Abstract
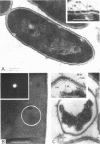
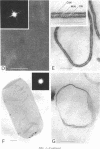
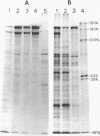
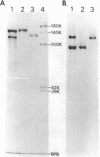
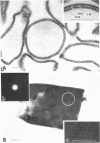
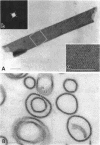
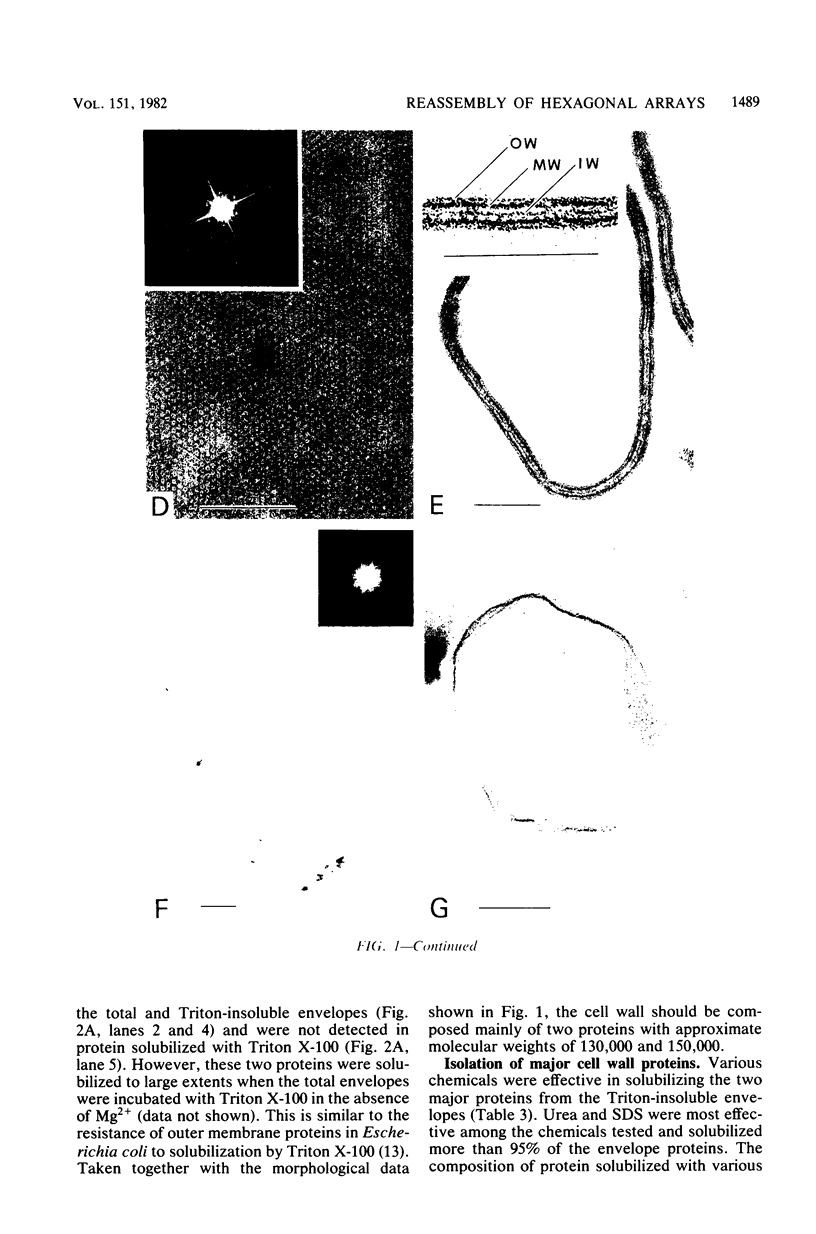
Bacillus brevis 47 had two protein layers (the outer and middle walls) and a peptidoglycan layer (the inner wall) and contained two major proteins with approximate molecular weights of 130,000 and 150,000 in the cell wall. Both the total and Triton-insoluble envelopes revealed a hexagonal lattice array with a lattice constant of 14.5 nm. The proteins of 130,000 and 150,000 molecular weight isolated from the Triton-insoluble envelopes were serologically different from each other and assembled in vitro on the peptidoglycan layer. A mixture of 130,000- and 150,000-molecular-weight proteins led to the formation of a five-layered cell wall structure, two layers on each side of the peptidoglycan layer, which resembled closely the Triton-insoluble envelopes. A three-layered cell wall structure, one layer on each side of the peptidoglycan layer, was reconstituted when only the 150,000-molecular-weight protein was used. Both five- and three-layered cell walls reconstituted in vitro also contained hexagonally arranged arrays with the same lattice constant as that of the total and Triton-insoluble envelopes. A mutant, strain 47-57, which was isolated as a phage-resistant colony, had a two-layered cell wall consisting of the middle and inner wall layers and contained only 150,000-molecular-weight protein as the major cell wall protein. The cell envelopes of the mutant revealed the hexagonal arrays with the same lattice constant as that of the wild-type cell envelopes. We conclude that the outer and middle wall layers consist of proteins with approximate molecular weights of 130,000 and 150,000, respectively. Furthermore, the 150,000-molecular-weight protein formed the hexagonal arrays in the middle wall layer.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi U., Smith P. R., Dubochet J., Henry C., Kellenberger E. A study of the structure of the T-layer of Bacillus brevis. J Supramol Struct. 1973;1(6):498–522. doi: 10.1002/jss.400010606. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Glauert A. M., Thornley M. J. The topography of the bacterial cell wall. Annu Rev Microbiol. 1969;23:159–198. doi: 10.1146/annurev.mi.23.100169.001111. [DOI] [PubMed] [Google Scholar]
- Holt S. C., Leadbetter E. R. Comparative ultrastructure of selected aerobic spore-forming bacteria: a freeze-etching study. Bacteriol Rev. 1969 Jun;33(2):346–378. doi: 10.1128/br.33.2.346-378.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leduc M., Rousseau M., van Heijenoort J. Structure of the cell wall of Bacillus species C.I.P. 76-111. Eur J Biochem. 1977 Oct 17;80(1):153–163. doi: 10.1111/j.1432-1033.1977.tb11867.x. [DOI] [PubMed] [Google Scholar]
- Nermut M. V., Murray R. G. Ultrastructure of the cell wall of Bacillus polymyxa. J Bacteriol. 1967 Jun;93(6):1949–1965. doi: 10.1128/jb.93.6.1949-1965.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OUCHTERLONY O. Antigen-antibody reactions in gels. IV. Types of reactions in coordinated systems of diffusion. Acta Pathol Microbiol Scand. 1953;32(2):230–240. [PubMed] [Google Scholar]
- Schnaitman C. A. Solubilization of the cytoplasmic membrane of Escherichia coli by Triton X-100. J Bacteriol. 1971 Oct;108(1):545–552. doi: 10.1128/jb.108.1.545-552.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz U., Asmus A., Frank H. Autolytic enzymes and cell division of Escherichia coli. J Mol Biol. 1969 May 14;41(3):419–429. doi: 10.1016/0022-2836(69)90285-x. [DOI] [PubMed] [Google Scholar]
- Sleytr U. B., Glauert A. M. Ultrastructure of the cell walls of two closely related clostridia that possess different regular arrays of surface subunits. J Bacteriol. 1976 May;126(2):869–882. doi: 10.1128/jb.126.2.869-882.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleytr U. B. Regular arrays of macromolecules on bacterial cell walls: structure, chemistry, assembly, and function. Int Rev Cytol. 1978;53:1–62. doi: 10.1016/s0074-7696(08)62240-8. [DOI] [PubMed] [Google Scholar]
- Sleytr U., Adam H., Klaushofer H. Die Feinstruktur der Zellwandoberfläche von zwei thermophilen Clostridienarten, dargestellt mit Hilfe der Gefrierätztechnik. Mikroskopie. 1968 Aug;23(1):1–10. [PubMed] [Google Scholar]
- Tsukagoshi N., Yamada H., Tsuboi A., Udaka S. Effects of Phosphate in Medium on Protein Secretion in a Protein-Producing Bacterium, Bacillus brevis 47. Appl Environ Microbiol. 1981 Aug;42(2):370–374. doi: 10.1128/aem.42.2.370-374.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H., Tsukagoshi N., Udaka S. Morphological alterations of cell wall concomitant with protein release in a protein-producing bacterium, Bacillus brevis 47. J Bacteriol. 1981 Oct;148(1):322–332. doi: 10.1128/jb.148.1.322-332.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]