Abstract
Background
Indian Asian men residing in the United Kingdom have a higher prevalence of hyperhomocysteinemia than do their European counterparts. This has been largely attributed to dietary deficiencies in cobalamin associated with vegetarianism among these Indian Asians.
Objective
We aimed to ascertain the prevalence of folate and cobalamin deficiencies and hyperhomocysteinemia in Bangladesh.
Design
Plasma concentrations of homocysteine, folate, and cobalamin and urinary concentrations of creatinine were assessed in 1650 adults in Bangladesh.
Results
The prevalence of hyperhomocysteinemia (men: >11.4 μmol/L; women: >10.4 μmol/L) was markedly (P < 0.0001) greater among men (63%; x̄ ± SD: 15.3 ± 9.5 μmol/L) than among women (26%; 9.5 ± 4.7 μmol/L). Folate was lower (9.8 ± 6.5 and 12.3 ± 7.6 nmol/L, respectively), whereas cobalamin was higher (281 ± 115 and 256 ± 118 pmol/L, respectively) (P < 0.0001 for both) among men than among women. Folate explained 15% and cobalamin explained 5% of the variation in homocysteine concentrations. For men, folate (P = 0.005) and cobalamin (P = 0.03) were positively correlated with urinary creatinine. Smoking (P < 0.0003) and betelnut use (P < 0.0002) were independent negative predictors of folate.
Conclusions
Bangladeshi men have a high prevalence of hyperhomocysteinemia, which is more closely associated with folate than with cobalamin, although other factors, eg, smoking and betelnut use, may also contribute to its cause. The positive correlations between urinary creatinine and plasma folate and cobalamin were unanticipated and could suggest that, in marginal nutrition, these vitamins may be limiting for creatine biosynthesis.
Keywords: Folate, folate deficiency, cobalamin, vitamin B-12, vitamin B-12 deficiency, micronutrient deficiency, homocysteine, hyperhomocysteinemia, one-carbon metabolism, creatinine, Bangladesh, betelnut
INTRODUCTION
Folate deficiency is associated with many adverse outcomes, including increased risk of neural tube defects (NTDs), colon cancer, and hyperhomocysteinemia, itself a potential risk factor for cardiovascular and cerebral vascular disease. Serum folate concentrations correlate inversely with homocysteine in all populations and age groups surveyed, and folate supplementation lowers homocysteine concentrations even in persons without obvious folate deficiency (1, 2). This is likely because of the remethylation of homocysteine, a process in which folate is a cosubstrate and cobalamin a cofactor. In most but not all studies, homocysteine correlates more strongly with folate than with cobalamin concentrations (1, 2). A notable exception is the high prevalence of hyperhomocysteinemia among Asian Indian men residing in the United Kingdom, which reportedly is associated with cobalamin deficiency that is attributed to vegetarian diets (3).
Because of the associated risk of NTDs, a great deal of attention has been given to suboptimal folate status in women of childbearing age who live in Western countries. In 1992, the US Public Health Service recommended that all women capable of becoming pregnant consume 400 μg folate/d to reduce the occurrence of spina bifida and other NTDs. Fortification of cereal-grain products in the United States became mandatory in January 1998. Similar programs have gone into effect in Canada, other parts of the Americas, and parts of Europe. As a result, the prevalence of spina bifida in the United States has declined 31%, the prevalence of anencephaly has declined by 16% (4), and the prevalence of hyperhomocysteinemia has declined from 18.7% to 9% (5). Although Asian Indians, Pakistanis, and Bangladeshis who reside in the United Kingdom have been reported to have lower mean erythrocyte folate concentrations than do white Europeans (6, 7), surprisingly little is known about the prevalence of folate deficiency in South and Southeast Asia, a region estimated to have more than half of the global burden of malnutrition (8, 9).
The prevalence of hyperhomocysteinemia among Asian Indian and Bangladeshi men residing in the United Kingdom is reportedly higher than that among their white European counterparts (3, 7) and the incidence of cardiovascular disease in Southeast Asia is progressively increasing (9), but the prevalence of hyperhomocysteinemia in Bangladesh has not been previously reported. The current study was undertaken to ascertain the prevalence of hyperhomocysteinemia and folate and cobalamin deficiencies among adults residing in Bangladesh.
SUBJECTS AND METHODS
The data presented are from the Nutritional Influences on Arsenic Toxicity study, an ongoing study on nutritional influences on arsenic metabolism, which works in collaboration with a larger ongoing multidisciplinary study by health, earth, and social scientists from Columbia University and in Bangladesh (the Columbia University Superfund Basic Research Program), the National Institute of Preventive and Social Medicine, and Dhaka University. The centerpiece of the public health research is a prospective cohort study of 12 000 adults exposed to a wide range of water arsenic concentrations, from which the current sample is derived.
The region
Bangladesh is a nation of 124 million people with an area of 56 000 square miles. It is divided into 64 districts, each of which is divided into 10–50 administrative units, or thanas. The study site is located in Araihazar, 1 of 464 thanas in Bangladesh, which has an area of 25 km2 and is located ≈30 km east of Dhaka. The study site was chosen because 1) it is known to have both high and low concentrations of arsenic in the drinking water, which provides a wide range of arsenic exposure and thus permits dose-response analyses for other studies, and 2) it is within a reasonable commuting distance from Dhaka. Our socioeconomic status data indicate that this region is not particularly poor by Bangladeshi standards.
Eligibility criteria, participant recruitment, and ethics
The cohort study includes 12 000 married men and women aged 20–65 y who were recruited between September 2000 and May 2002. Marriage was chosen as an inclusion criterion to minimize the likelihood of loss to follow-up because of a change of residence after marriage. For the current study, recruitment of 1650 of the 12 000 cohort participants was centered around visits of one of the investigators (MVG).
Oral informed consent was obtained by our Bangladeshi field staff physicians, who read an institutional review board–approved assent form to the study participants. Ethical approval was obtained from the Institutional Review Board of Columbia Presbyterian Medical Center and the Bangladesh Medical Research Council.
Plasma collection and handling
Plasma samples for total homocysteine (tHcy), folate, and total cobalamin were obtained by venipuncture after the participant had been sitting for 10–15 min for an interview. Blood was collected into EDTA-containing tubes, which were immediately placed in IsoRack cool packs (Brinkmann Instruments, Westbury, NY) designed to maintain samples at 0 °C for 6 h. Within 4 h, samples were transported in hand-carried coolers that also contained additional ice packs to our local laboratory, which is situated in the Columbia University Superfund Basic Research Program’s field clinic in Araihazar. Samples were spun at 3000 ×g for 10 min at 4 °C, and plasma was separated from cells. Plasma was stored in aliquots at −80 °C and shipped in a frozen state on dry ice to Columbia University for analysis.
Analytic techniques
Plasma folate and cobalamin
Folate and cobalamin were analyzed in 1648 plasma samples (2 samples had insufficient plasma for these analyses) by radio-proteinbinding assay (Quantaphase II radioimmunoassay; Bio-Rad Laboratories, Richmond, CA). This method requires heating to 100 °C to denature endogenous binding substances. For folate determination, folic acid as pteroylglutamic acid was used for calibration, and its 125I-labeled analog was used as the tracer. Cyanocobalamin was used for calibration, and its 57Co-labeled analog was the tracer for cobalamin assays. The within- and between-day CVs for folate were 3% and 11%, respectively, and those for cobalamin were 4% and 8%, respectively.
Although the Quantaphase II radioimmunoassay provides precise measurement of plasma folate concentrations, the reference values are lower than those obtained with other kits (10) and methods (11, 12), and, in agreement with other studies (13), they were not used for ascertaining the prevalence of folate deficiency. Normal reference values (95% CIs) for cobalamin and plasma folate have been reported by Christenson et al (10) from ostensibly healthy blood donors with the use of the same Bio-Rad Quantaphase II radioimmunoassay, and their reference values were used for the purposes of estimating the prevalence of folate deficiency in this study.
Plasma homocysteine concentrations
Plasma tHcy concentrations were measured in 1644 plasma samples by HPLC with fluorescence detection according to the method described by Pfeiffer et al (14). Six samples either were hemolyzed or had insufficient plasma for these analyses. Briefly, protein-bound thiols were reduced and released by incubating plasma with TCEP [tris(2-carboxyethyl)phosphine]–reducing reagent along with cystamine dihydrochloride internal standard. Plasma was then deproteinized by the addition of trichloroacetic acid. After centrifugation, the derivatized sample was transferred to an HPLC vial containing NaOH, borate, and SBD-F (ammonium 7-fluorobenzofurazane-4-sulfonate). After incubation for 1 h at 60 °C under dim red light, 10 μL of the derivatized sample was injected onto the HPLC. The mobile phase consisted of 0.1 mol acetic acid-acetate/L (pH 5.5), containing 30 mL methanol/L at a flow rate of 0.7 mL/min. Separation was achieved by using a 150 × 3.2–mm, 5-μm Prodigy ODS2 analytic column (Phenomenex, Torrance, CA). Fluorescence was detected with the use of a Waters 474 fluorescence detector (Waters Corporation, Milford, MA) with excitation at 385 nm and emission at 515 nm. L-Homocysteine and L -cysteine were used as external calibrators (14). The within- and between-day CVs for tHcy were 5% and 8%, respectively.
Statistical analysis
Descriptive statistics for characteristics of the study sample were calculated separately by sex. Sex differences in quantitative variables were tested by using Wilcoxon’s rank-sum test without distribution assumptions. Chi-square tests were used to test for sex differences in the categorical variables. Because the distributions of plasma concentrations of tHcy, folate, and cobalamin and of urine creatinine concentrations were skewed, we used log-transformed data in the linear regression analyses to study associations between continuous outcomes and independent variables of interest, after control for other variables (SAS software, version 9.1; SAS Institute Inc, Cary, NC).
RESULTS
Characteristics of the population
The characteristics of the study sample are presented in Table 1. A total of 1650 participants, 973 women and 677 men, were evaluated. As a crude indicator of social class, housing type, from lowest to highest, was recorded as 1) thatched roof, 2) corrugated tin roof, or 3) cement roof. On average, men (4 y) had slightly more education than did women (3.2 y). Cigarette smoking was much more common in men (76%) than in women (6%). Betelnut use was also more common among men (40%) than among women (30%), but the difference was far less striking than that for smoking.
TABLE 1.
Characteristics of the study sample1
| Women (n = 973) | Men (n = 677) | |||
|---|---|---|---|---|
| Age (y) | 34.6 ± 8.82,3 | 33 (28–41)4 | 42.2 (9.8) | 41 (35–49) |
| Weight (kg) | 45.3 ± 7.93 | 44.3 (39.8–50) | 50.8 (9.1) | 49.3 (45–55.3) |
| Height (cm) | 149.6 ± 5.23 | 149.6 (146.2–152.9) | 161.6 (6.0) | 161.5 (157.6–165.5) |
| BMI (kg/m2) | 20.2 ± 3.23 | 19.8 (21.8–18.0) | 19.4 (3.0) | 18.7 (17.4–21.0) |
| Education (y) | 3.2 ± 3.63 | 2 (0–5) | 4.0 (4.2) | 3 (0–7) |
| Children in home (n) | 3.1 ± 1.7 | 3 (2–4) | — | — |
| Cigarette smokers (%) | 63 | 76 | ||
| Betelnut users (%) | 303 | 40 | ||
| Type of housing (%) | ||||
| Thatched | 5.9 | 5.9 | ||
| Corrugated tin | 78.5 | 78.3 | ||
| Cement | 13.8 | 13.4 | ||
| Other | 1.9 | 2.4 | ||
| Plasma tHcy (μmol/L) | 9.5 ± 4.73 | 8.6 (7–10.7) | 15.3 (9.5) | 12.8 (10.2–17.1) |
| Hyperhomocysteinemia (%)5 | 263 | 63 | ||
| Plasma folate (nmol/L) | 12.3 ± 7.63 | 10.4 (7.4–14.5) | 9.8 (6.5) | 8.3 (6.1–11.5) |
| Plasma folate < 9 nmol/L (%) | 393 | 57 | ||
| Plasma cobalamin (pmol/L) | 256 ± 1183 | 233.3 (180.4–306.2) | 281 (115) | 257.9 (200.1–339) |
| Plasma cobalamin < 151 pmol/L (%) | 136 | 8 | ||
| Urinary creatinine (mg/dL) | 55.8 ± 41.23 | 45.8 (25.2–76.1) | 69.8 (51.7) | 55.4 (30–95.1) |
tHcy, total homocysteine.
x̄ ± SD (all such values).
Significantly different from men (Wilcoxon’s rank-sum test): 3P < 0.0001, 6P < 0.01.
Median:interquartile range in parentheses (all such values). For the interquartile range, the lower and upper 25th percentile values are reported.
Defined as ≥ 10.4 umol/L for women and ≥ 11.4 umol/L for men.
Total homocysteine, folate, and cobalamin
Sex-specific frequency distributions for concentrations of tHcy, folate, and cobalamin are provided in Figure 1, and mean values for these metabolites are provided in Table 1. These data indicate a high prevalence of hyperhomocysteinemia, particularly among men. When we used sex-specific cutoffs derived from the third National Health and Nutrition Examination Survey (NHANES III; 13), 63% of men and 26% of women studied had hyperhomocysteinemia (men: ≥11.4 μmol/L; women: ≥10.4 μmol/L). When we used the published reference values for plasma folate and cobalamin described in Methods (10), 39% of women and 57% of men had low plasma folate concentrations (<9 nmol/L). The prevalence of cobalamin deficiency (cobalamin <151 nmol/L) was 8% for men and 13% for women. Because men were significantly older than women (42 ± 10 and 35 ± 9 y, respectively; P < 0.0001), the data were reanalyzed after adjustment for age; the differences remained highly significant (P < 0.0001 for tHcy, folate, and cobalamin).
FIGURE 1.
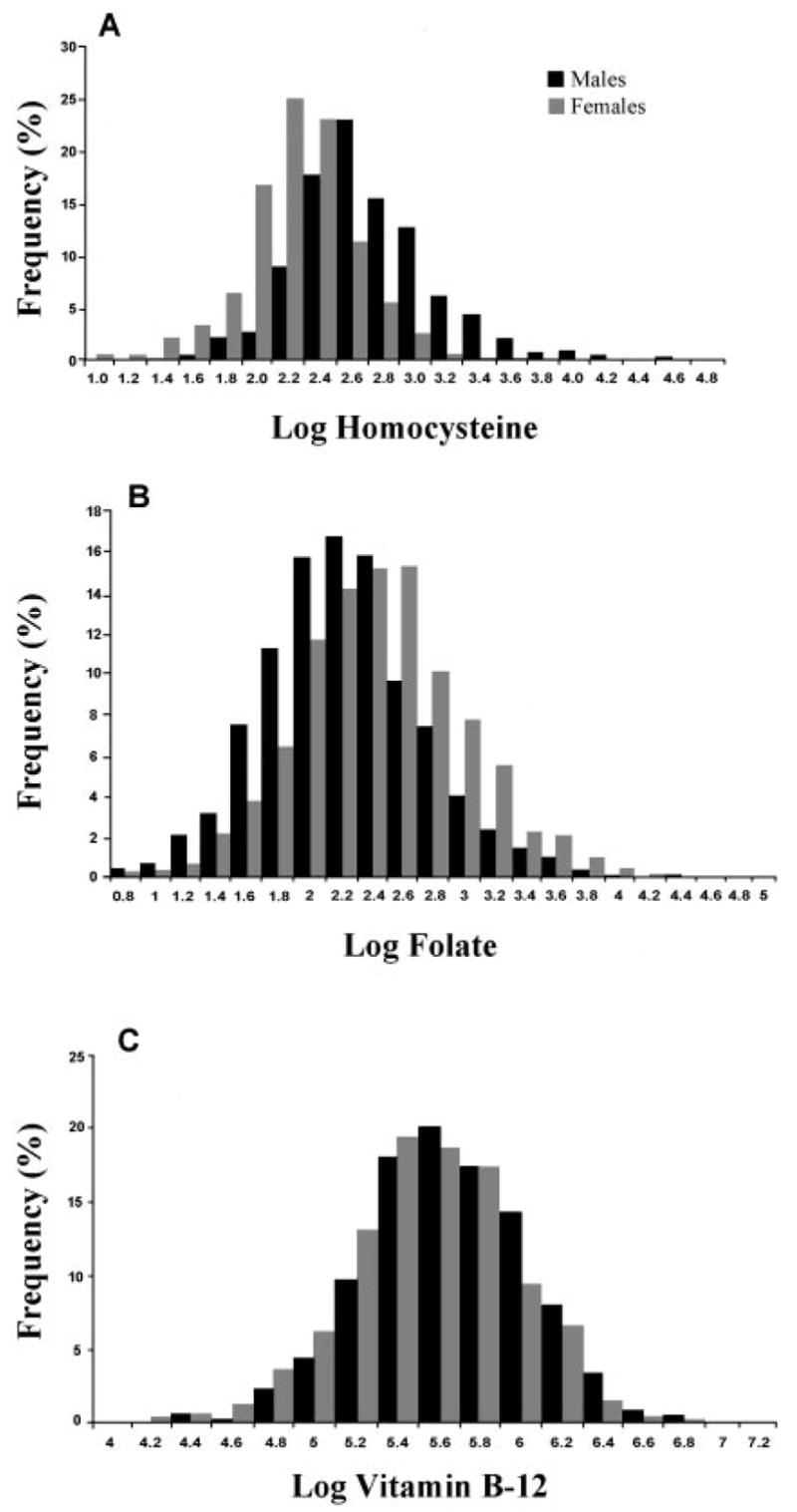
Frequency distributions for women (
 ) and men (
) and men (
 ) for homocysteine (A; n = 676 M; n = 968 F), folate (B; n = 676 M; n = 972 F), and cobalamin (C; n = 676 M; n = 972 F). Homocysteine, folate, and cobalamin were skewed and were, therefore, log transformed.
) for homocysteine (A; n = 676 M; n = 968 F), folate (B; n = 676 M; n = 972 F), and cobalamin (C; n = 676 M; n = 972 F). Homocysteine, folate, and cobalamin were skewed and were, therefore, log transformed.
As expected, an inverse association was observed between plasma folate and tHcy for both men and women (r = −0.46 and −0.43, respectively; P < 0.0001). Plasma cobalamin and tHcy concentrations were also correlated, although, as is consistent with the literature, this relation was less strong (r = −0.30 and −0.19 for men and women, respectively, P < 0.0001; Table 2). After adjustment for age and sex, plasma folate explained 15.1% and cobalamin explained 5.0% of the variation in plasma homocysteine concentrations.
TABLE 2.
Spearman rank-order correlation coefficients1
| tHcy | Folate | Cobalamin | Creatinine | |
|---|---|---|---|---|
| Men (n = 677) | ||||
| tHcy | 1 | |||
| Folate | −0.462 | 1 | ||
| Cobalamin | −0.302,3 | 0.2172,3 | 1 | |
| Urinary creatinine | −0.03 | 0.1173,4 | 0.0913,5 | 1 |
| Age | 0.2182 | −0.06 | −0.133,4 | 0.0943,5 |
| Weight | 0.0063 | 0.06 | 0.03 | 0.154 |
| Height | −0.02 | −0.011 | −0.024 | 0.002 |
| BMI | 0.0263 | 0.0835 | 0.038 | 0.182 |
| Education | 0.0683 | 0.052 | 0.1034 | 0.030 |
| Women (n = 973) | ||||
| tHcy | 1 | |||
| Folate | −0.4272 | 1 | ||
| Cobalamin | −0.1872 | 0.1084 | 1 | |
| Urinary creatinine | −0.003 | 0.011 | −0.020 | 1 |
| Age | 0.242 | 0.02 | 0.023 | −0.056 |
| Weight | −0.1064 | 0.0884 | 0.0745 | 0.1144 |
| Height | −0.018 | 0.051 | 0.031 | −0.002 |
| BMI | −0.104 | 0.0715 | 0.0695 | 0.1214 |
| Education | −0.1562 | 0.1322 | 0.0665 | 0.0745 |
tHcy; total homocysteine.
P < 0.0001.
Significantly different from men, P < 0.05.
P < 0.01.
P < 0.05.
Plasma folate concentrations were lower for cigarette smokers than for nonsmokers (9.3 ± 5.5 and 12.3 ± 7.8 nmol/L, respectively; P < 0.0001) and for betelnut users than for nonusers (9.9 ± 6.1 and 12.0 ± 7.7 nmol/L, respectively; P < 0.0001). In multiple regression models, cigarette smoking (P = 0.0003) and betelnut (P = 0.0002) use were both independent negative predictors of plasma folate for men, and betelnut use was a negative predictor of plasma folate for women (P < 0.0001). Only 10 of the 54 women who smoked did not use betelnut. Participants who both smoked and chewed betelnut were more than twice as likely to be within the lowest quartile for plasma folate and less than half as likely to be within the highest quartile for plasma folate concentrations than were nonsmokers and nonbetelnut users, as shown in Figure 2. In a regression model that controlled for age and sex, the cigarette smoking × betelnut use interaction was significant (P = 0.04). Cigarette smoking × sex or betelnut use × sex interaction was not significant. The tHcy concentrations were significantly higher for smokers than for nonsmokers (15.0 ± 9.5 and 10.2 ± 5.9 μmol/L, respectively; P < 0.0001) and for betelnut users than for nonusers (13.2 ± 7.5 and 11.2 ± 7.6μmol/L, respectively; P < 0.0001). Neither cigarette smoking nor betelnut use was associated with plasma cobalamin concentrations.
FIGURE 2.
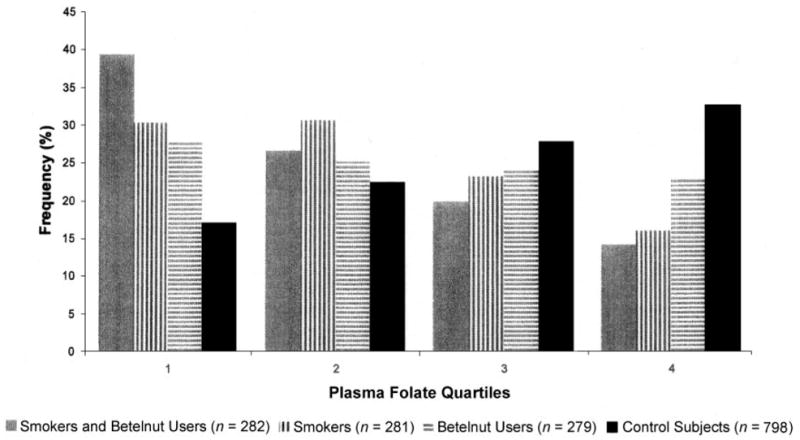
Plasma folate quartiles by smoking and betelnut use among smokers, betelnut users, smokers and betelnut users, and control subjects. The subgroups in each folate quartile were as follows: quartile 1: 39% (n = 111), 30% (n = 85), 28% (n = 78), and 17% (n = 136); quartile 2: 27% (n = 75), 31% (n = 86), 25% (n = 70), and 22% (n = 179); quartile 3: 20% (n = 56), 23% (n = 65), 24% (n = 67), and 28% (n = 222); and quartile 4: 14% (n = 40), 16% (n = 45), 23% (n = 64), and 33% (n = 261), respectively. In the regression model that controlled for age and sex, the cigarette smoking × betelnut use interaction was significant (P = 0.04).
Because this study was conducted to provide the framework for a folate intervention trial to study arsenic methylation, urinary arsenic and arsenic metabolites were also measured (data not shown). Total urinary arsenic is typically expressed per gram urinary creatinine to adjust for variations in urine concentration. We observed a significant inverse correlation between plasma folate concentrations and urinary arsenic per gram creatinine (Spearman correlation coefficients: −0.22 and −0.17 for men and women, respectively; P < 0.0001 for both). On closer inspection, it became apparent that the correlation was due in part to a positive correlation between plasma folate and urinary creatinine. Spearman correlation coefficients between urinary creatinine and plasma folate, tHcy, and cobalamin, as well as other covariates, are provided in Table 2.
DISCUSSION
In 1998, Michie et al (6) reported that healthy Indian or Pakistani men and women had significantly lower concentrations of erythrocyte folate than did age-matched whites. They also alluded to data from the Congenital Malformation Register that suggested that there may be a higher prevalence of pregnancies with NTDs in women of Indian or Pakistani origin (6). Later that same year, another report described higher plasma homocysteine concentrations among 170 healthy East London Bangladeshis than the published values for whites with ischemic heart disease (7). Mean serum tHcy concentrations reported in that study were 13.3 μmol/L, a value that is slightly higher than the overall average of 11.9 μmol/L that we observed in Bangladesh, but lower than the average of 15.3 μmol/L among men. Two years later, these reports were confirmed in a larger study conducted by Chambers et al (3), who found that, among healthy male subjects, plasma tHcy concentrations were 6% higher in Indian Asian men residing in the United Kingdom than in their white European counterparts. Geometric mean tHcy concentrations among Indian Asian men were 10.8 μmol/L. By comparison, geometric mean tHcy concentrations for Bangladeshi men in the current study were 13.5 μmol/L. In 2001, a study of 17 women and 83 men from Pune, India, reported that 77% had plasma tHcy concentrations >15 μmol/L, and that the x̄ ± SD was 23.2 ± 13.1 μmol/L (15). In that population, 38% of whom were vegetarian, 47% had serum cobalamin concentrations <150 pmol/L (15). This finding contrasts with findings in Bangladesh, where vegetarianism is uncommon, and only 11% of our study participants were found to have cobalamin concentrations <150 pmol/L. A limitation of our study is that serum concentrations of methylmalonic acid were not measured, and it is possible that we have underestimated the prevalence of cobalamin deficiency. However, because vegetarianism in our study region is uncommon, we consider gross underestimation to be unlikely. We have ascertained that the prevalence of the common 677C→T polymorphism in methylenetetrahydrofolate reductase in this region is similar or slightly less than that seen in white populations (data not shown), as has been seen in Indian Asians in the United Kingdom (16).
The negative association between cigarette smoking and plasma folate is consistent with other reports in the literature that attribute the observation to differences in dietary intake between smokers and nonsmokers or to oxidatively induced degradation of folate, as has been reported for antioxidant micronutrients, including vitamin C and carotenoids (17). One study, which reported short-term changes in plasma folate concentrations in subjects who had last smoked <1 h before the blood sample was drawn, suggested that smoking had an acute effect (18).
Chewing of betelnut, consisting of betel leaf (Piper betel), areca nut, and slaked lime (calcium hydroxide), is common throughout South Asia and the Pacific (19). Areca nut is thought to be the fourth most commonly used psychoactive substance in the world—exceeded only by caffeine, nicotine, and alcohol—and it has an estimated 600 million users, a number equivalent to 10% of the world’s population (20). Betelnut chewing is a predominant cause of oral cancer, and the principal carcinogenic and genotoxic agents include nitrosamines, reactive oxygen species, and arecoline (20). Although the association between betelnut chewing and oral cancers is well documented, to our knowledge the associations between betelnut use and lower plasma folate concentrations and hyperhomocysteinemia have not previously been reported. These associations are highly significant, even when the analyses are restricted to nonsmokers. Unfortunately, betelnut use was not reported in the aforementioned studies on Asians who reside in the United Kingdom, but it is quite possible that betelnut use accounts for some portion of the excess hyperhomocysteinemia that has been described in those studies.
Although malnutrition is known to be a substantial problem in Bangladesh, deficiencies in the B vitamins have not been rigorously assessed. A report of 861 adolescents living in Rupganj thana, Narayanganj, a rural district that neighbors Araihazar thana (our study site), indicated a high prevalence of malnutrition as assessed by clinical outcomes (21, 22). We estimate a relatively high prevalence of suboptimal folate status, particularly among men, when we used the 95% reference intervals for plasma folate and cobalamin published by Christenson et al (10) from ostensibly healthy blood donors with the use of the same Quantaphase II radioimmunoassay. NHANES III (1988–1994, ie, before mandated folate fortification) used the Quantaphase I radioimmunoassay and found folate values comparable to those of our study among men and women aged 20–29 y (men: 4.9 compared with 4.3 ng/mL; women: 5.7 compared with 5.4 ng/mL, respectively). In contrast, however, they observed an increase in folate with age that was not observed in Bangladesh. Mean folate for all age groups in NHANES III and in Bangladesh was 6.8 and 4.3 ng/mL for men and 7.5 and 5.4 ng/mL for women, respectively (23). Although folate is relatively ubiquitous in the food supply, traditional Bangladeshi methods of food preparation involve prolonged cooking, a process that is known to result in the oxidation of up to 95% of naturally occurring food folates (24). This rural region is not representative of the poorest areas of Bangladesh; thus, we speculate that the nationwide burden of impaired one-carbon metabolism could be substantially higher.
In agreement with several other studies, including NHANES III, men were found to have lower plasma folate concentrations than were women (13, 25, 26), although the reasons for this difference are unclear. The observation that men, on average, have higher plasma tHcy concentrations than do women is well documented and may be attributed in part to steroid hormones. However, the sex differences in tHcy in this Bangladeshi population are quite striking (men: 15.3 ± 9.5 μmol/L; women: 9.5 ± 4.7 μmol/L). Sex differences in tHcy are also related to differences in plasma creatine, and growing evidence indicates that creatine biosynthesis poses a significant drain on the labile methyl pool that is largely underappreciated. In 1975, Mudd and Poole (27) ascertained that the formation of creatine from methylation of guanidinoacetate accounts for ≈75% of all transmethylation reactions. Creatine is a precursor of creatinine, and both are synthesized and circulate at concentrations that are proportional to muscle mass, which is generally greater in men than in women and conspicuously so in Bangladesh. Thus, we speculate that sex differences in muscle mass may account in part for the observation that men have higher tHcy concentrations than do women; that is, higher demands on the methyl pool for creatine biosynthesis may be in competition with the need to remethylate homocysteine to methionine.
It is likewise possible that, under conditions of nutritional deficiency, folate or cobalamin could become somewhat limiting for creatine biosynthesis, which would explain the correlations between urinary creatinine and concentrations of these vitamins observed in Bangladesh. To our knowledge, these correlations have not previously been reported. It is possible that the relation is not apparent in populations in which foods are fortified with folic acid and folate deficiency is less common. The observed association between urinary creatinine and plasma concentrations of folate and cobalamin provides support to the notion that creatine biosynthesis may play an underappreciated role in the regulation of one-carbon metabolism.
In summary, this study shows that a high prevalence of hyperhomocysteinemia exists in Bangladesh. Hyperhomocysteinemia is markedly pronounced among men and is attributable more to folate deficiency than to cobalamin deficiency. This finding is in contrast to reports on Asian Indians residing in the United Kingdom, where hyperhomocysteinemia is more likely due to cobalamin deficiency associated with vegetarian diets. It is quite possible that other factors—eg, renal function, smoking, and betelnut use—also contribute to hyperhomocysteinemia among Bangladeshi men. Little is known about the incidence of NTDs, colon cancer, cardiovascular disease, and other outcomes associated with folate deficiency and hyperhomocysteinemia in Bangladesh. Certainly, assessment of these outcomes warrants further study.
Acknowledgments
We thank Shafiul Alam for overseeing laboratory operations in Araihazar and our staff, fieldworkers, and the study participants in Bangladesh, without whom this work would not be possible.
MVG, HA, PF, and JHG were responsible for the concept and design of the study. XL was responsible for the statistical analyses. VI, VS, and FP were responsible for the collection, shipment, and processing of samples and for the laboratory analyses. None of the authors had a conflict of interest in relation to this study.
Footnotes
Supported by grants from the National Institutes of Health (RO1 ES011601, 5P30ES09089, and 1 P42 ES10349).
References
- 1.Carmel R. Folate deficiency. In: Carmel R, Jacobsen DW, editors. Homocysteine in health and disease. Cambridge, United Kingdom: Cambridge University Press; 2001. pp. 271–88. [Google Scholar]
- 2.Ubbink JB. What is a desirable homocysteine level? In: Carmel R, Jacobson CF, editors. Homocysteine in health and disease. Cambridge, United Kingdom: Cambridge University Press; 2001. pp. 485–90. [Google Scholar]
- 3.Chambers JC, Obeid OA, Refsum H, et al. Plasma homocysteine concentrations and risk of coronary heart disease in UK Indian Asian and European men. Lancet. 2000;355:523–7. doi: 10.1016/S0140-6736(99)93019-2. [DOI] [PubMed] [Google Scholar]
- 4.Williams LJ, Mai CT, Edmonds LD, et al. Prevalence of spina bifida and anencephaly during the transition to mandatory folic acid fortification in the United States. Teratology. 2002;66:33–9. doi: 10.1002/tera.10060. [DOI] [PubMed] [Google Scholar]
- 5.Jacques PF, Selhub J, Bostom AG, Wilson PW, Rosenberg IH. The effect of folic acid fortification on plasma folate and total homocysteine concentrations. N Engl J Med. 1999;340:1449–54. doi: 10.1056/NEJM199905133401901. [DOI] [PubMed] [Google Scholar]
- 6.Michie CA, Chambers J, Abramsky L, Kooner JS. Folate deficiency, neural tube defects, and cardiac disease in UK Indians and Pakistanis. Lancet. 1998;351:1105. doi: 10.1016/s0140-6736(05)79386-7. (letter) [DOI] [PubMed] [Google Scholar]
- 7.Obeid OA, Mannan N, Perry G, Iles RA, Boucher BJ. Homocysteine and folate in healthy east London Bangladeshis. Lancet. 1998;352:1829–30. doi: 10.1016/s0140-6736(05)79892-5. [DOI] [PubMed] [Google Scholar]
- 8.Black RE, Morris SS, Bryce J. Where and why are 10 million children dying every year? Lancet. 2003;361:2226–34. doi: 10.1016/S0140-6736(03)13779-8. [DOI] [PubMed] [Google Scholar]
- 9.Nath I, Reddy KS, Dinshaw KA, et al. Country profile: India. Lancet. 1998;351:1265–75. doi: 10.1016/s0140-6736(98)03010-4. [DOI] [PubMed] [Google Scholar]
- 10.Christenson RH, Dent GA, Tuszynski A. Two radioassays for serum vitamin B12 and folate determination compared in a reference interval study. Clin Chem. 1985;31:1358–60. [PubMed] [Google Scholar]
- 11.Gunter EW, Bowman BA, Caudill SP, Twite DB, Adams MJ, Sampson EJ. Results of an international round robin for serum and whole-blood folate. Clin Chem. 1996;42:1689–94. [PubMed] [Google Scholar]
- 12.Pfeiffer CM, Gunter EW, Caudill SP. Comparison of serum and whole blood folate measurements in 12 laboratories: an international study. Clin Chem. 2001;47(suppl):A62. (abstr) [Google Scholar]
- 13.Selhub J, Jacques PF, Rosenberg IH, et al. Serum total homocysteine concentrations in the third National Health and Nutrition Examination Survey (1991–1994): population reference ranges and contribution of vitamin status to high serum concentrations. Ann Intern Med. 1999;131:331–9. doi: 10.7326/0003-4819-131-5-199909070-00003. [DOI] [PubMed] [Google Scholar]
- 14.Pfeiffer CM, Huff DL, Gunter EW. Rapid and accurate HPLC assay for plasma total homocysteine and cysteine in a clinical laboratory setting. Clin Chem. 1999;45:290–2. [PubMed] [Google Scholar]
- 15.Refsum H, Yajnik CS, Gadkari M, et al. Hyperhomocysteinemia and elevated methylmalonic acid indicate a high prevalence of cobalamin deficiency in Asian Indians. Am J Clin Nutr. 2001;74:233–41. doi: 10.1093/ajcn/74.2.233. [DOI] [PubMed] [Google Scholar]
- 16.Chambers JC, Ireland H, Thompson E, et al. Methylenetetrahydrofolate reductase 677 C→T mutation and coronary heart disease risk in UK Indian Asians. Arterioscler Thromb Vasc Biol. 2000;20:2448–52. doi: 10.1161/01.atv.20.11.2448. [DOI] [PubMed] [Google Scholar]
- 17.Alberg A. The influence of cigarette smoking on circulating concentrations of antioxidant micronutrients. Toxicology. 2002;180:121–37. doi: 10.1016/s0300-483x(02)00386-4. [DOI] [PubMed] [Google Scholar]
- 18.Piyathilake CJ, Macaluso M, Hine RJ, Richards EW, Krumdieck CL. Local and systemic effects of cigarette smoking on folate and vitamin B-12. Am J Clin Nutr. 1994;60:559–66. doi: 10.1093/ajcn/60.4.559. [DOI] [PubMed] [Google Scholar]
- 19.Gupta PC, Ray CS. Epidemiology of betel quid usage. Ann Acad Med Singapore. 2004;33:31–6. [PubMed] [Google Scholar]
- 20.Nair U, Bartsch H, Nair J. Alert for an epidemic of oral cancer due to use of the betel quid substitutes gutkha and pan masala: a review of agents and causative mechanisms. Mutagenesis. 2004;19:251–62. doi: 10.1093/mutage/geh036. [DOI] [PubMed] [Google Scholar]
- 21.Chowdhury S, Shahabuddin AK, Seal AJ, et al. Nutritional status and age at menarche in a rural area of Bangladesh. Ann Hum Biol. 2000;27:249–56. doi: 10.1080/030144600282136. [DOI] [PubMed] [Google Scholar]
- 22.Shahabuddin AK, Talukder K, Talukder MK, et al. Adolescent nutrition in a rural community in Bangladesh. Indian J Pediatr. 2000;67:93–8. doi: 10.1007/BF02726173. [DOI] [PubMed] [Google Scholar]
- 23.Wright JD, Bialostosky K, Gunter EW, et al. Blood folate and vitamin B12: United States, 1988–94. Vital Health Stat 11. 1998:1–78. [PubMed] [Google Scholar]
- 24.Herbert V, Colman N. Folic acid and vitamin B12. In: Shils M, Young VR, editors. Modern nutrition in health and disease. Philadelphia, PA: Lea & Febiger; 1988. pp. 388–416. [Google Scholar]
- 25.Ford ES, Bowman BA. Serum and red blood cell folate concentrations, race, and education: findings from the third National Health and Nutrition Examination Survey. Am J Clin Nutr. 1999;69:476–81. doi: 10.1093/ajcn/69.3.476. [DOI] [PubMed] [Google Scholar]
- 26.Lussier-Cacan S, Xhignesse M, Piolot A, Selhub J, Davignon J, Genest JJ. Plasma total homocysteine in healthy subjects: sex-specific relation with biological traits. Am J Clin Nutr. 1996;64:587–93. doi: 10.1093/ajcn/64.4.587. [DOI] [PubMed] [Google Scholar]
- 27.Mudd SH, Poole JR. Labile methyl balances for normal humans on various dietary regimens. Metabolism. 1975;24:721–35. doi: 10.1016/0026-0495(75)90040-2. [DOI] [PubMed] [Google Scholar]


