Abstract
The S gene of bacteriophage lambda is a late gene required for cell lysis, but unlike the other two lysis genes, R and Rz, it does not code for an endolysin. Earlier studies have shown that the S gene product inhibits respiration and macromolecular synthesis and makes the inner membrane permeable to sucrose. In this study, the effect of the S gene product on a number of Escherichia coli membrane functions (active transport, permeability, respiration, and transhydrogenase and ATPase activity) were measured, and a product of the lambda S gene was identified in the inner membrane fraction by two-dimensional polyacrylamide gel electrophoresis. The results of these experiments indicate that the lambda S product is present in the inner membrane, that it increased the permeability of the membrane for all of the small molecules that were tested, and that its action is reversible. The simplest explanation of these results is that the S gene product forms a hydrophilic pore through the inner membrane, allowing small molecules and lambda lysozyme to pass through.
Full text
PDF

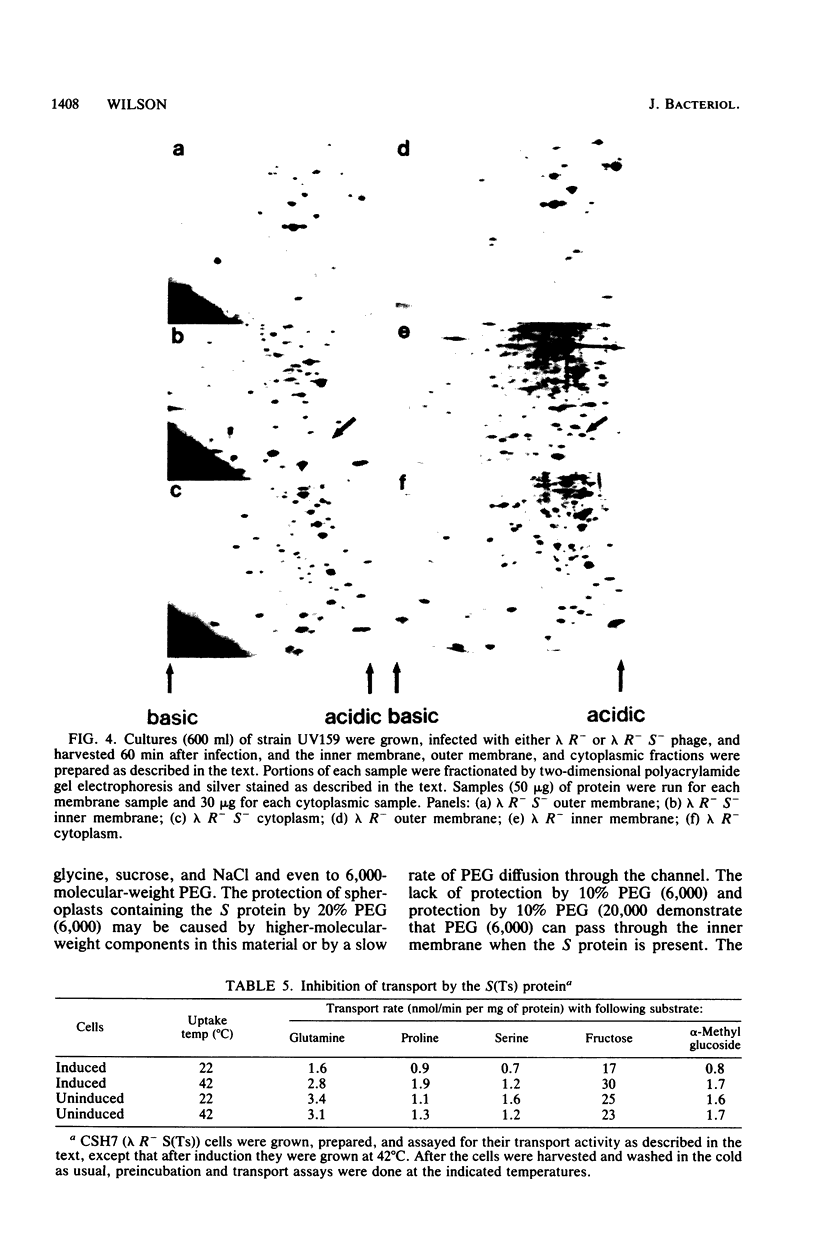


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Nikaido K. Two-dimensional gel electrophoresis of membrane proteins. Biochemistry. 1976 Feb 10;15(3):616–623. doi: 10.1021/bi00648a026. [DOI] [PubMed] [Google Scholar]
- Baker R., Tessman I. The circular genetic map of phage S13. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1438–1445. doi: 10.1073/pnas.58.4.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black L. W., Hogness D. S. The lysozyme of bacteriophage lambda. I. Purification and molecular weight. J Biol Chem. 1969 Apr 25;244(8):1968–1975. [PubMed] [Google Scholar]
- Britton J. R., Haselkorn R. Permeability lesions in male Escherichia coli infected with bacteriophage T7. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2222–2226. doi: 10.1073/pnas.72.6.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J. H., Rolfe B. G. Evidence for a dual control of the initiation of host-cell lysis caused by phage lambda. Mol Gen Genet. 1975 Aug 5;139(1):1–8. doi: 10.1007/BF00267990. [DOI] [PubMed] [Google Scholar]
- Futai M. Reconstitution of transport dependent on D-lactate or glycerol 3-phosphate in membrane vesicles of Escherichia coli deficient in the corresponding dehydrogenases. Biochemistry. 1974 May 21;13(11):2327–2333. doi: 10.1021/bi00708a014. [DOI] [PubMed] [Google Scholar]
- GROMAN N. B., SUZUKI G. QUANTITATIVE STUDY OF ENDOLYSIN SYNTHESIS DURING REPRODUCTION OF LAMBDA PHAGES. J Bacteriol. 1963 Aug;86:187–194. doi: 10.1128/jb.86.2.187-194.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt J. Positive control of endolysin synthesis in vitro by the gene N protein of phage lambda. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3606–3610. doi: 10.1073/pnas.69.12.3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzberg E. L., Hinkle P. C. Oxidative phosphorylation and proton translocation in membrane vesicles prepared from Escherichia coli. Biochem Biophys Res Commun. 1974 May 7;58(1):178–184. doi: 10.1016/0006-291x(74)90908-5. [DOI] [PubMed] [Google Scholar]
- Josslin R. The lysis mechanism of phage T4: mutants affecting lysis. Virology. 1970 Mar;40(3):719–726. doi: 10.1016/0042-6822(70)90216-3. [DOI] [PubMed] [Google Scholar]
- Kaback H. R. Transport across isolated bacterial cytoplasmic membranes. Biochim Biophys Acta. 1972 Aug 4;265(3):367–416. doi: 10.1016/0304-4157(72)90014-7. [DOI] [PubMed] [Google Scholar]
- Lengyel J. A., Calendar R. Control of bacteriophage P2 protein and DNA synthesis. Virology. 1974 Feb;57(2):305–313. doi: 10.1016/0042-6822(74)90170-6. [DOI] [PubMed] [Google Scholar]
- Mukai F., Streisinger G., Miller B. The mechanism of lysis in phage T4-infected cells. Virology. 1967 Nov;33(3):398–404. doi: 10.1016/0042-6822(67)90115-8. [DOI] [PubMed] [Google Scholar]
- Mukherjee P. K., Mandal R. K. Role of S gene of bacteriophage lambda in host lysis. Biochem Biophys Res Commun. 1976 May 3;70(1):302–309. doi: 10.1016/0006-291x(76)91142-6. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Reader R. W., Siminovitch L. Lysis defective mutants of bacteriophage lambda: genetics and physiology of S cistron mutants. Virology. 1971 Mar;43(3):607–622. doi: 10.1016/0042-6822(71)90286-8. [DOI] [PubMed] [Google Scholar]
- Reader R. W., Siminovitch L. Lysis defective mutants of bacteriophage lambda: on the role of the S function in lysis. Virology. 1971 Mar;43(3):623–637. doi: 10.1016/0042-6822(71)90287-x. [DOI] [PubMed] [Google Scholar]
- Rolfe B. G., Campbell J. H. A relationship between tolerance to colicin K and the mechanism of phage-induced host cell lysis. Mol Gen Genet. 1974;133(4):293–297. doi: 10.1007/BF00332705. [DOI] [PubMed] [Google Scholar]
- Sato K. Genetic map of bacteriophage phi80: genes on the right arm. Virology. 1970 Apr;40(4):1067–1069. doi: 10.1016/0042-6822(70)90156-x. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A. Protein composition of the cell wall and cytoplasmic membrane of Escherichia coli. J Bacteriol. 1970 Nov;104(2):890–901. doi: 10.1128/jb.104.2.890-901.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Lerner S. A., Lin E. C. Replacement of a phosphoenolpyruvate-dependent phosphotransferase by a nicotinamide adenine dinucleotide-linked dehydrogenase for the utilization of mannitol. J Bacteriol. 1967 Feb;93(2):642–648. doi: 10.1128/jb.93.2.642-648.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. B. Source of energy for the Escherichia coli galactose transport systems induced by galactose. J Bacteriol. 1974 Nov;120(2):866–871. doi: 10.1128/jb.120.2.866-871.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R., Way J., Way S., Yin J., Syvanen M. Transposition mutagenesis of bacteriophage lambda: a new gene affecting cell lysis. J Mol Biol. 1979 Aug 15;132(3):307–322. doi: 10.1016/0022-2836(79)90262-6. [DOI] [PubMed] [Google Scholar]