Abstract
Janus kinases (JAKs) are cytoplasmic tyrosine kinases critical for signaling by growth hormone (GH) and many other ligands that bind to members of the cytokine receptor superfamily. SH2-Bβ was previously identified as a JAK2-interacting protein that is tyrosyl phosphorylated in response to GH and other cytokines that activate JAK2. In this study, we examined whether SH2-Bβ alters the activity of JAK2. SH2-Bβ, when coexpressed with JAK2, significantly increased the tyrosyl phosphorylation of JAK2 and multiple other cellular proteins and stimulated the kinase activity of JAK2 by ≈20-fold. Coexpression of SH2-Bβ with JAK2 dramatically increased tyrosyl phosphorylation of signal transducer and activator of transcription (Stat)5B and Stat3, physiological substrates of JAK2. SH2-Bβ(R555E) with a defective Src homology 2 domain was unable to stimulate JAK2 and JAK2-mediated tyrosyl phosphorylation of Stat5B and Stat3. More importantly, SH2-Bβ enhanced GH-induced tyrosyl phosphorylation of endogenous JAK2 and ligand-induced tyrosyl phosphorylation of Stat5B by endogenous JAK2. In contrast, SH2-Bβ did not potentiate the activation of other tyrosine kinases including the receptors for platelet-derived growth factor, epidermal growth factor, or nerve growth factor (TrkA), tyrosine kinases that also bind SH2-Bβ. These data demonstrate that SH2-Bβ is a potent cytoplasmic activator of JAK2 and is thereby expected to be an important cellular regulator of signaling by GH and other hormones and cytokines that activate JAK2.
Members of the Janus kinase (JAK) family of tyrosine kinases (JAK1, JAK2, JAK3, and Tyk2) play a critical role in cellular responses to growth hormones (GH) and the many other ligands that bind to the receptors in the cytokine receptor superfamily (1). On ligand binding, one or more of the JAKs is activated, which in turn phosphorylates multiple cellular proteins, including the JAKs themselves, the associated cytokine receptors, and the latent cytoplasmic transcription factors, designated signal transducers and activators of transcription (Stats) (2–5). Seven Stats (Stat1, 2, 3, 4, 5A, 5B, and 6) have been described to date. On phosphorylation by JAKs of the conserved tyrosine in their C termini, Stats form either homo- or heterodimers, accumulate in the nucleus, bind to their response elements in the promoter of their target genes, and activate the expression of their target genes (4, 5). This JAK/Stat pathway plays a pivotal role in the cellular actions of cytokines, which activates JAKs (1, 6). For example, both GH and prolactin activate JAK2 (7–9). The activated JAK2 then tyrosyl phosphorylates and activates Stat1, 3, 5A, and 5B, which in turn stimulate the expression of a variety of target genes (10–14). Stat5B is required for many actions of GH (15, 16), whereas Stat5A plays a pivotal role in the action of prolactin (16, 17).
Of the 25 or so ligands known to activate JAKs via members of the cytokine receptor superfamily, more than two-thirds activate JAK2. These include GH, prolactin, erythropoietin, granulocyte colony-stimulating factor, IL-3, IL-5, IL-12, granulocyte macrophage colony-stimulating factor (GM-CSF), leptin, thrombopoietin, IFN-γ, and ligands whose receptor includes gp130 (IL-6, oncostatin M, leukemia inhibitory factor, IL-11, cardiotropin, and ciliary neurotrophic factor). Some of these ligands show a marked preference and/or requirement for JAK2, including GH (7), prolactin, erythropoietin, IFN-γ, IL-3, thrombopoietin, IL-5, and GM-CSF (2, 18). Deletion of JAK2 by gene disruption causes embryonic lethality (18, 19), indicating that JAK2 is fundamental for animal development. JAK2-deficient mice lack definitive erythropoiesis in fetal liver, presumably because of a deficiency in response to critical cytokines such as erythropoietin and thrombopoietin (18, 19). Taken together, these findings indicate that JAK2 plays an essential role in many body functions. Thus, understanding the mechanisms by which JAK2 is regulated is of vital importance.
We previously identified SH2-Bβ, an Src homology 2 (SH2) and pleckstrin homology (PH) domain-containing protein, as a JAK2-interacting protein (20). Three splicing variants of SH2-B, designated α, β, and γ, have been described to date (20–23) (K. Nelms, personal communication). The three splicing variants of SH2-B have an identical N-terminal portion of 631 amino acids, which contains a PH domain, an SH2 domain, and multiple proline-rich motifs (20–23) (K. Nelms, personal communication). SH2-Bβ binds via its SH2 domain to tyrosyl-phosphorylated JAK2 and serves as a substrate of JAK2 (20). In this study, we show that overexpression of SH2-Bβ dramatically stimulates the kinase activity of JAK2, resulting in a significant increase of tyrosyl phosphorylation of JAK2 and multiple other cellular proteins. Overexpression of SH2-Bβ also enhances GH-induced tyrosyl phosphorylation of endogenous JAK2. The SH2 domain of SH2-Bβ is required for the stimulatory effect of SH2-Bβ on the kinase activity of JAK2. Finally, SH2-Bβ stimulates JAK2-mediated tyrosyl phosphorylation of Stat3 and Stat5B and enhances GH-induced tyrosyl phosphorylation of Stat5B by endogenous JAK2. Our data provide strong evidence that SH2-Bβ is a potent cytoplasmic activator of JAK2 and enhances cytokine signaling via JAK2/Stat pathways.
MATERIALS AND METHODS
Reagents.
Recombinant human GH was a gift of Eli Lilly. Murine epidermal growth factor (EGF) was from Collaborative Biomedical Products (Bedford, MA). Recombinant protein A-agarose was from Repligen. Aprotinin, leupeptin, and Triton X-100 were purchased from Boehringer Mannheim. Enhanced chemiluminescence detection system and [γ-32P]-ATP were from Amersham. Polyclonal antibodies to SH2-Bβ (αSH2-B) were raised against glutathione S-transferase fusion proteins containing the C terminus of SH2-Bβ (20). Anti-JAK2 antiserum (αJAK2) was raised in rabbits against a synthetic peptide corresponding to amino acids 758–776 (24) and was used at a dilution of 1:500 for immunoprecipitation and 1:15,000 for immunoblotting. Monoclonal antiphosphotyrosine antibody 4G10 (αPY) was purchased from Upstate Biotechnology (Lake Placid, NY) and was used at a dilution of 1:7,500 for immunoblotting. Monoclonal antibody against Myc-tag (αMyc, 9E10), anti-Stat3, and anti-Stat5B were from Santa Cruz Biotechnology and were used at a dilution of 1:100 for immunoprecipitation and 1:1,000 (αMyc and αStat3) or 1:5,000 (αStat5B) for immunoblotting. Monoclonal antiphosphoStat5 (αpStat5) was from Zymed and was used at 1 μg/ml in immunoblotting.
Plasmids.
cDNAs encoding both wild-type murine JAK2 and mutant murine JAK2 in which the critical lysine in the ATP-binding domain is mutated to glutamate (K882E) were previously cloned into a mammalian expression vector and were provided to us by J. Ihle and B. Witthuhn (St. Jude Children’s Research Hospital, Memphis, TN). The cDNA encoding full-length rat SH2-Bβ with a Myc-tag at its N terminus was cloned into a mammalian expression vector. Arg-555 in SH2-Bβ was mutated to Glu by using the QuickChange site-directed mutagenesis kit (Stratagene). The sequence of the primer (sense) used in site-directed mutagenesis is: 5′-GTCTTCTTGGTAGAACAGAGTGAGACAAGA-3′. The mutation was verified by DNA sequencing (Sequenase 2.0; United States Biochemical). Rat GH receptor (GHR) cDNA was from G. Norstedt (Karolinska Institute, Stockholm) (25). Expression vector encoding platelet-derived growth factor (PDGF) receptor β subunit was provided by A. Kazlauskas (Harvard University, Cambridge, MA). Plasmids encoding Stat3 (26) and Stat5B were from R. Jove (University of South Florida College of Medicine, Tampa, FL) and L. Yu-Lee (Baylor College of Medicine, Waco, TX), respectively.
Cell Culture, Transfection, and Lysis.
COS cells were grown in DMEM (supplemented with 1 mM l-glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin, 0.25 mg/ml amphotericin, and 10% FCS). 293T cells were grown in DMEM (supplemented as above and with 1 μM sodium pyruvate). Cells were transiently transfected by calcium phosphate precipitation (27) and assayed 48 hr after transfection.
Cells were deprived of serum overnight in DMEM supplemented with 1 mM l-glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin, 0.25 mg/ml amphotericin, and 1% BSA. The deprived cells were treated for various times with or without GH at 37°C, then rinsed three times with 10 mM sodium phosphate, pH 7.4/150 mM NaCl/1 mM Na3VO4. Cells were solubilized in lysis buffer (50 mM Tris, pH 7.5/0.1% Triton X-100/150 mM NaCl/2 mM EGTA/1 mM Na3VO4/1 mM phenylmethylsulfonyl fluoride/10 μg/ml aprotinin/10 μg/ml leupeptin) and centrifuged at 14,000 × g for 10 min at 4°C. The supernatant (cell lysate) was used for immunoprecipitation and immunoblotting. Proteins in cell lysates were quantified by using BCA* Protein Assay Reagent (Pierce).
Immunoprecipitation and Immunoblotting.
Cell lysates were incubated on ice with the indicated antibody for 2 hr. The immune complexes were collected on protein A-agarose (50 μl) during 1-hr incubation at 4°C. The beads were washed three times with washing buffer (50 mM Tris, pH 7.5/0.1% Triton X-100/150 mM NaCl/2 mM EGTA) and boiled for 5 min in a mixture (80:20) of lysis buffer and SDS/PAGE sample buffer (250 mM Tris⋅HCl, pH 6.8/10% SDS/10% β-mercaptoethanol/40% glycerol/0.01% bromophenol blue). The solubilized proteins were separated by SDS/PAGE (5–12% gradient gel unless noted otherwise) followed by immunoblotting with the indicated antibody by using the enhanced chemiluminescence detection system.
In Vitro Kinase Assay.
JAK2 was immunoprecipitated with αJAK2 from COS cells coexpressing JAK2 with SH2-Bβ, SH2-Bβ(R555E), or control expression vector. After being washed twice with lysis buffer and twice with kinase buffer (50 mM Hepes, pH 7.6/5 mM MgCl2/5 mM MnCl2/0.5 mM DTT/100 mM NaCl/1 mM Na3VO4), αJAK2 immunoprecipitates were incubated at 30°C for 30 min in 50 μl of kinase buffer supplemented with 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 10 μCi [γ-32P]-ATP. After the in vitro kinase assay, proteins in the reaction mixture were resolved by SDS/PAGE and visualized by autoradiography.
For quantification, autoradiographs or immunoblots were scanned by using an Agfa ArcusII scanner and fotolook sa software (Agfa, Mortsel, Belgium). The resulting image was analyzed by using molecular analyst image analysis software from Bio-Rad.
RESULTS
SH2-Bβ Stimulates Tyrosyl Phosphorylation of JAK2 and Multiple Other Cellular Proteins.
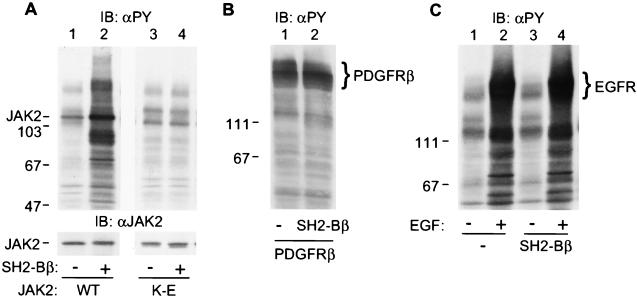
We have shown previously that SH2-Bβ directly interacts with activated tyrosyl-phosphorylated JAK2 via the SH2 domain of SH2-Bβ (20). To determine whether SH2-Bβ regulates the kinase activity of JAK2, we first examined whether overexpression of SH2-Bβ alters tyrosyl phosphorylation of JAK2, because tyrosyl phosphorylation of JAK2 is thought to be a consequence of autophosphorylation and thus an indicator of JAK2 activity (7, 28). JAK2 was transiently coexpressed with SH2-Bβ in COS cells. Proteins in the cell lysates were resolved by SDS/PAGE and immunoblotted with antibody to phosphotyrosine. Consistent with our previous report (20), JAK2 was constitutively activated and phosphorylated on tyrosines when overexpressed in COS cells (Fig. 1A Upper, lane 1). Coexpression of SH2-Bβ with JAK2 dramatically increased tyrosyl phosphorylation of JAK2 as well as multiple other cellular proteins (Fig. 1A Upper, lane 2). This SH2-Bβ-induced increase in tyrosyl phosphorylation is not caused by a difference in level of JAK2 expression, because an equal amount of JAK2 was expressed in cells transfected with either control plasmid or plasmid encoding SH2-Bβ (Fig. 1A Lower, lanes 1 and 2). Tyrosyl phosphorylation of cellular proteins depended on the amount of SH2-Bβ cDNA used in the transfection. By using 2 μg JAK2 cDNA, it was detectable at 0.1 μg SH2-Bβ cDNA and reached a maximal level at 5 μg (data not shown). It also depended on active JAK2, because overexpression of SH2-Bβ alone (Fig. 1C, lane 1 vs. 3) or with kinase-inactive JAK2 (Fig. 1A, lanes 3 and 4) did not cause an increase in tyrosyl phosphorylation of cellular proteins. These results suggest that SH2-Bβ stimulates the kinase activity of JAK2, resulting in increased tyrosyl phosphorylation of JAK2 and multiple other cellular proteins.
Figure 1.
SH2-Bβ stimulates tyrosyl phosphorylation of JAK2 and multiple other cellular proteins. (A) COS cells were cotransfected with plasmid (4 μg) encoding wild-type (lanes 1 and 2) or kinase-inactive JAK2 (lanes 3 and 4) and either control plasmid (10 μg) or plasmid (10 μg) encoding SH2-Bβ. After 48 hr, cells were lysed in lysis buffer. Proteins (40 μg) in the lysates were resolved by SDS/PAGE and immunoblotted with αPY (Upper) and reprobed with αJAK2 (Lower). (B) COS cells were cotransfected with plasmid (5 μg) encoding the β subunit of PDGF receptor with control plasmid (10 μg) or plasmid (10 μg) encoding SH2-Bβ. Proteins (40 μg) in the cell lysates were resolved by SDS/PAGE and immunoblotted with αPY. (C) COS cells were transfected with either control plasmid (10 μg) or plasmid (10 μg) encoding SH2-Bβ. Twenty-four hours after transfection, cells were deprived of serum overnight and stimulated with 100 ng/ml EGF for 10 min. Proteins (40 μg) in the lysates were resolved by SDS/PAGE and immunoblotted with αPY. The migration of molecular weight standards (×10−3) is indicated.
Because SH2-Bβ binds to multiple tyrosine kinases including receptors for nerve growth factor (NGF) (29, 30), PDGF (22), and EGF (L.R. and C.C.-S., unpublished data), we examined whether SH2-Bβ also stimulates tyrosyl phosphorylation of these receptor tyrosine kinases. PDGF receptor β subunit was coexpressed with SH2-Bβ in COS cells, and cell lysates were immunoblotted with antibody to phosphotyrosine. When overexpressed, PDGF receptor β subunit was autoactivated and autophosphorylated on tyrosines (Fig. 1B and data not shown). In contrast to what was observed with JAK2, SH2-Bβ did not stimulate tyrosyl phosphorylation of PDGF receptor β subunit or of any other cellular proteins (Fig. 1B). Similarly, SH2-Bβ overexpressed in COS cells did not enhance EGF-induced tyrosyl phosphorylation of endogenous EGF receptor or of other cellular proteins (Fig. 1C, lanes 2 and 4), nor did it enhance NGF-induced tyrosyl phosphorylation of the NGF receptor TrkA when SH2-Bβ was stably overexpressed in PC12 cells (29). These results suggest that the stimulatory action of SH2-Bβ on kinase activity is relatively specific to JAK2.
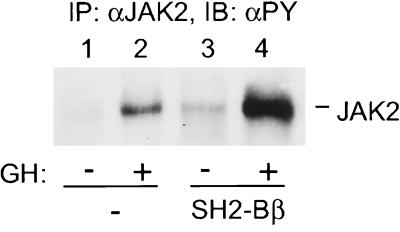
To address whether SH2-Bβ potentiates ligand-induced activation of endogenous JAK2, SH2-Bβ was coexpressed in COS cells with rat GH receptor (GHR). GH binding to GHR is known to activate JAK2. The activated JAK2 is thought to phosphorylate GHR, JAK2 itself, and multiple other cellular proteins, including SH2-Bβ, thereby initiating the multiple signaling pathways important for GH action (7, 20, 31–34). Cells were treated with 500 ng/ml GH for 10 min, and JAK2 was immunoprecipitated with antibody to JAK2 and immunoblotted with antibody to phosphotyrosine. Overexpression of SH2-Bβ enhanced GH-induced tyrosyl phosphorylation of endogenous JAK2 by more than 3-fold (Fig. 2, lanes 2 and 4), suggesting that SH2-Bβ is a potent enhancer of GH-induced activation of JAK2.
Figure 2.
SH2-Bβ potentiates GH-induced tyrosyl phosphorylation of endogenous JAK2. COS cells were cotransfected with plasmid (5 μg) encoding GHR and either control plasmid (10 μg) or plasmid (10 μg) encoding SH2-Bβ. Twenty-four hours after transfection, cells were deprived of serum overnight and stimulated with 500 ng/ml GH for 10 min. Proteins (700 μg) in the lysates were immunoprecipitated with αJAK2 and immunoblotted with αPY. Levels of endogenous JAK2 in COS cells are too low to be detected by αJAK2 in immunoblots.
The SH2 Domain of SH2-Bβ Is Required for SH2-Bβ-Induced Tyrosyl Phosphorylation of JAK2 and JAK2-Dependent Phosphorylation of Other Cellular Proteins.
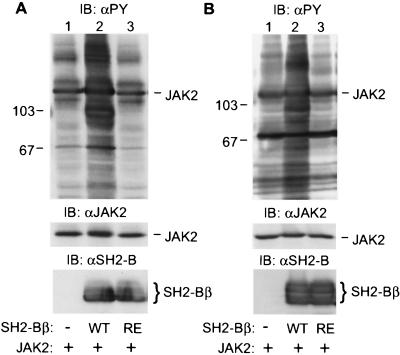
Because the SH2 domain of SH2-Bβ mediates the interaction of SH2-Bβ with tyrosyl-phosphorylated JAK2 (20), we examined whether the SH2 domain of SH2-Bβ is required for its stimulatory effect on JAK2. Within SH2-Bβ, the critical Arg-555 within the FLVR motif required for binding of SH2 domains to phosphorylated tyrosines was replaced with Glu. SH2-Bβ(R555E) does not bind to tyrosyl-phosphorylated PDGF receptor (22) or TrkA (29) and has a dramatically reduced affinity for tyrosyl-phosphorylated JAK2 compared with wild-type SH2-Bβ (L.R. and C.C.-S., unpublished data). Overexpression of SH2-Bβ with JAK2 in either COS cells (Fig. 3A, lane 2) or 293T cells (Fig. 3B, lane 2) increased dramatically tyrosyl phosphorylation of JAK2 and multiple other cellular proteins, indicating that SH2-Bβ-induced activation of JAK2 is not specific to COS cells. In contrast, overexpression of SH2-Bβ(R555E) did not promote tyrosyl phosphorylation of JAK2 or of other cellular proteins in either COS cells (Fig. 3A, lane 3) or 293T cells (Fig. 3B, lane 3), although equal amounts of wild-type SH2-Bβ and SH2-Bβ(R555E) were expressed (Fig. 3A Bottom, lanes 2 and 3; Fig. 3B Bottom, lanes 2 and 3). These results indicate that the SH2 domain of SH2-Bβ is crucial for the stimulatory effect of SH2-Bβ on JAK2.
Figure 3.
The SH2 domain of SH2-Bβ is required for SH2-Bβ-induced tyrosyl phosphorylation of JAK2 and multiple other cellular proteins. (A) COS cells were cotransfected with plasmid (4 μg) encoding JAK2 and either control plasmid (10 μg) or plasmid (10 μg) encoding SH2-Bβ or SH2-Bβ(R555E). After 48 hr, cells were lysed, and proteins (40 μg) in the lysates were resolved by SDS/PAGE, immunoblotted with αPY (Top), and reprobed with αJAK2 (Middle). In a parallel experiment, proteins (40 μg) in the lysates were immunoblotted with αSH2-B (Bottom). (B) 293T cells were cotransfected with plasmid (4 μg) encoding JAK2 and either control plasmid (10 μg) or plasmid (10 μg) encoding SH2-Bβ or SH2-Bβ(R555E). After 48 hr, cells were lysed, and proteins (40 μg) in the lysates were resolved by SDS/PAGE and immunoblotted with αPY. In parallel experiments, proteins (40 μg) in cell lysates were immunoblotted with αJAK2 (Middle) or αSH2-B (Bottom). The migration of molecular weight standards (×10−3) (Top) is indicated.
SH2-Bβ Stimulates the Kinase Activity of JAK2.
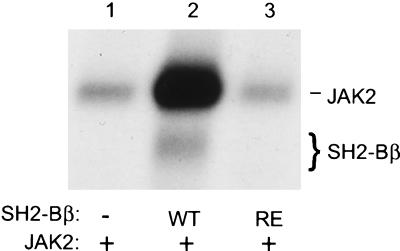
To provide more direct evidence that SH2-Bβ stimulates the kinase activity of JAK2, wild-type SH2-Bβ or SH2-Bβ(R555E) was coexpressed with JAK2 in COS cells, JAK2 was immunoprecipitated with antibody to JAK2, and the kinase activity of JAK2 was assessed by an in vitro kinase assay by using [γ-32P]-ATP. Coexpression of wild-type SH2-Bβ with JAK2 stimulated the autophosphorylation of JAK2 by more than 20-fold (n = 3) (Fig. 4, lanes 1 and 2). SH2-Bβ coimmunoprecipitated with JAK2 and was phosphorylated in vitro by JAK2 (Fig. 4, lane 2). As expected, coexpression of SH2-Bβ(R555E) with JAK2 did not stimulate the kinase activity of JAK2 (Fig. 4, lane 3). These results show that SH2-Bβ is a strong activator of JAK2.
Figure 4.
SH2-Bβ stimulates the kinase activity of JAK2. COS cells were cotransfected with plasmid (4 μg) encoding JAK2 and either control plasmid (10 μg) or plasmid (10 μg) encoding SH2-Bβ or SH2-Bβ(R555E). Forty-eight hours after transfection, proteins (600 μg) in the cell lysates were immunoprecipitated with αJAK2. αJAK2 immunoprecipitates were subjected to an in vitro kinase assay followed by autoradiography.
SH2-Bβ Stimulates JAK2-Mediated Tyrosyl Phosphorylation of Stat5B and Stat3.
Because SH2-Bβ stimulates JAK2, we hypothesized that SH2-Bβ would also stimulate phosphorylation of JAK2 substrates. To test this hypothesis, Stat5B was coexpressed in COS cells with JAK2 and either SH2-Bβ or SH2-Bβ(R555E). Stat5B was immunoprecipitated with antibody to Stat5B and immunoblotted with antibody to phosphotyrosine. Expression of wild-type SH2-Bβ significantly enhanced (≈3-fold) tyrosyl phosphorylation of Stat5B (Fig. 5A Upper, lanes 1 and 2). In contrast, overexpression of SH2-Bβ(R555E) did not increase tyrosyl phosphorylation of Stat5B (Fig. 5A Upper, lane 3). Immunoblotting with antibody to Stat5B revealed a similar level of Stat5B expression in the different cells (Fig. 5A Lower). Coexpression of wild-type SH2-Bβ also reduced significantly the mobility of Stat5B (Fig. 5A Lower, lane 2). The multiple forms of Stat5B have been shown to be caused by differential phosphorylation of Stat5B on serines/threonines and tyrosines (35). When Stat5B was coexpressed with kinase-inactive JAK2, tyrosyl phosphorylation of Stat5B was not detectable even when wild-type SH2-Bβ was coexpressed (Fig. 5A, lane 5), suggesting that SH2-Bβ stimulation of tyrosyl phosphorylation of Stat5B requires kinase-active JAK2.
Figure 5.
SH2-Bβ stimulates tyrosyl phosphorylation of Stat5B and Stat3 via JAK2. (A) COS cells were cotransfected with plasmids encoding Stat5B (5 μg), wild-type (WT) (0.5 μg) or kinase-inactive (K-E) (0.5 μg) JAK2 and SH2-Bβ (WT) (6 μg), or SH2-Bβ(R555E) (RE) (6 μg), as indicated. Twenty-four hours after transfection, cells were deprived of serum overnight. Stat5B was immunoprecipitated with αStat5B, immunoblotted with αPY (Upper), and reprobed with αStat5B (Lower). (B) 293T cells were cotransfected with plasmids encoding Stat3 (5 μg), wild-type (0.5 μg) or kinase-inactive (0.5 μg) JAK2 and SH2-Bβ (6 μg), or SH2-Bβ(R555E) (6 μg) as indicated. Twenty-four hours after transfection, cells were deprived of serum overnight. Stat3 was immunoprecipitated with αStat3, immunoblotted with αPY (Upper), and reprobed with αStat3 (Lower).
To provide further evidence that SH2-Bβ stimulates phosphorylation of JAK2 substrates, we examined the effect of SH2-Bβ overexpression on tyrosyl phosphorylation of Stat3. As with Stat5B, Stat3 was tyrosyl phosphorylated when coexpressed in 293T cells with wild-type JAK2 (Fig. 5B, lane 1), but not with kinase-inactive JAK2 (Fig. 5B, lane 4). Coexpression of wild-type SH2-Bβ with JAK2 and Stat3 increased JAK2-mediated tyrosyl phosphorylation of Stat3 by more than 4-fold (Fig. 5B, lane 2). SH2-Bβ(R555E) only slightly increased tyrosyl phosphorylation of Stat3 (Fig. 5B, lane 3). Taken together, our data show that SH2-Bβ stimulates JAK2-mediated tyrosyl phosphorylation of Stat proteins and that the SH2 domain of SH2-Bβ is critical for that phosphorylation.
SH2-Bβ Enhances GH-Induced Tyrosyl Phosphorylation of Stat5B.
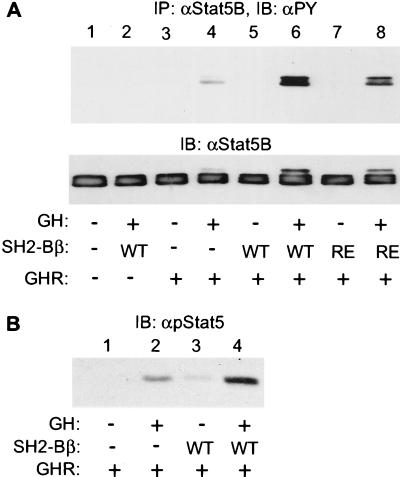
From the above results, we predicted that SH2-Bβ would stimulate ligand-induced signaling events downstream of JAK2. We therefore examined whether SH2-Bβ stimulates ligand-induced tyrosyl phosphorylation of Stat5B. Stat5B was coexpressed in COS cells with GHR and either wild-type SH2-Bβ or SH2-Bβ(R555E). Cells were stimulated with 50 ng/ml GH for 15 min. Stat5B was immunoprecipitated with antibody to Stat5B and immunoblotted with antibody to phosphotyrosine. As reported previously (35), GH stimulated tyrosyl phosphorylation of Stat5B, presumably via endogenous JAK2 (Fig. 6A, lanes 3 and 4). Coexpression of SH2-Bβ increased GH-induced tyrosyl phosphorylation of Stat5B by more than 5-fold (n = 3) (Fig. 6A, lanes 5 and 6). Interestingly, coexpression of SH2-Bβ(R555E) also slightly but reproducibly increased tyrosyl phosphorylation of Stat5B (Fig. 6A, lanes 7 and 8), suggesting that SH2-Bβ may have a second function in GH signaling other than simply activating JAK2. Without exogenous coexpression of GHR, GH was unable to stimulate tyrosyl phosphorylation of Stat5B (Fig. 6A, lanes 1 and 2), indicating that GH-induced tyrosyl phosphorylation of Stat5B is mediated by GHR. We also examined whether overexpression of SH2-Bβ enhances GH-induced phosphorylation of Stat5B on Tyr-699 that has been shown to be phosphorylated in response to cytokines and is required for the nuclear translocation and transcriptional activation of Stat5B (4, 36). Proteins from cell lysates were immunoblotted with antibody that specifically recognizes Stat5B phosphorylated on Tyr-699. Overexpression of SH2-Bβ increased the amount of Stat5B phosphorylated on Tyr-699 in response to GH (Fig. 6B, lane 2 vs. 4). SH2-Bβ also enhanced GH-induced tyrosyl phosphorylation of Stat5A and prolactin-induced tyrosyl phosphorylation of Stat5B and Stat3 (data not shown). These results indicate that SH2-Bβ stimulates JAK2/Stat pathways in response to ligands that activate JAK2.
Figure 6.
SH2-Bβ enhances GH-induced tyrosyl phosphorylation of Stat5B. (A) COS cells were cotransfected with plasmids encoding GHR (5 μg), Stat5B (5 μg) and SH2-Bβ (WT) (6 μg), or SH2-Bβ(R555E) (RE) (6 μg), as indicated. Twenty-four hours after transfection, cells were deprived of serum overnight and treated with 50 ng/ml GH for 15 min. Stat5B was immunoprecipitated with αStat5B, immunoblotted with αPY (Upper), and reprobed with αStat5B (Lower). (B) COS cells were cotransfected with plasmids encoding GHR (5 μg), Stat5B (5 μg), and SH2-Bβ (6 μg), as indicated. Twenty-four hours after transfection, cells were deprived of serum overnight and treated with 50 ng/ml GH for 15 min. Proteins (40 μg) in the lysates were resolved by SDS/PAGE and immunoblotted with antiphosphoStat5 (αpStat5).
DISCUSSION
In this study, we report that SH2-Bβ is a potent activator of JAK2 and potentiates cytokine-induced activation of JAK2/Stat pathways. These conclusions are supported by multiple findings. First, SH2-Bβ has been shown to interact directly with JAK2 in response to GH at endogenous levels of GHR, JAK2, and SH2-Bβ (20). Second, coexpression of SH2-Bβ dramatically increases tyrosyl phosphorylation of ectopically expressed JAK2 and many other cellular proteins. Third, SH2-Bβ stimulates the kinase activity of JAK2 by more than 20-fold when coexpressed with JAK2. Fourth, overexpression of SH2-Bβ increases GH-induced tyrosyl phosphorylation of endogenous JAK2. Fifth, SH2-Bβ enhances JAK2-mediated tyrosyl phosphorylation of Stat5B and Stat3, physiological substrates of JAK2. Sixth, SH2-Bβ significantly enhances GH- and prolactin-induced tyrosyl phosphorylation of Stat5B, Stat5A, and Stat3 by endogenous JAK2. In support of an activating role of SH2-Bβ on JAK2, transient overexpression of SH2-Bβ in 3T3-F442A cells, which express endogenous GHR and JAK2, strongly potentiates GH-induced changes in cellular morphology (J. Herrington, L.R., M. Diakonova, and C.C.-S., unpublished work). In combination, these results indicate that SH2-Bβ is a potent activator of JAK2 that plays an important role in the regulation of the JAK2 and its downstream signaling pathways, including those that involve Stat proteins.
The stimulatory effect of SH2-Bβ on the kinase activity of JAK2 requires the SH2 domain of SH2-Bβ. The SH2 domain of SH2-B also appears to be sufficient to activate JAK2 (L.R. and C.C.-S., unpublished work). The SH2 domain of SH2-Bβ was shown previously to be essential for the high-affinity interaction of SH2-Bβ with activated JAK2 and other tyrosyl-phosphorylated tyrosine kinases including PDGF receptor and TrkA (20, 22, 29). These results suggest that high-affinity binding of SH2-Bβ via its SH2 domain to phosphotyrosine-containing motif(s) in JAK2 may stabilize JAK2 in a conformation that has greater kinase activity. Alternatively, binding of SH2-Bβ to JAK2 may release an inhibitor(s) from JAK2. However, a stimulatory effect of SH2-Bβ on JAK2 activity was not detected when bacterially produced glutathione S-transferase (GST)-SH2-Bβ was incubated in vitro with JAK2 immunoprecipitated from COS cells or 3T3-F442A cells (data not shown). This observation raises the possibilities that factor(s) in addition to SH2-Bβ are required for SH2-Bβ-promoted activation of JAK2, or that SH2-Bβ stimulates JAK2 indirectly. Alternatively, a posttranslational modification of SH2-Bβ such as phosphorylation, which is not present in GST-SH2-Bβ, may be required for the stimulatory effect of SH2-Bβ on JAK2, and/or the experimental conditions used in these in vitro assays may not have allowed SH2-Bβ and JAK2 to interact appropriately. Experiments are under way to differentiate between these possibilities.
SH2-Bβ did not stimulate tyrosyl phosphorylation of the receptors for PDGF and EGF and NGF receptor TrkA, suggesting that the stimulatory effect of SH2-Bβ on kinase activity may be relatively specific for JAK2 and not shared by all tyrosine kinases that interact with SH2-Bβ. The absence of a stimulatory effect of SH2-Bβ on tyrosyl phosphorylation of these other receptor tyrosine kinases, despite its ability to bind to these receptors and be phosphorylated on tyrosines, serines, and/or threonines in response to ligand stimulation of these receptors, suggests that SH2-Bβ may serve functions in addition to its activation of JAK2. Several observations are in agreement with this idea. SH2-B has been shown to be required for NGF-induced neuronal differentiation of PC12 cells (29) and NGF-mediated axonal growth and survival of primary sympathetic neurons (30). SH2-Bβ has been shown to associate with multiple other proteins in response to PDGF, indicating that it acts as an adapter or scaffolding protein for PDGF signaling (22). Different ligands stimulate tyrosines and serine/threonine phosphorylation of SH2-Bβ to significantly different extents (20, 22, 29), suggesting that different ligands may use SH2-Bβ in different signaling pathways. In this study, although SH2-Bβ(R555E) is unable to stimulate the kinase activity of JAK2 when coexpressed with JAK2 in COS cells, it still enhanced GH-induced tyrosyl phosphorylation of Stat5B, but to a lesser extent than wild-type SH2-Bβ. This result suggests that SH2-Bβ might enhance ligand-induced activation of Stats through multiple mechanisms in addition to activation of JAK2.
Although it has now been known for more than 5 years that ligand binding to cytokine receptors present in the plasma membrane can activate JAK2, SH2-Bβ is, to our knowledge, the first cytoplasmic protein to be identified as an activator of JAK2. SOCS-1/JAB/SSI-1, the only other cytoplasmic protein found in a yeast two-hybrid system to bind directly to JAK2, was identified as an inhibitor of JAKs (37–39). SOCS-1/JAB/SSI-1 is a cytokine-inducible gene product that, like SH2-Bβ, interacts with JAK2 via its SH2 domain. SOCS-1/JAB/SSI-1 is proposed to act in a negative feedback control loop to regulate signaling by cytokines that activate JAK/Stat pathways (37–39). Our identification of SH2-Bβ as a potent activator of JAK2 suggests that levels of SH2-Bβ, its access to JAK2, and its ability to bind to JAK2 will have a significant impact on the degree to which a given ligand will activate JAK2. SH2-Bβ is differentially phosphorylated on tyrosines as well as on serines/threonines in response to different ligands (20, 22, 29). It is intriguing to speculate that the different levels of phosphorylation of the different sites in SH2-Bβ affect its ability to stimulate JAK2. If so, SH2-Bβ would provide a powerful mechanism for crosstalk among different cytokines, hormones, and growth factors that regulate cellular kinases and phosphatases.
In summary, we report that SH2-Bβ binds to JAK2 and stimulates the kinase activity of JAK2. SH2-Bβ represents the only cytoplasmic protein known to date that binds to and activates a protein tyrosine kinase. As a result of that activation, SH2-Bβ stimulates tyrosyl phosphorylation of JAK2 and substrates of JAK2, including multiple Stats, and potentiates activation of Stat and other JAK2-dependent pathways in response to GH and presumably other hormones and cytokines that activate JAK2. Thus, the regulation of cellular levels of SH2-Bβ or its availability for binding to JAK2 should provide an important mechanism by which the cell modulates JAK2 activity and thereby its response to cytokine stimulation.
Acknowledgments
We thank Drs. J. B. Herrington, L. S. Argetsinger, J. A. VanderKuur, and M. Stofega for helpful discussions and X. Wang for technical assistance. We thank Drs. J. Ihle, G. Norstedt, L. Yu-Lee, R. Jove, and A. Kazlauskas for providing us with cDNA encoding JAK2, GHR, Stat5B, Stat3, and PDGFRβ, respectively. This paper was supported by National Institutes of Health grants DK 34171 and DK 48283. Oligonucleotides were synthesized by the Biomedical Research Core Facilities, University of Michigan, and supported in part by grants to University of Michigan Comprehensive Cancer Center (P30 CA 46592), Michigan Diabetes Research and Training Center (P60-DK 20572), and University of Michigan–Multipurpose Arthritis Center (P60-AR20557).
ABBREVIATIONS
- SH2
Src homology 2
- Stat
signal transducers and activators of transcription
- GH
growth hormone
- GHR
GH receptor
- EGF
epidermal growth factor
- PDGF
platelet-derived growth factor
- NGF
nerve growth factor
References
- 1.Ihle J N. Adv Immunol. 1995;60:1–35. doi: 10.1016/s0065-2776(08)60582-9. [DOI] [PubMed] [Google Scholar]
- 2.Argetsinger L S, Carter-Su C. Physiol Rev. 1996;76:1089–1107. doi: 10.1152/physrev.1996.76.4.1089. [DOI] [PubMed] [Google Scholar]
- 3.Ihle J N. Philos Trans R Soc London B. 1996;351:159–166. doi: 10.1098/rstb.1996.0012. [DOI] [PubMed] [Google Scholar]
- 4.Darnell J E., Jr Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 5.Ihle J N. Cell. 1996;84:331–334. doi: 10.1016/s0092-8674(00)81277-5. [DOI] [PubMed] [Google Scholar]
- 6.Ihle J N, Kerr I M. Trends Genet. 1995;11:69–74. doi: 10.1016/s0168-9525(00)89000-9. [DOI] [PubMed] [Google Scholar]
- 7.Argetsinger L S, Campbell G S, Yang X, Witthuhn B A, Silvennoinen O, Ihle J N, Carter-Su C. Cell. 1993;74:237–244. doi: 10.1016/0092-8674(93)90415-m. [DOI] [PubMed] [Google Scholar]
- 8.Campbell G S, Argetsinger L S, Ihle J N, Kelly P A, Rillema J A, Carter-Su C. Proc Natl Acad Sci USA. 1994;91:5232–5236. doi: 10.1073/pnas.91.12.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lebrun J J, Ali S, Ullrich A, Kelly P A. J Biol Chem. 1995;270:10664–10670. doi: 10.1074/jbc.270.18.10664. [DOI] [PubMed] [Google Scholar]
- 10.Campbell G S, Meyer D J, Raz R, Levy D E, Schwartz J, Carter-Su C. J Biol Chem. 1995;270:3974–3979. doi: 10.1074/jbc.270.8.3974. [DOI] [PubMed] [Google Scholar]
- 11.Gronowski A M, Zhong Z, Wen Z, Thomas M J, Darnell J E, Jr, Rotwein P. Mol Endocrinol. 1995;9:171–177. doi: 10.1210/mend.9.2.7776967. [DOI] [PubMed] [Google Scholar]
- 12.Smit L S, Meyer D J, Billestrup N, Norstedt G, Schwartz J, Carter-Su C. Mol Endocrinol. 1996;10:519–533. doi: 10.1210/mend.10.5.8732683. [DOI] [PubMed] [Google Scholar]
- 13.Smit L S, VanderKuur J A, Stimage A, Han Y, Luo G, Yu-Lee L-y, Schwartz J, Carter-Su C. Endocrinology. 1997;138:3426–3434. doi: 10.1210/endo.138.8.5332. [DOI] [PubMed] [Google Scholar]
- 14.Yi W, Kim S O, Jiang J, Park S H, Kraft A S, Waxman D J, Frank S J. Mol Endocrinol. 1996;10:1425–1443. doi: 10.1210/mend.10.11.8923468. [DOI] [PubMed] [Google Scholar]
- 15.Udy G B, Towers R P, Snell R G, Wilkins R J, Park S H, Ram P A, Waxman D J, Davey H W. Proc Natl Acad Sci USA. 1997;94:7239–7244. doi: 10.1073/pnas.94.14.7239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teglund S, McKay C, Schuetz E, van Deursen J M, Stravopodis D, Wang D, Brown M, Bodner S, Grosveld G, Ihle J N. Cell. 1998;93:841–850. doi: 10.1016/s0092-8674(00)81444-0. [DOI] [PubMed] [Google Scholar]
- 17.Liu X, Robinson G W, Wagner K U, Garrett L, Wynshaw-Boris A, Hennighausen L. Genes Dev. 1997;11:179–186. doi: 10.1101/gad.11.2.179. [DOI] [PubMed] [Google Scholar]
- 18.Parganas E, Wang D, Stravopodis D, Topham D J, Marine J C, Teglund S, Vanin E F, Bodner S, Colamonici O R, van Deursen J M, et al. Cell. 1998;93:385–395. doi: 10.1016/s0092-8674(00)81167-8. [DOI] [PubMed] [Google Scholar]
- 19.Neubauer H, Cumano A, Muller M, Wu H, Huffstadt U, Pfeffer K. Cell. 1998;93:397–409. doi: 10.1016/s0092-8674(00)81168-x. [DOI] [PubMed] [Google Scholar]
- 20.Rui L, Mathews L S, Hotta K, Gustafson T A, Carter-Su C. Mol Cell Biol. 1997;17:6633–6644. doi: 10.1128/mcb.17.11.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osborne M A, Dalton S, Kochan J P. Bio/Technology. 1995;13:1474–1478. doi: 10.1038/nbt1295-1474. [DOI] [PubMed] [Google Scholar]
- 22.Rui L, Carter-Su C. J Biol Chem. 1998;273:21239–21245. doi: 10.1074/jbc.273.33.21239. [DOI] [PubMed] [Google Scholar]
- 23.Wang J, Riedel H. J Biol Chem. 1998;273:3136–3139. doi: 10.1074/jbc.273.6.3136. [DOI] [PubMed] [Google Scholar]
- 24.Silvennoinen O, Witthuhn B, Quelle F W, Cleveland J L, Yi T, Ihle J N. Proc Natl Acad Sci USA. 1993;90:8429–8433. doi: 10.1073/pnas.90.18.8429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Uhler M D, Billestrup N, Norstedt G, Talamantes F, Nielsen J H, Carter-Su C. J Biol Chem. 1992;267:17390–17396. [PubMed] [Google Scholar]
- 26.Turkson J, Bowman T, Garcia R, Caldenhoven E, De Groot R P, Jove R. Mol Cell Biol. 1998;18:2545–2552. doi: 10.1128/mcb.18.5.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen C, Okayama H. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Witthuhn B A, Quelle F W, Silvennoinen O, Yi T, Tang B, Miura O, Ihle J N. Cell. 1993;74:227–236. doi: 10.1016/0092-8674(93)90414-l. [DOI] [PubMed] [Google Scholar]
- 29.Rui L, Herrington J B, Carter-Su C. J Biol Chem. 1999;274:10590–10594. doi: 10.1074/jbc.274.15.10590. [DOI] [PubMed] [Google Scholar]
- 30.Qian X, Riccio A, Zhang Y, Ginty D D. Neuron. 1998;21:1017–1029. doi: 10.1016/s0896-6273(00)80620-0. [DOI] [PubMed] [Google Scholar]
- 31.Argetsinger L S, Hsu G W, Myers M G, Jr, Billestrup N, Norstedt G, White M F, Carter-Su C. J Biol Chem. 1995;270:14685–14692. doi: 10.1074/jbc.270.24.14685. [DOI] [PubMed] [Google Scholar]
- 32.Argetsinger L S, Billestrup N, Norstedt G, White M F, Carter-Su C. J Biol Chem. 1996;271:29415–29421. doi: 10.1074/jbc.271.46.29415. [DOI] [PubMed] [Google Scholar]
- 33.VanderKuur J, Allevato G, Billestrup N, Norstedt G, Carter-Su C. J Biol Chem. 1995;270:7587–7593. doi: 10.1074/jbc.270.13.7587. [DOI] [PubMed] [Google Scholar]
- 34.Stofega M R, Wang H, Ullrich A, Carter-Su C. J Biol Chem. 1998;273:7112–7117. doi: 10.1074/jbc.273.12.7112. [DOI] [PubMed] [Google Scholar]
- 35.Herrington J, Rui L, Luo G, Yu-Lee L-y, Carter-Su C. J Biol Chem. 1999;274:5138–5145. doi: 10.1074/jbc.274.8.5138. [DOI] [PubMed] [Google Scholar]
- 36.Gouilleux F, Wakao H, Mundt M, Groner B. EMBO J. 1994;13:4361–4369. doi: 10.1002/j.1460-2075.1994.tb06756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Endo T A, Masuhara M, Yokouchi M, Suzuki R, Sakamoto H, Mitsui K, Matsumoto A, Tanimura S, Ohtsubo M, Misawa H, et al. Nature (London) 1997;387:921–924. doi: 10.1038/43213. [DOI] [PubMed] [Google Scholar]
- 38.Starr R, Willson T A, Viney E M, Murray L J, Rayner J R, Jenkins B J, Gonda T J, Alexander W S, Metcalf D, Nicola N A, et al. Nature (London) 1997;387:917–921. doi: 10.1038/43206. [DOI] [PubMed] [Google Scholar]
- 39.Naka T, Narazaki M, Hirata M, Matsumoto T, Minamoto S, Aono A, Nishimoto N, Kajita T, Taga T, Yoshizaki K, et al. Nature (London) 1997;387:924–929. doi: 10.1038/43219. [DOI] [PubMed] [Google Scholar]