Abstract
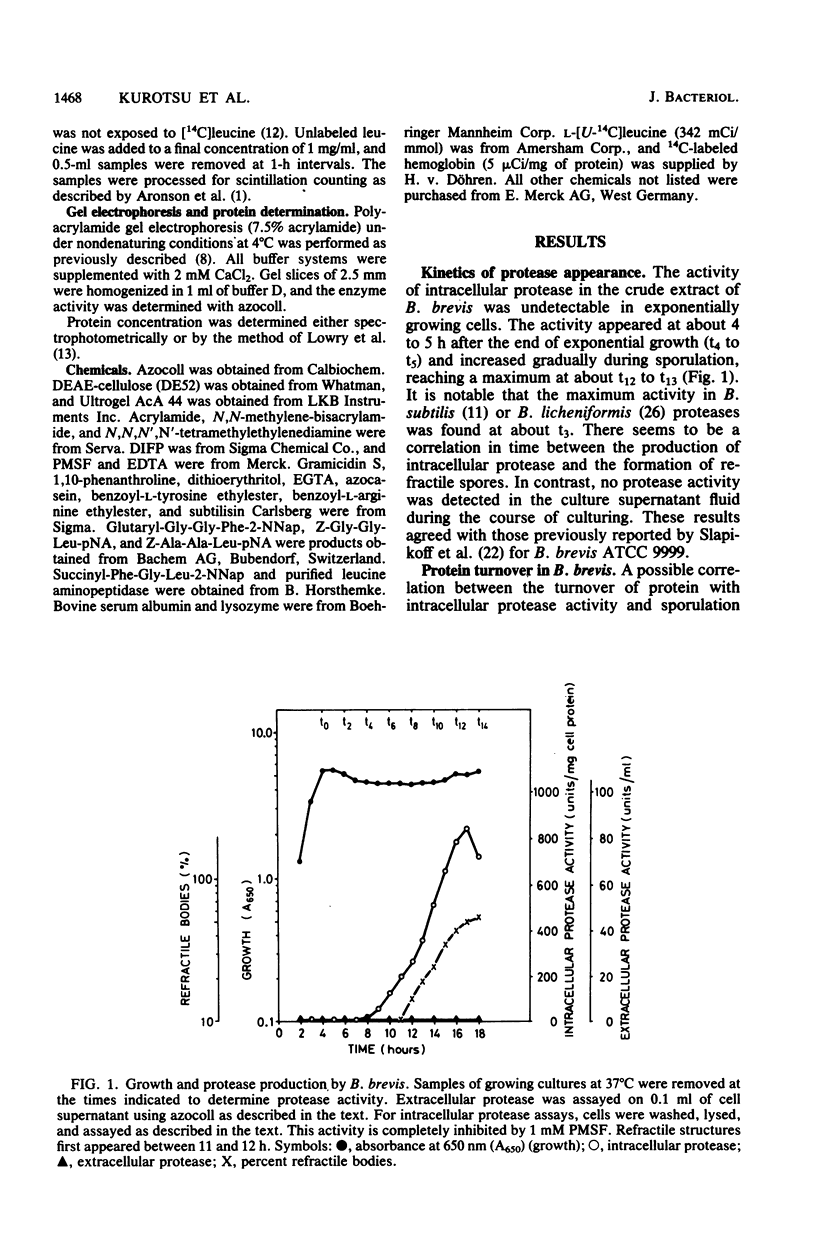
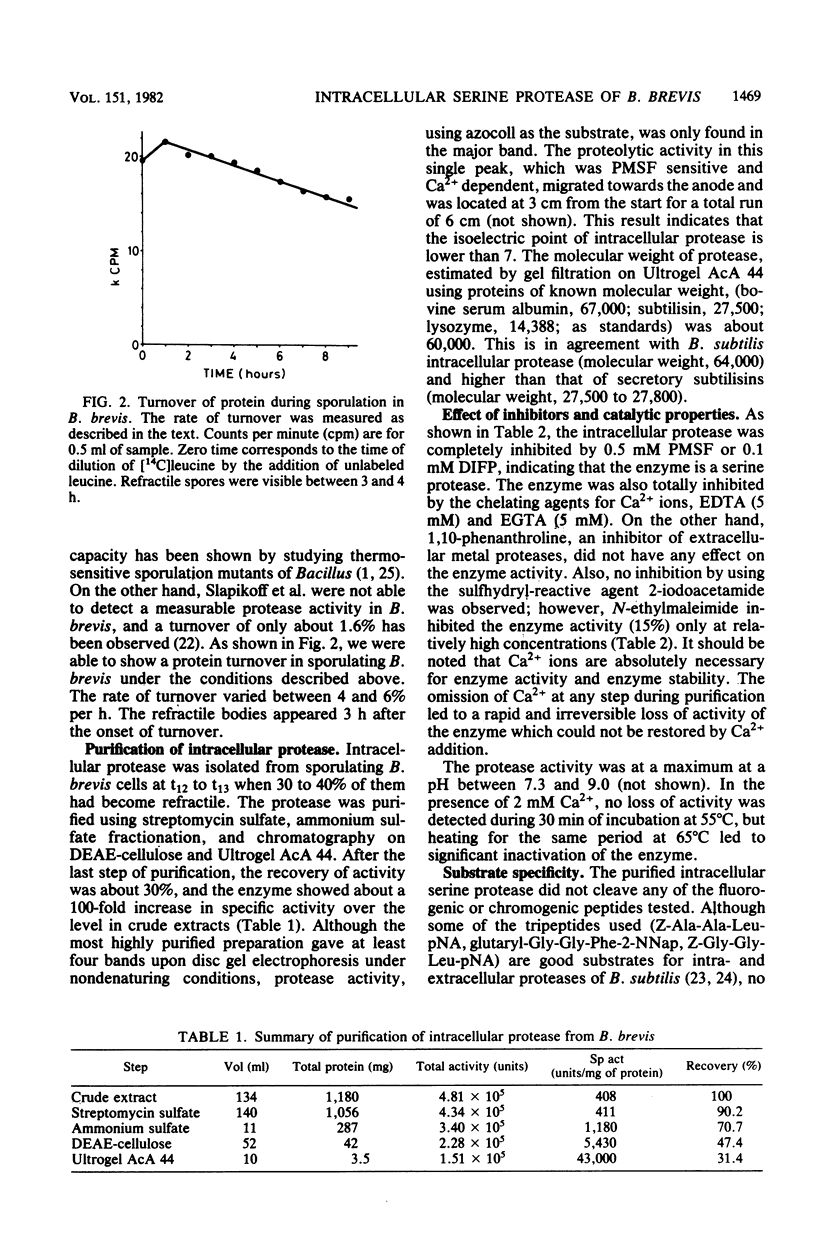
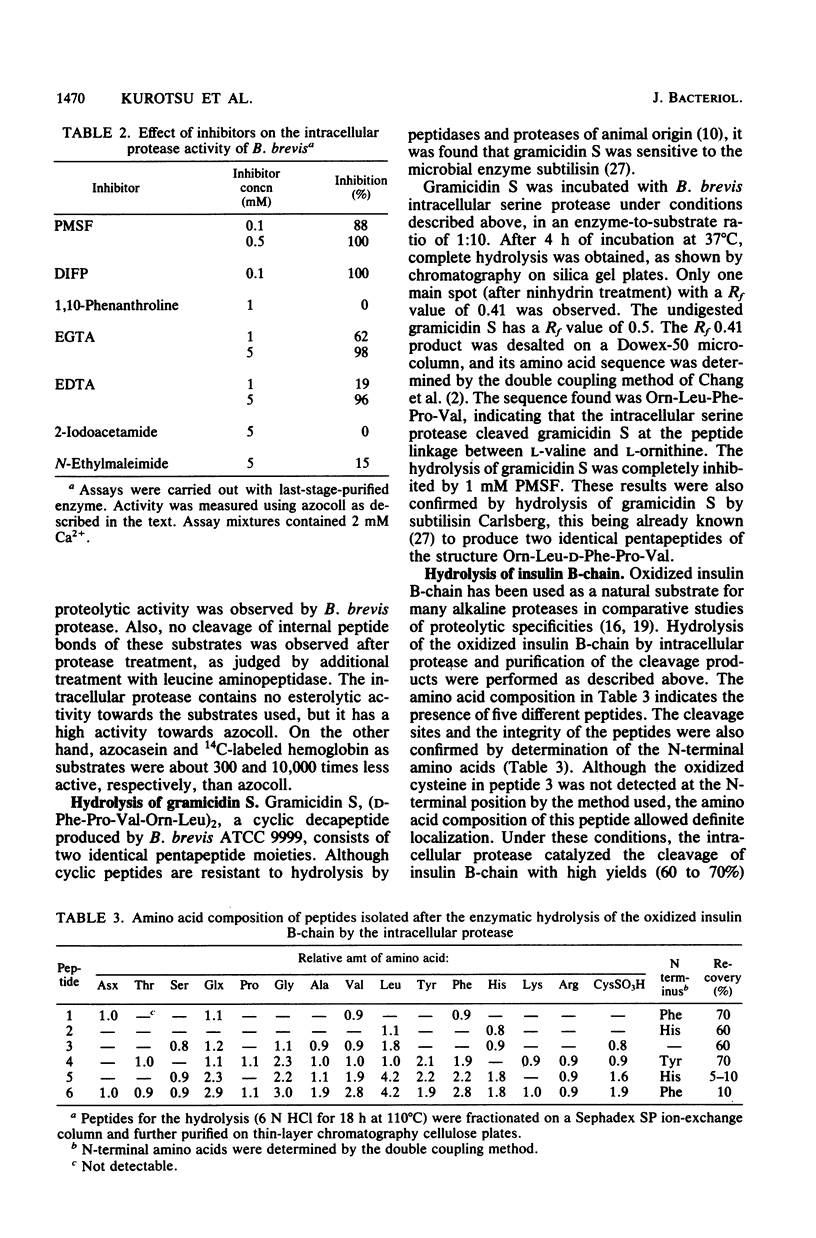
Sporulating cells of Bacillus brevis ATCC 9999 produced a high level of an intracellular serine protease when grown in nutrient medium. The protease activity in the crude extracts of this strain appeared at hour 5 (t5) after the end of exponential growth and increased gradually during sporulation, reaching a maximum at t12 to t13. The enzyme isolated in a partially purified state showed a pH optimum between 7.3 and 9.0 and had an apparent molecular weight of about 60,000. The activity was completely inhibited by phenylmethylsulfonyl fluoride, diisopropyl fluorophosphate, EDTA, and ethylene glycol-bis(beta-aminoethyl ether)-N,N-tetraacetic acid. The protease possessed a high activity for azocoll and low activities for azocasein and 14C-labeled hemoglobin. It cleaved the cyclic decapeptide gramicidin S specifically at the peptide linkage between valine and ornithine and hydrolyzed the oxidized insulin B-chain mainly at peptide bonds 4-5 (Glu-His), 6-7 (Leu-CysSO3H), and 15-16 (Leu-Tyr). No catalysis of bond cleavage by the enzyme on a variety of small peptides or esters was detected. Unlike other Bacillus species, B. brevis ATCC 9999 grown in nutrient medium excreted no extracellular proteases.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson A. I., Angelo N., Holt S. C. Regulation of extracellular protease production in Bacillus cereus T: characterization of mutants producing altered amounts of protease. J Bacteriol. 1971 Jun;106(3):1016–1025. doi: 10.1128/jb.106.3.1016-1025.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. S., Aronson A. I. Alterations of spore coat processing and protein turnover in a Bacillus cereus mutant with a defective postexponential intracellular protease. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1254–1258. doi: 10.1073/pnas.74.3.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond E. P., Starnes W. L., Behal F. J. Aminopeptidases of Bacillus subtilis. J Bacteriol. 1975 Oct;124(1):353–363. doi: 10.1128/jb.124.1.353-363.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- Horsthemke B., Bauer K. Characterization of a nonchymotrypsin-like endopeptidase from anterior pituitary that hydrolyzes luteining hormone-releasing hormone at the tyrosyl-glycine and histidyl-tryptophan bonds. Biochemistry. 1980 Jun 24;19(13):2867–2873. doi: 10.1021/bi00554a008. [DOI] [PubMed] [Google Scholar]
- Katz E., Demain A. L. The peptide antibiotics of Bacillus: chemistry, biogenesis, and possible functions. Bacteriol Rev. 1977 Jun;41(2):449–474. doi: 10.1128/br.41.2.449-474.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjan P., Keryer E., Szulmajster J. Characterization of a thermosensitive sporulation mutant of Bacillus subtilis affected in the structural gene of an intracellular protease. Eur J Biochem. 1979 Aug 1;98(2):353–362. doi: 10.1111/j.1432-1033.1979.tb13194.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marahiel M. A., Danders W., Krause M., Kleinkauf H. Biological role of gramicidin S in spore functions. Studies on gramicidin-S-negative mutants of Bacillus brevis ATCC9999. Eur J Biochem. 1979 Aug 15;99(1):49–55. doi: 10.1111/j.1432-1033.1979.tb13229.x. [DOI] [PubMed] [Google Scholar]
- Millet J. Caractérisation d'une endopeptidase cytoplasmique chez Bacillus megaterium en voie de sporulation. C R Acad Sci Hebd Seances Acad Sci D. 1971 Mar 29;272(13):1806–1809. [PubMed] [Google Scholar]
- Millet J. Characterization of proteinases excreted by Bacillus subtilis Marburg strain during sporulation. J Appl Bacteriol. 1970 Mar;33(1):207–219. doi: 10.1111/j.1365-2672.1970.tb05245.x. [DOI] [PubMed] [Google Scholar]
- Morihara K. Comparative specificity of microbial proteinases. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):179–243. doi: 10.1002/9780470122860.ch5. [DOI] [PubMed] [Google Scholar]
- Murakami K., Voellmy R., Goldberg A. L. Protein degradation is stimulated by ATP in extracts of Escherichia coli. J Biol Chem. 1979 Sep 10;254(17):8194–8200. [PubMed] [Google Scholar]
- Perlman D., Bodanszky M. Biosynthesis of peptide antibiotics. Annu Rev Biochem. 1971;40:449–464. doi: 10.1146/annurev.bi.40.070171.002313. [DOI] [PubMed] [Google Scholar]
- Slapikoff S., Spitzer J. L., Vaccaro D. Sporulation in Bacillus brevis: studies on protease and protein turnover. J Bacteriol. 1971 Jun;106(3):739–744. doi: 10.1128/jb.106.3.739-744.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanov V. M., Strongin A. Y., Izotova L. S., Abramov Z. T., Lyublinskaya L. A., Ermakova L. M., Baratova L. A., Belyanova L. P. Intracellular serine protease from Bacillus subtilis. Structural comparison with extracellular serine proteases-subtilisins. Biochem Biophys Res Commun. 1977 Jul 11;77(1):298–305. doi: 10.1016/s0006-291x(77)80196-4. [DOI] [PubMed] [Google Scholar]
- Strongin A. Y., Izotova L. S., Abramov Z. T., Gorodetsky D. I., Ermakova L. M., Baratova L. A., Belyanova L. P., Stepanov V. M. Intracellular serine protease of Bacillus subtilis: sequence homology with extracellular subtilisins. J Bacteriol. 1978 Mar;133(3):1401–1411. doi: 10.1128/jb.133.3.1401-1411.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitković L., Sadoff H. L. Purification of the extracellular protease of Bacillus licheniformis and its inhibition by bacitracin. J Bacteriol. 1977 Sep;131(3):891–896. doi: 10.1128/jb.131.3.891-896.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yukioka M., Saito Y., Otani S. Enzymatic hydrolysis of gramicidin S. J Biochem. 1966 Sep;60(3):295–302. doi: 10.1093/oxfordjournals.jbchem.a128436. [DOI] [PubMed] [Google Scholar]



