Abstract
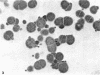
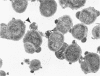
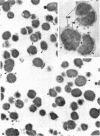
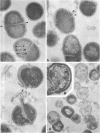
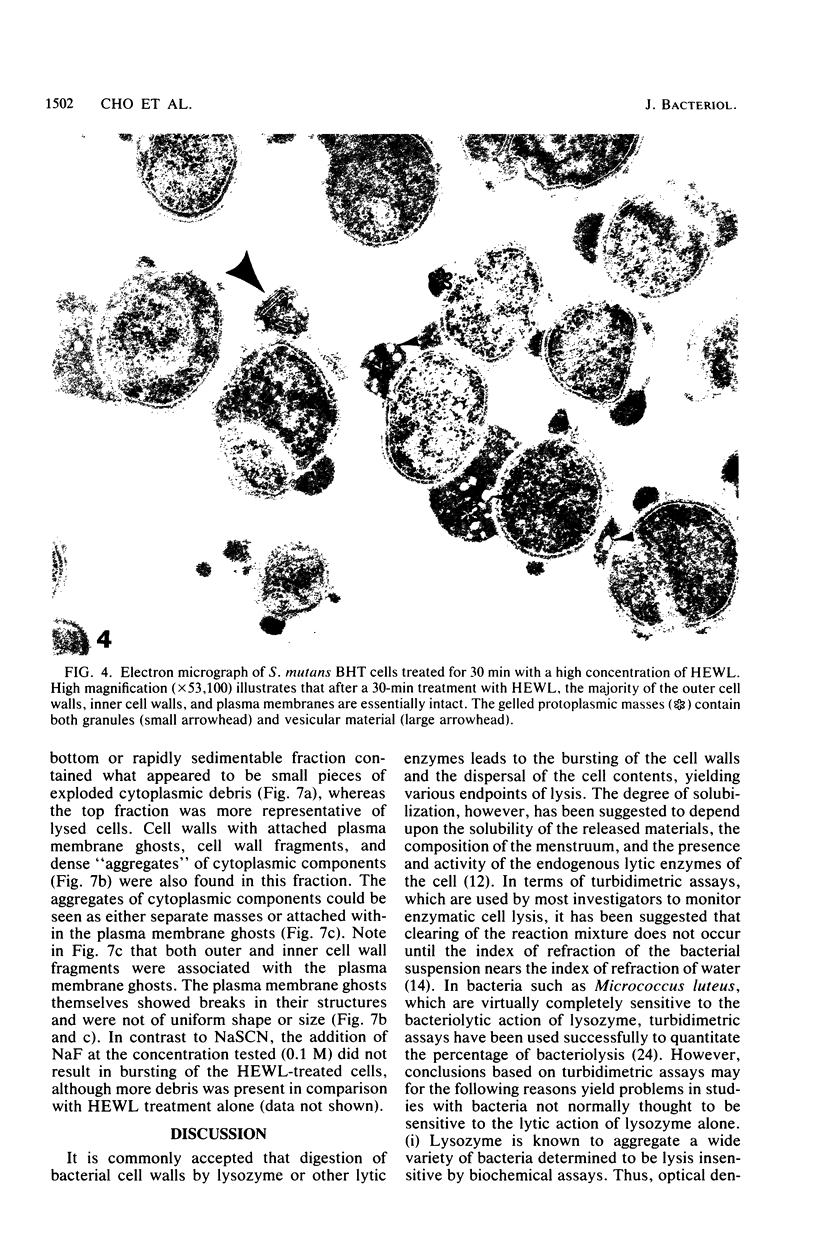
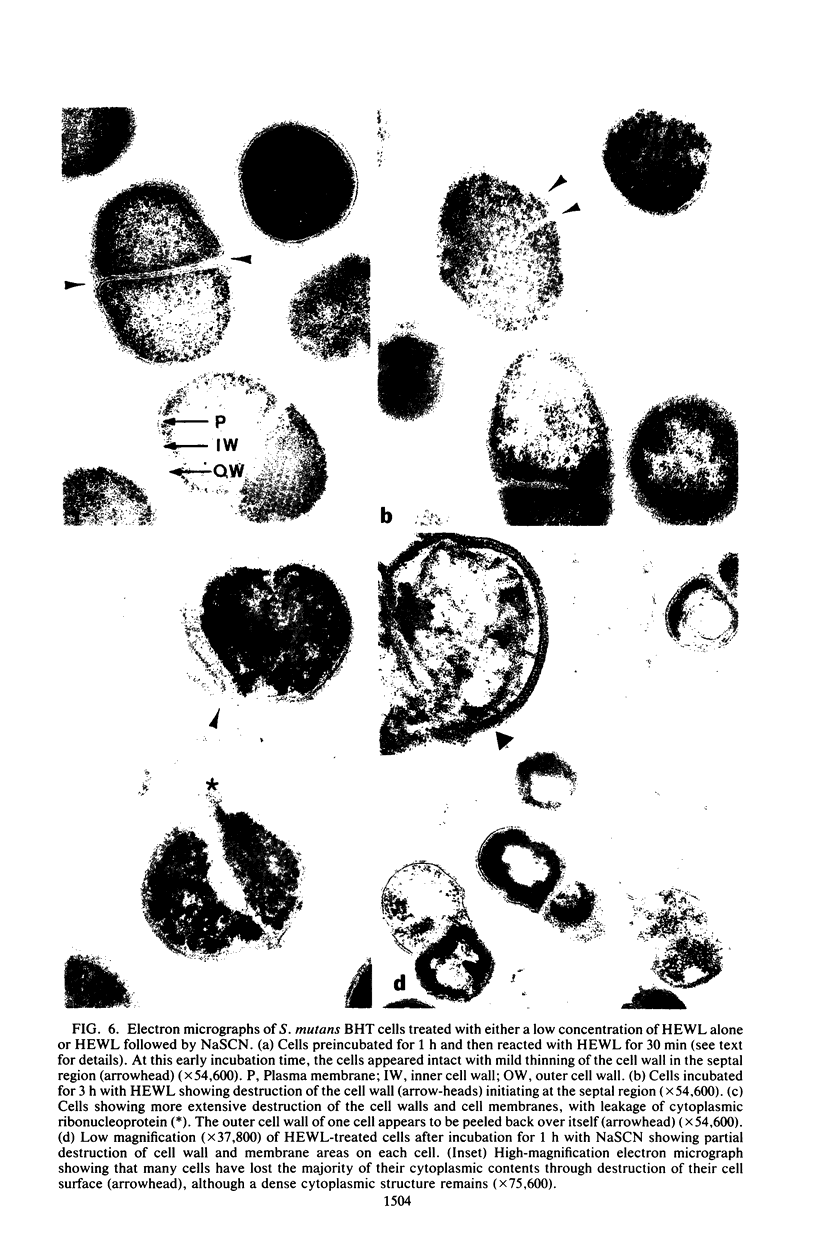
The effects of hen egg white lysozyme and the inorganic salt sodium thiocyanate on the integrity of Streptococcus mutans BHT were studied by transmission electron microscopy. Both control cells and cells exposed to NaSCN possessed thick outer cell walls and densely staining inner cell walls juxtaposed to the plasma membranes. In the presence of NaSCN, however, the S. mutans BHT nucleoid was coagulated into thick electron-dense filaments. Exposure of S. mutans BHT to 150 μg of hen egg white lysozyme per ml resulted in the progressive destruction of both the cell walls and the plasma membranes. The enzyme appeared to affect the region of the cell wall septum, and exposure to 150 μg of hen egg white lysozyme per ml for as short a time as 10 min resulted in visible morphological cell wall alterations. At 30 min, ultrastructural observations revealed that the majority of the cells were in the process of expelling a portion of their cytoplasmic contents from the septal and other regions of the cells at the time of fixation. After 3 h of incubation in the presence of this high lysozyme concentration, gelled protoplasmic masses, which were free from the cells, were evident. In addition, extensive damage to the outer and inner cell walls and to the plasma membranes was apparent, although the cells maintained their shape. On some areas of the cell surface, the outer cell wall and plasma membrane were completely absent, whereas at other locations the outer cell wall was either split away from the inner cell wall and plasma membrane or distended from an area free of inner cell wall and plasma membrane. Upon addition of NaSCN to the hen egg white lysozyme-treated cells, both the gelled protoplasmic masses and the damaged cells exhibited an exploded appearance and existed as membrane ghosts, cell wall fragments, or dense aggregates of cytoplasmic components. The effects of a low lysozyme concentration (22.5 μg/ml) on S. mutans morphology were less pronounced at short incubation times (i.e., 10 and 30 min) than those that were observed with a high enzyme concentration; however, breaks in the cell walls and dissolution of the plasma membranes with resulting cell lysis were visible after a prolonged (3-h) incubation and after subsequent addition of NaSCN.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLADEN H. A., MERGENHAGEN S. E. ULTRASTRUCTURE OF VEILLONELLA AND MORPHOLOGICAL CORRELATION OF AN OUTER MEMBRANE WITH PARTICLES ASSOCIATED WITH ENDOTOXIC ACTIVITY. J Bacteriol. 1964 Nov;88:1482–1492. doi: 10.1128/jb.88.5.1482-1492.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D. C., Thorne K. J. Spheroplasts of Lactobacillus casei and the cellular distribution of bactoprenol. J Cell Sci. 1970 Nov;7(3):755–785. doi: 10.1242/jcs.7.3.755. [DOI] [PubMed] [Google Scholar]
- Bladen H., Hageage G., Harr R., Pollock F. Lysis of certain organisms by the synergistic action of complement and lysozyme. J Dent Res. 1973 Mar-Apr;52(2):371–376. doi: 10.1177/00220345730520023101. [DOI] [PubMed] [Google Scholar]
- Chassy B. M., Giuffrida A. Method for the lysis of Gram-positive, asporogenous bacteria with lysozyme. Appl Environ Microbiol. 1980 Jan;39(1):153–158. doi: 10.1128/aem.39.1.153-158.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman S. E., van de Rijn I., Bleiweis A. S. Lysis of grouped and ungrouped streptococci by lysozyme. Infect Immun. 1970 Nov;2(5):563–569. doi: 10.1128/iai.2.5.563-569.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLADSTONE G. P., JOHNSTON H. H. The effect of cultural conditions on the susceptibility of Bacillus anthracis to lysozyme. Br J Exp Pathol. 1955 Aug;36(4):363–372. [PMC free article] [PubMed] [Google Scholar]
- GRULA E. A., HARTSELL S. E. Lysozyme action and its relation to the Nakamura effect. J Bacteriol. 1954 Sep;68(3):302–306. doi: 10.1128/jb.68.3.302-306.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRULA E. A., HARTSELL S. E. Lysozyme and morphological alterations induced in Micrococcus lysodeikticus. J Bacteriol. 1954 Aug;68(2):171–177. doi: 10.1128/jb.68.2.171-177.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRULA E. A., HARTSELL S. E. Lysozyme in the bacteriolysis of gram-negative bacteria. I. Morphological changes during use of Nakamura's technique. Can J Microbiol. 1957 Feb;3(1):13–21. doi: 10.1139/m57-002. [DOI] [PubMed] [Google Scholar]
- GRULA E. A., HARTSELL S. E. Lysozyme in the bacteriolysis of gram-negative bacteria. II. Factors influencing clearing during the Nakamura treatment. Can J Microbiol. 1957 Feb;3(1):23–34. doi: 10.1139/m57-003. [DOI] [PubMed] [Google Scholar]
- Gilpin R. W., Chatterjee A. N., Young F. E. Autolysis of microbial cells: salt activation of autolytic enzymes in a mutant of Staphylococcus aureus. J Bacteriol. 1972 Jul;111(1):272–283. doi: 10.1128/jb.111.1.272-283.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman H., Pollock J. J., Iacono V. J., Wong W., Shockman G. D. Peptidoglycan loss during hen egg white lysozyme-inorganic salt lysis of Streptococcus mutans. J Bacteriol. 1981 May;146(2):755–763. doi: 10.1128/jb.146.2.755-763.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman H., Pollock J. J., Katona L. I., Iacono V. J., Cho M. I., Thomas E. Lysis of Streptococcus mutans by hen egg white lysozyme and inorganic sodium salts. J Bacteriol. 1981 May;146(2):764–774. doi: 10.1128/jb.146.2.764-774.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono V. J., MacKay B. J., DiRienzo S., Pollock J. J. Selective antibacterial properties of lysozyme for oral microorganisms. Infect Immun. 1980 Aug;29(2):623–632. doi: 10.1128/iai.29.2.623-632.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu S., Ikeda K., Hamaguchi K., Miwa S., Nakashima K. Binding of N-acetyl-chitotriose to human lysozyme. J Biochem. 1975 Aug;78(2):327–333. doi: 10.1093/oxfordjournals.jbchem.a130911. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perch B., Kjems E., Ravn T. Biochemical and serological properties of Streptococcus mutans from various human and animal sources. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Jun;82(3):357–370. doi: 10.1111/j.1699-0463.1974.tb02338.x. [DOI] [PubMed] [Google Scholar]
- Pollock J. J., Katona L. I., Goodman H., Cho M. I., Iacono V. J. Bacteriolysis of Streptococcus mutans BHT by lysozyme and inorganic anions normally present in human saliva. Arch Oral Biol. 1981;26(9):711–716. doi: 10.1016/0003-9969(81)90187-4. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALTON M. R. Cell structure and the enzymic lysis of bacteria. J Gen Microbiol. 1953 Dec;9(3):512–523. doi: 10.1099/00221287-9-3-512. [DOI] [PubMed] [Google Scholar]
- SALTON M. R. Studies of the bacterial cell wall. V. The action of lysozyme on cell walls of some lysozyme-sensitive bacteria. Biochim Biophys Acta. 1956 Dec;22(3):495–506. doi: 10.1016/0006-3002(56)90060-9. [DOI] [PubMed] [Google Scholar]
- Terleckyj B., Willett N. P., Shockman G. D. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun. 1975 Apr;11(4):649–655. doi: 10.1128/iai.11.4.649-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne K. J., Barker D. C. The occurrence of bactoprenol in the mesosome and plasma membranes of Lactobacillus casei and Lactobacillus plantarum. J Gen Microbiol. 1972 Apr;70(1):87–98. doi: 10.1099/00221287-70-1-87. [DOI] [PubMed] [Google Scholar]
- Tortosa M., Cho M. I., Wilkens T. J., Iacono V. J., Pollock J. J. Bacteriolysis of Veillonella alcalescens by lysozyme and inorganic anions present in saliva. Infect Immun. 1981 Jun;32(3):1261–1273. doi: 10.1128/iai.32.3.1261-1273.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgilio R., González C., Muñoz N., Mendoza S. Electron microscopy of Staphylococcus aureus cell wall lysis. J Bacteriol. 1966 May;91(5):2018–2024. doi: 10.1128/jb.91.5.2018-2024.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westmacott D., Perkins H. R. Effects of lysozyme on Bacillus cereus 569: rupture of chains of bacteria and enhancement of sensitivity to autolysins. J Gen Microbiol. 1979 Nov;115(1):1–11. doi: 10.1099/00221287-115-1-1. [DOI] [PubMed] [Google Scholar]