We have isolated cya-lac operon and protein fusions in Escherichia coli K-12, and we used these to study the regulation of cya, the structural gene for adenylate cyclase. Data obtained from these fusion strains suggest that neither cyclic AMP (cAMP) nor the cAMP receptor protein plays a major role in transcriptional or translational regulation of cya expression. Modulation of intracellular cAMP concentrations elicited only weak repression of cya-lac fusion activity under conditions of high intracellular cAMP, relative to fusion activity under conditions of low intracellular cAMP. The functional cAMP receptor protein was required for this effect. Incorporation of delta crp into cya-lac fusion strains did not affect fusion expression in glucose-grown cells as compared with similarly cultured isogenic crp+ strains. Furthermore, 20 independently obtained mutants derived from a cya-lacZ protein fusion strain exhibiting a weak Lac+ phenotype were isolated, and it was determined that the mutants had beta-galactosidase activities ranging from 2- to 77-fold greater than those of the parental strain. None of the mutations responsible for this increase in fusion activity map in the crp locus. We used these mutants to aid in the identification of a 160,000-dalton cya-lacZ hybrid protein. Finally, chromosome mobilization experiments, using cya-lac fusion strains, allowed us to infer a clockwise direction of transcription for the cya gene relative to the standard E. coli genetic map.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassford P. J., Jr, Diedrich D. L., Schnaitman C. L., Reeves P. Outer membrane proteins of Escherichia coli. VI. Protein alteration in bacteriophage-resistant mutants. J Bacteriol. 1977 Aug;131(2):608–622. doi: 10.1128/jb.131.2.608-622.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassford P. J., Jr, Silhavy T. J., Beckwith J. R. Use of gene fusion to study secretion of maltose-binding protein into Escherichia coli periplasm. J Bacteriol. 1979 Jul;139(1):19–31. doi: 10.1128/jb.139.1.19-31.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckwith J. A genetic approach to characterizing complex promoters in E. coli. Cell. 1981 Feb;23(2):307–308. doi: 10.1016/0092-8674(81)90125-2. [DOI] [PubMed] [Google Scholar]
- Berman M. L., Beckwith J. Fusions of the lac operon to the transfer RNA gene tyrT of Escherichia coli. J Mol Biol. 1979 May 25;130(3):285–301. doi: 10.1016/0022-2836(79)90542-4. [DOI] [PubMed] [Google Scholar]
- Botsford J. L. Cyclic nucleotides in procaryotes. Microbiol Rev. 1981 Dec;45(4):620–642. doi: 10.1128/mr.45.4.620-642.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botsford J. L., Drexler M. The cyclic 3',5'-adenosine monophosphate receptor protein and regulation of cyclic 3',5'-adenosine monophosphate synthesis in Escherichia coli. Mol Gen Genet. 1978 Sep 20;165(1):47–56. doi: 10.1007/BF00270375. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivo probe for transcriptional control sequences. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4530–4533. doi: 10.1073/pnas.76.9.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Chumley F. G., Menzel R., Roth J. R. Hfr formation directed by tn10. Genetics. 1979 Apr;91(4):639–655. doi: 10.1093/genetics/91.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debarbouille M., Schwartz M. The use of gene fusions to study the expression of malT the positive regulator gene of the maltose regulon. J Mol Biol. 1979 Aug 15;132(3):521–534. doi: 10.1016/0022-2836(79)90273-0. [DOI] [PubMed] [Google Scholar]
- Dessein A., Schwartz M., Ullmann A. Catabolite repression in Escherichia coli mutants lacking cyclic AMP. Mol Gen Genet. 1978 Jun 1;162(1):83–87. doi: 10.1007/BF00333853. [DOI] [PubMed] [Google Scholar]
- Epstein W., Rothman-Denes L. B., Hesse J. Adenosine 3':5'-cyclic monophosphate as mediator of catabolite repression in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2300–2304. doi: 10.1073/pnas.72.6.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
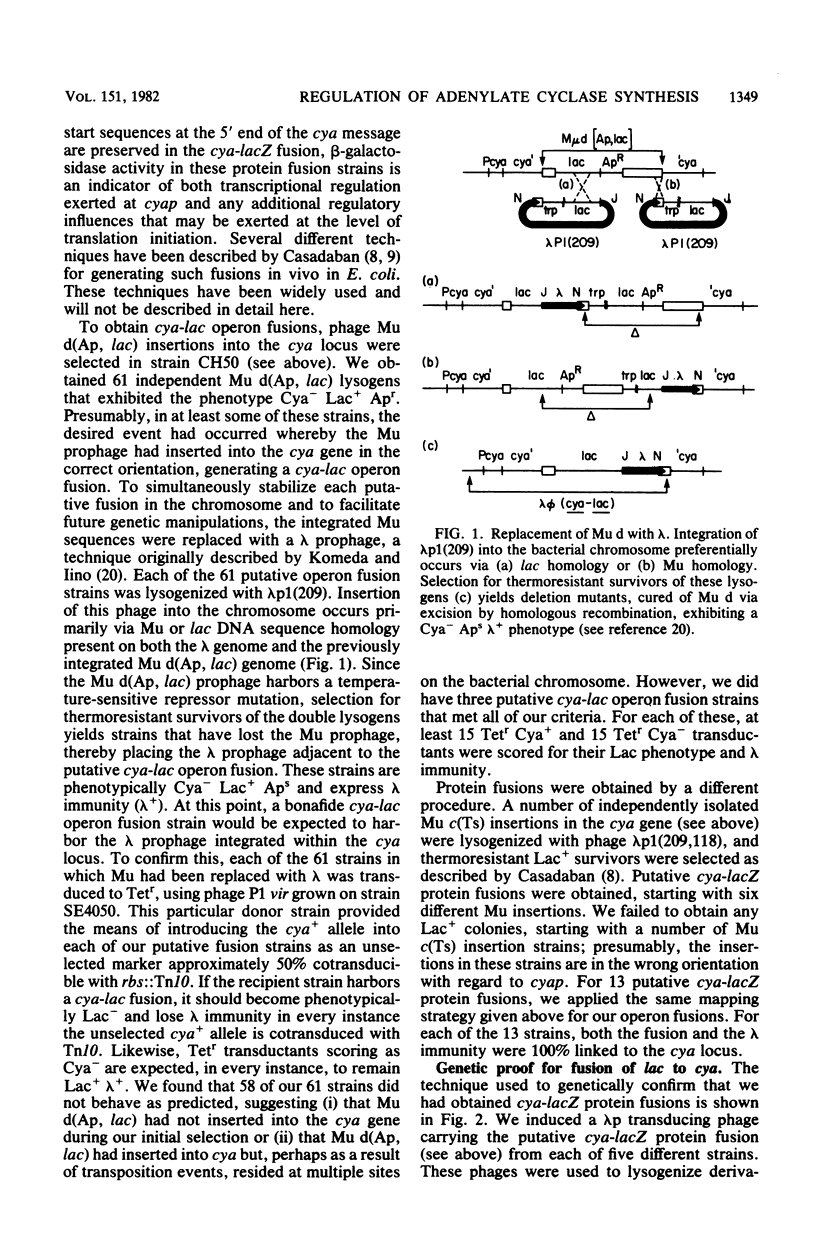
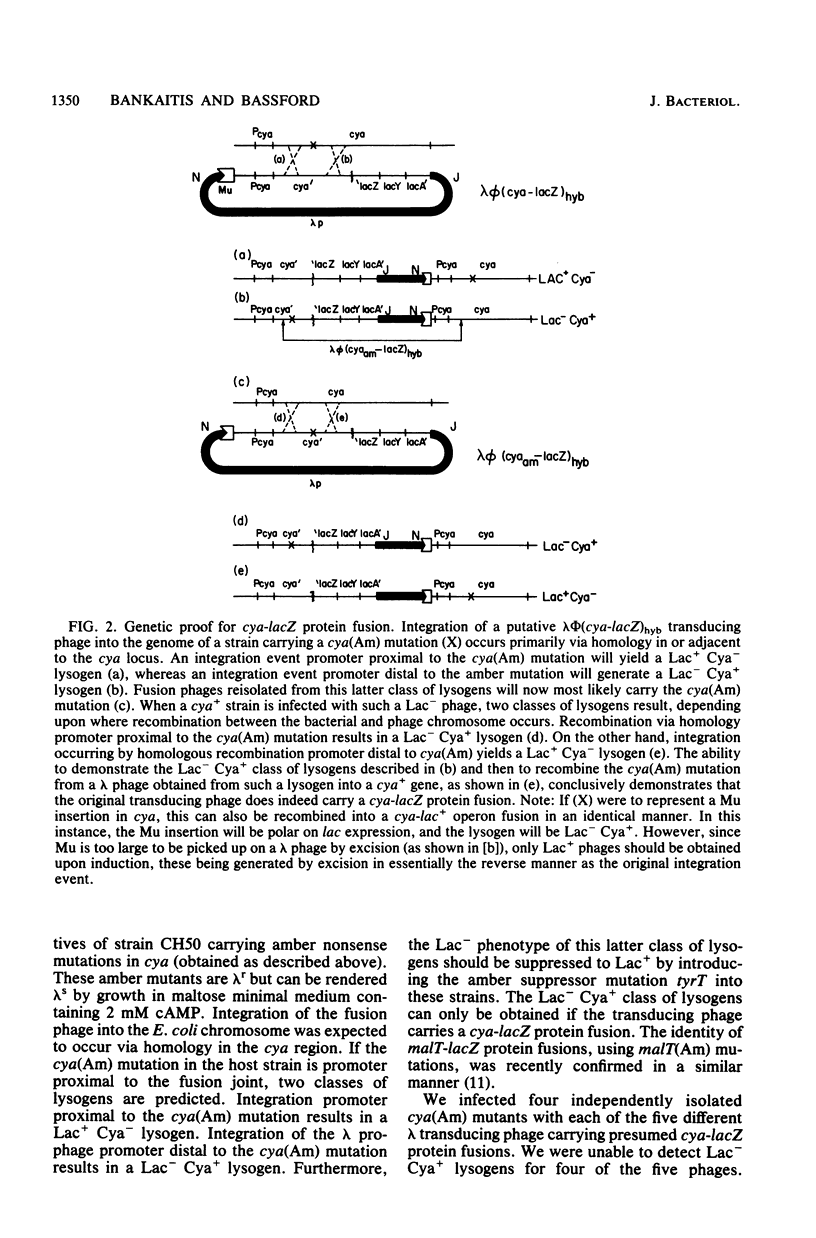
- Gottesman S., Beckwith J. R. Directed transposition of the arabinose operon: a technique for the isolation of specialized transducing bacteriophages for any Escherichia coli gene. J Mol Biol. 1969 Aug 28;44(1):117–127. doi: 10.1016/0022-2836(69)90408-2. [DOI] [PubMed] [Google Scholar]
- Guidi-Rontani C., Danchin A., Ullmann A. Catabolite repression in Escherichia coli mutants lacking cyclic AMP receptor protein. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5799–5801. doi: 10.1073/pnas.77.10.5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidi-Rontani C., Danchin A., Ullmann A. Isolation and characterization of an Escherichia coli mutant affected in the regulation of adenylate cyclase. J Bacteriol. 1981 Dec;148(3):753–761. doi: 10.1128/jb.148.3.753-761.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M. N., Silhavy T. J. Transcriptional regulation of Escherichia coli K-12 major outer membrane protein 1b. J Bacteriol. 1979 Nov;140(2):342–350. doi: 10.1128/jb.140.2.342-350.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidecker G., Müller-Hill B. Synthetic multifunctional proteins: isolation of covalently linked tryptophan synthetase alpha-subunit-lac-repressor-beta-galactosidase chimeras. Mol Gen Genet. 1977 Oct 24;155(3):301–307. doi: 10.1007/BF00272809. [DOI] [PubMed] [Google Scholar]
- Ito K., Bassford P. J., Jr, Beckwith J. Protein localization in E. coli: is there a common step in the secretion of periplasmic and outer-membrane proteins? Cell. 1981 Jun;24(3):707–717. doi: 10.1016/0092-8674(81)90097-0. [DOI] [PubMed] [Google Scholar]
- Komeda Y., Iino T. Regulation of expression of the flagellin gene (hag) in Escherichia coli K-12: analysis of hag-lac gene fusions. J Bacteriol. 1979 Sep;139(3):721–729. doi: 10.1128/jb.139.3.721-729.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAKMAN R. S., SUTHERLAND E. W. ADENOSINE 3',5'-PHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1965 Mar;240:1309–1314. [PubMed] [Google Scholar]
- Pastan I., Adhya S. Cyclic adenosine 5'-monophosphate in Escherichia coli. Bacteriol Rev. 1976 Sep;40(3):527–551. doi: 10.1128/br.40.3.527-551.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman R. L., Pastan I. Pleiotropic deficiency of carbohydrate utilization in an adenyl cyclase deficient mutant of Escherichia coli. Biochem Biophys Res Commun. 1969 Sep 24;37(1):151–157. doi: 10.1016/0006-291x(69)90893-6. [DOI] [PubMed] [Google Scholar]
- Perlman R., Pastan I. Cyclic 3'5-AMP: stimulation of beta-galactosidase and tryptophanase induction in E. coli. Biochem Biophys Res Commun. 1968 Mar 27;30(6):656–664. doi: 10.1016/0006-291x(68)90563-9. [DOI] [PubMed] [Google Scholar]
- Peterkofsky A., Gazdar C. Escherichia coli adenylate cyclase complex: regulation by the proton electrochemical gradient. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1099–1103. doi: 10.1073/pnas.76.3.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterkofsky A., Gazdar C. Interaction of enzyme I of the phosphoenolpyruvate:sugar phosphotransferase system with adenylate cyclase of Escherichia coli. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2920–2924. doi: 10.1073/pnas.72.8.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter K., Chaloner-Larsson G., Yamazaki H. Abnormally high rate of cyclic AMP excretion from an Escherichia coli mutant deficient in cyclic AMP receptor protein. Biochem Biophys Res Commun. 1974 Mar 25;57(2):379–385. doi: 10.1016/0006-291x(74)90941-3. [DOI] [PubMed] [Google Scholar]
- Rephaeli A. W., Saier M. H., Jr Effects of crp mutations on adenosine 3',5'-monophosphate metabolism in Salmonella typhimurium. J Bacteriol. 1976 Jul;127(1):120–127. doi: 10.1128/jb.127.1.120-127.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Yanofsky C. Metabolic regulation of the tryptophan operon of Escherichia coli: repressor-independent regulation of transcription initiation frequency. J Mol Biol. 1972 Aug 14;69(1):103–118. doi: 10.1016/0022-2836(72)90026-5. [DOI] [PubMed] [Google Scholar]
- Roy A., Danchin A. Restriction map of the cya region of the Escherichia coli K12 chromosome. Biochimie. 1981 Aug-Sep;63(8-9):719–722. doi: 10.1016/s0300-9084(81)80220-9. [DOI] [PubMed] [Google Scholar]
- Sabourin D., Beckwith J. Deletion of the Escherichia coli crp gene. J Bacteriol. 1975 Apr;122(1):338–340. doi: 10.1128/jb.122.1.338-340.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr, Feucht B. U. Coordinate regulation of adenylate cyclase and carbohydrate permeases by the phosphoenolpyruvate:sugar phosphotransferase system in Salmonella typhimurium. J Biol Chem. 1975 Sep 10;250(17):7078–7080. [PubMed] [Google Scholar]
- Shattuck-Eidens D. M., Kadner R. J. Exogenous induction of the Escherichia coli hexose phosphate transport system defined by uhp-lac operon fusions. J Bacteriol. 1981 Oct;148(1):203–209. doi: 10.1128/jb.148.1.203-209.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuman H. A., Silhavy T. J., Beckwith J. R. Labeling of proteins with beta-galactosidase by gene fusion. Identification of a cytoplasmic membrane component of the Escherichia coli maltose transport system. J Biol Chem. 1980 Jan 10;255(1):168–174. [PubMed] [Google Scholar]
- Silhavy T. J., Brickman E., Bassford P. J., Jr, Casadaban M. J., Shuman H. A., Schwartz V., Guarente L., Schwartz M., Beckwith J. R. Structure of the malB region in Escherichia coli K12. II. Genetic map of the malE,F,G operon. Mol Gen Genet. 1979 Jul 24;174(3):249–259. doi: 10.1007/BF00267797. [DOI] [PubMed] [Google Scholar]
- Ullmann A., Joseph E., Danchin A. Cyclic AMP as a modulator of polarity in polycistronic transcriptional units. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3194–3197. doi: 10.1073/pnas.76.7.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Y., Clegg D. O., Koshland D. E., Jr Molecular cloning and amplification of the adenylate cyclase gene. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4684–4688. doi: 10.1073/pnas.78.8.4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne P. K., Rosen O. M. Cyclic 3':5'-adenosine monophosphate in Escherichia coli during transient and catabolite repression. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1436–1440. doi: 10.1073/pnas.71.4.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]