Abstract
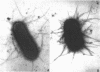
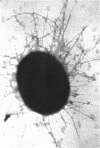
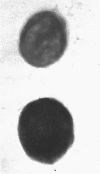
Previous work on the control of fimbriation in bacteria has demonstrated the importance of environmental factors such as static versus shaking broth and the absence versus the presence of glucose on the degree of fimbriation. When the Pil+ K-12 strain of Escherichia coli CSH50 was grown in static broth, the bacteria grown with glucose were less fimbriate (as determined by electron microscopy) than those grown without glucose. In contrast, a derivative, the pil-lac operon fusion strain VL361, gave off similar proportions of Lac+ and Lac- colonies when grown with or without glucose. Introduction of delta cya into either CSH50 or VL361 did not affect synthesis of either fimbriae or beta-galactosidase, respectively. When total synthesis of fimbriae by strain CSH50 was assayed, using an enzyme-linked immunosorbent inhibition test, glucose-grown bacteria made less antigen when they were grown in static broth but not when they were grown in shaking broth. When results are taken together, we interpret them as showing that glucose does not suppress fimbrial synthesis by classic catabolite repression but rather merely prevents the outgrowth or fimbriate bacteria in static broth.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRINTON C. C., Jr Non-flagellar appendages of bacteria. Nature. 1959 Mar 21;183(4664):782–786. doi: 10.1038/183782a0. [DOI] [PubMed] [Google Scholar]
- Bar-Shavit Z., Ofek I., Goldman R., Mirelman D., Sharon N. Mannose residues on phagocytes as receptors for the attachment of Escherichia coli and Salmonella typhi. Biochem Biophys Res Commun. 1977 Sep 9;78(1):455–460. doi: 10.1016/0006-291x(77)91276-1. [DOI] [PubMed] [Google Scholar]
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Brinton C. C., Jr The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans N Y Acad Sci. 1965 Jun;27(8):1003–1054. doi: 10.1111/j.2164-0947.1965.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivo probe for transcriptional control sequences. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4530–4533. doi: 10.1073/pnas.76.9.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Duguid J. P., Anderson E. S., Campbell I. Fimbriae and adhesive properties in Salmonellae. J Pathol Bacteriol. 1966 Jul;92(1):107–138. doi: 10.1002/path.1700920113. [DOI] [PubMed] [Google Scholar]
- Eisenstein B. I., Beachey E. H., Solomon S. S. Divergent effects of cyclic adenosine 3',5'-monophosphate on formation of type 1 fimbriae in different K-12 strains of Escherichia coli. J Bacteriol. 1981 Jan;145(1):620–623. doi: 10.1128/jb.145.1.620-623.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein B. I., Ofek I., Beachey E. H. Interference with the mannose binding and epithelial cell adherence of Escherichia coli by sublethal concentrations of streptomycin. J Clin Invest. 1979 Jun;63(6):1219–1228. doi: 10.1172/JCI109417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein B. I. Phase variation of type 1 fimbriae in Escherichia coli is under transcriptional control. Science. 1981 Oct 16;214(4518):337–339. doi: 10.1126/science.6116279. [DOI] [PubMed] [Google Scholar]
- Epstein W., Rothman-Denes L. B., Hesse J. Adenosine 3':5'-cyclic monophosphate as mediator of catabolite repression in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2300–2304. doi: 10.1073/pnas.72.6.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson R. E. Factors affecting expression of the Escherichia coli pilus K99. Infect Immun. 1980 Apr;28(1):190–194. doi: 10.1128/iai.28.1.190-194.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallick U., Herrlich P. Regulation of synthesis of a major outer membrane protein: cyclic AMP represses Escherichia coli protein III synthesis. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5520–5523. doi: 10.1073/pnas.76.11.5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny C., Carnahan J., Brinton C. C., Jr Mechanical removal of F pili, type I pili, and flagella from Hfr and RTF donor cells and the kinetics of their reappearance. J Bacteriol. 1969 Jun;98(3):1294–1306. doi: 10.1128/jb.98.3.1294-1306.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H. Mannose binding and epithelial cell adherence of Escherichia coli. Infect Immun. 1978 Oct;22(1):247–254. doi: 10.1128/iai.22.1.247-254.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Mosek A., Sharon N. Mannose-specific adherence of Escherichia coli freshly excreted in the urine of patients with urinary tract infections, and of isolates subcultured from the infected urine. Infect Immun. 1981 Dec;34(3):708–711. doi: 10.1128/iai.34.3.708-711.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old D. C., Duguid J. P. Selective outgrowth of fimbriate bacteria in static liquid medium. J Bacteriol. 1970 Aug;103(2):447–456. doi: 10.1128/jb.103.2.447-456.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottow J. C. Ecology, physiology, and genetics of fimbriae and pili. Annu Rev Microbiol. 1975;29:79–108. doi: 10.1146/annurev.mi.29.100175.000455. [DOI] [PubMed] [Google Scholar]
- Salit I. E., Gotschlich E. C. Type I Escherichia coli pili: characterization of binding to monkey kidney cells. J Exp Med. 1977 Nov 1;146(5):1182–1194. doi: 10.1084/jem.146.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaran L., Pogell B. M. Differential inhibition of catabolite-sensitive enzyme induction by intercalating dyes. Nat New Biol. 1973 Oct 31;245(148):257–260. doi: 10.1038/newbio245257a0. [DOI] [PubMed] [Google Scholar]
- Silverblatt F. J. Host-parasite interaction in the rat renal pelvis: a possible role for pili in the pathogenesis of pyelonephritis. J Exp Med. 1974 Dec 1;140(6):1696–1711. doi: 10.1084/jem.140.6.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman M., Simon M. Phase variation: genetic analysis of switching mutants. Cell. 1980 Apr;19(4):845–854. doi: 10.1016/0092-8674(80)90075-6. [DOI] [PubMed] [Google Scholar]
- Silverman M., Zieg J., Mandel G., Simon M. Analysis of the functional components of the phase variation system. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):17–26. doi: 10.1101/sqb.1981.045.01.005. [DOI] [PubMed] [Google Scholar]
- Swaney L. M., Liu Y. P., Ippen-Ihler K., Brinton C. C., Jr Genetic complementation analysis of Escherichia coli type 1 somatic pilus mutants. J Bacteriol. 1977 Apr;130(1):506–511. doi: 10.1128/jb.130.1.506-511.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaney L. M., Liu Y. P., To C. M., To C. C., Ippen-Ihler K., Brinton C. C., Jr Isolation and characterization of Escherichia coli phase variants and mutants deficient in type 1 pilus production. J Bacteriol. 1977 Apr;130(1):495–505. doi: 10.1128/jb.130.1.495-505.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T., Gots J. S. Requirement of adenosine 3', 5'-cyclic phosphate for flagella formation in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1970 Aug;103(2):513–516. doi: 10.1128/jb.103.2.513-516.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieg J., Silverman M., Hilmen M., Simon M. Recombinational switch for gene expression. Science. 1977 Apr 8;196(4286):170–172. doi: 10.1126/science.322276. [DOI] [PubMed] [Google Scholar]