Abstract
Gene transfer into nervous tissue is a powerful tool for the analysis of gene function. By using a rat hippocampal slice culture preparation, we show here that Semliki Forest virus (SFV) and Sindbis virus (SIN) vectors are useful for the effective infection of neurons. The stratum pyramidale and/or the granular cell layer were injected with recombinant virus encoding β-galactosidase (LacZ) or green fluorescent protein (GFP). By using low concentrations of injected SFV-LacZ or SIN-LacZ, we detected LacZ staining of pyramidal cells, interneurons, and granule cells. About 60% of the infected cells showed clear neuronal morphology; thus, relatively few glial cells expressed the transgene. Expression of GFP from SFV and SIN vectors gave similar results, with an even higher percentage (>90%) of the GFP-positive cells identified as neurons. Infected pyramidal cells were readily recognized in living slices, displaying GFP fluorescence in dendrites of up to fourth order and in dendritic spines. They appeared morphologically normal and viable at 1–5 days postinfection. We conclude that both SFV and SIN vectors efficiently transfer genes into neurons in hippocampal slice cultures. In combination with the GFP reporter, SFV and SIN vectors will allow the physiological examination of identified neurons that have been modified by overexpression or suppression of a specific gene product.
Difficulties in neuronal transfection continue to impede the application of molecular approaches to neurobiology. The development of strategies for efficient neuronal transfection in tissue slices and in vivo is particularly important. Genetic manipulation of postmitotic neurons in intact tissue has been achieved by using viral vectors including adenovirus (1), herpes simplex virus (2), vaccinia virus (3), adeno-associated virus (4), and lentivirus (5) and by bombardment with DNA-coated gold particles (6). These methods, however, are often compromised by extensive transfection of glial cells (1, 2) and by cytotoxic effects (7). This is particularly problematic when one attempts to use dominant-negative mutants or antisense technology to suppress the function of proteins with slow turnover rates.
Semliki Forest virus (SFV) and Sindbis virus (SIN) are two closely related, plus-strand RNA viruses belonging to the alphavirus genus (8). Wild-type strains of both viruses infect neurons in the central nervous system, induce neuronal apoptosis, and cause encephalitis in mice (9, 10). Vectors for both SFV and SIN have been developed to express high levels of foreign genes in vitro and in vivo (11, 12). In one approach (12), a heterologous gene in the vector RNA replaces the viral structural genes that are required for encapsidation of viral RNA. For packaging into infectious particles, the vector RNA must be cotransfected with defective helper RNA that lacks nonstructural genes (which are necessary for viral RNA replication) but provides the structural genes in trans to the vector RNA. Infectious particles derived by using this approach will infect target cells but, lacking structural genes, not lead to the release of new virions. They are therefore termed “suicide vectors” (11). SIN vectors have been shown to effectively infect nerve cells in cocultures of dissociated neurons and glial cells and to infect granule cells of the dentate gyrus in vivo (13). Similarly, recombinant SFV has been used to infect dissociated hippocampal neurons in culture (14). Recently, in vivo transfer of a reporter gene with SFV vectors into rat brain was shown to result in local transgene expression that was mainly restricted to neurons (15).
In the present study, we aimed to determine whether SFV and SIN vectors, in which the structural viral genes had been replaced by reporter genes (suicide vectors), are useful for transfecting neurons in rat hippocampal slices cultured on glass coverslips. These cultures possess the following advantages: long-term (weeks to months) survival of the preparation, excellent microscopic visualization of cells, well characterized morphology and synaptic connections, and the possibility to study synaptic plasticity including long-term potentiation (16).
MATERIALS AND METHODS
Preparation of Alphavirus Expression Vectors.
Recombinant SFV particles were prepared as described (17). For the green fluorescent protein (GFP) reporter constructs, pSFV2 gen was used for insertion of cDNA encoding EGFP (CLONTECH) either directly or under the control of a cap-independent ribosomal entry site (IRES) to obtain pSFV2 gen–GFP and pSFV2 gen–IRES–GFP, respectively. In vitro-transcribed RNA molecules from pSFV3 encoding Escherichia coli β-galactosidase (LacZ) (12), pSFV2 gen–GFP, pSFV2 gen–IRES–GFP, and pSFV–Helper2 (18) were coelectroporated into baby hamster kidney (BHK)-21 cells. Stocks of the in vivo packaged particles were harvested after 24 h and had a high titer of ≈109 infectious particles per ml (tested on BHK-21 cells). These vectors were activated with α-chymotrypsin (100 μg/ml) before infection of the slices.
Sindbis virus replicons encoding LacZ (SIN–LacZ) or GFP (SIN–GFP) were prepared by cotransfecting RNA from SINrep5–LacZ or SINrep5–GFP (containing pGreen Lantern-1 GFP, GIBCO/BRL) together with RNA from DH(26S)5′SIN helper into BHK-21 cells as described (19). SIN–LacZ had a titer of 5 × 108 infectious particles per ml, as determined in a cytopathic effect assay on chicken cells (20).
Infection of Hippocampal Slice Cultures.
Hippocampal slices from 5- to 7-day postnatal rats were prepared and cultured by means of the roller-tube technique as described (21). Stocks of virus particles were diluted in culture medium supplemented with 10 mM MgCl2 and 0.5 μM tetrodotoxin (to reduce excitotoxic injury during the infection procedure). For virus injections, in vitro slice cultures [average age: 23 ± 11 days in culture, (mean ± SD), 21 batches; 2–8 sister cultures per batch] were transferred from the roller tubes to 35-mm plastic Petri dishes containing 2–3 ml of culture medium supplemented with 10 mM MgCl2 and 0.5 μM tetrodotoxin. Infections were performed at room temperature under biosafety containment level 2 by microinjection of virus into the extracellular space of the slice cultures. The tip of autoclaved glass micropipettes pulled from borosilicate glass (Clark Electromedical Instruments, Pangbourne, U.K.) was broken to a diameter of ≈20 μm. Pipettes were filled with viral solution and lowered into the pyramidal and/or granule cell layer by using a micromanipulator. For each position, one injection of short duration (<2 s) was performed by applying pressure from a 1-ml syringe. Typically, 3–10 injection sites were selected per slice, and a total of 1–2 μl of the virus solution was applied. After injection, slices were washed with culture medium, returned to the roller tubes, and cultured for 1–10 days.
Imaging of Cultured Slices.
Slices infected with SFV–LacZ or SIN–LacZ were fixed in 0.75% glutaraldehyde (Sigma) in PBS for 30 min and then stained for LacZ expression by using 5-bromo-4-chloro-3-indolyl β-d-galactoside (X-Gal) (22). Slices infected with SFV–GFP were bathed in saline (137 mM NaCl/2.7 mM KCl/11.6 mM NaHCO3/0.4 mM NaH2PO4/2 mM MgCl2/2.8 mM CaCl2/10 mg/ml phenol red, pH ≈7.4). Cell viability was tested by using propidium iodide (5 μg/ml) exclusion (23). All slices were viewed and photographed by using an upright fluorescence microscope (Zeiss Axiophot or Axioskop). GFP fluorescence was excited by using an FITC filterset (Zeiss) or with the polychrome I monochromator (TILL Photonics, Planegg, Germany) excited at 470 nm. Video images were acquired with a cooled frame transfer charge-coupled device camera (Pentamax 512-EFT, Princeton Instruments, Trenton, NJ). Images were binned twice during acquisition. Postprocessing included contrast enhancement and transformation of grayscale-GFP images into dual-tone false-color images by using the photoshop program (Adobe Systems, Mountain View, CA).
RESULTS
We tested the efficacy of SFV- and SIN-mediated gene transfer into organotypic hippocampal slices from postnatal rats. Slices were cultured on glass coverslips and examined by conventional bright field, differential interference contrast, and fluorescence microscopy.
Infection Using SFV–LacZ.
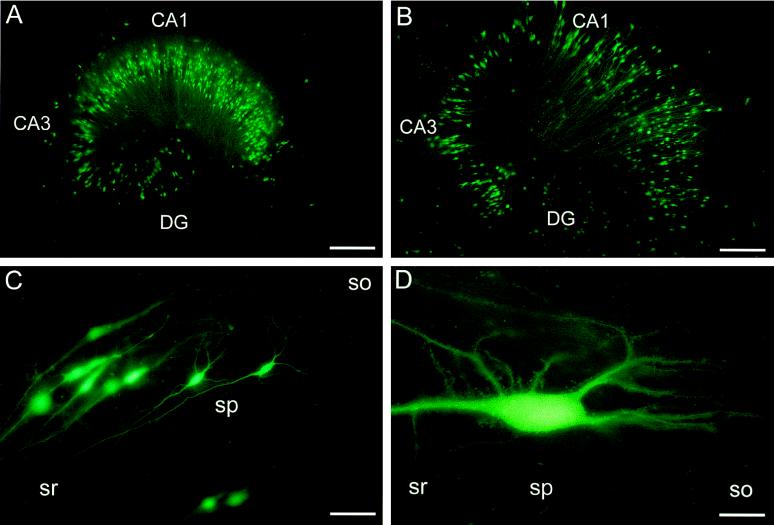
A total of 48 slices was injected with five different concentrations of SFV–LacZ (105–109 infectious particles per ml). CA1 and CA3 pyramidal cells as well as granule cells (identified by their characteristic morphology and location) expressed high LacZ levels 1 day after infection with SFV–LacZ (Fig. 1 A–C). When virus at a concentration of 105–106 infectious particles per ml had been injected (100–2,000 applied infectious particles), reporter gene expression within single cells was extensive and could even be detected in more distal dendrites (Fig. 1). In the injected areas of six slices that had been injected with 100–200 infectious SFV–LacZ particles and analyzed 1 day postinfection, a total of 38 ± 10 cells per slice expressed LacZ. Of these cells, 60 ± 17% had characteristic neuronal morphology, demonstrating that under our conditions, SFV particles efficiently infect nerve cells. The injection procedure we used does not completely prevent virus from infecting cells outside the principal cell layers. Based on their morphology and location at the periphery of stratum oriens (cf. ref. 24), we could also identify putative interneurons (Fig. 1D) that had been infected with SFV–LacZ. At high concentrations of injected virus (107–109 infectious particles per ml), single cell bodies could not easily be recognized because the X-Gal staining covered extensive parts of the slices (data not shown).
Figure 1.
Expression of LacZ in neurons by using recombinant SFV. Bright-field illuminations of SFV–LacZ-injected (105 infectious particles per ml) hippocampal slice cultures fixed 1 day postinfection and stained with X-Gal. (A) Whole slice. (B) CA1 and CA3 pyramidal cells. (C) Granule cell. (D) Interneuron in stratum oriens of CA1 region; arrow indicates the alvear border of the slice. DG, dentate gyrus; so, stratum oriens; sp, stratum pyramidale; sr, stratum radiatum; sg, stratum granulosum; sm, stratum moleculare. [Bar = 330 μm (A), 130 μm (B and D), and 70 μm (C).]
Infection Using SIN–LacZ.
When we infected 17 slices with four different concentrations of SIN–LacZ (5 × 105–5 × 108 infectious particles per ml), results comparable to the SFV data were obtained. Infected CA1 and CA3 pyramidal cells, granule cells, and interneurons could be discerned based on their morphology and location within the slice (Fig. 2). One day after injecting 50–100 infectious SIN–LacZ particles, 26 ± 13 cells in the injected areas of each slice expressed LacZ (n = 4), and 56 ± 6% of these cells had unequivocal neuronal morphology. As for SFV–LacZ, single cell bodies could not easily be identified by X-Gal staining in slices infected with higher SIN–LacZ concentrations (5 × 107 and 5 × 108 infectious particles per ml). Our data thus suggest that both alphaviruses are useful vectors for efficient infection of neurons in hippocampal slices.
Figure 2.
Expression of LacZ by using recombinant SIN vectors. Bright-field illuminations of SIN–LacZ-injected (105 infectious particles per ml) hippocampal slice cultures fixed 1 day postinfection and stained with X-Gal. (A) Whole slice. (B) CA3 pyramidal cell. (C) Granule cells. (D) Interneuron in stratum oriens of CA3 region; arrow indicates the perimeter of the slice. Abbreviations are as in Fig. 1. [Bar = 330 μm (A), 100 μm (B and D), 80 μm (C).]
GFP-Expressing Neurons in Living Slice Cultures.
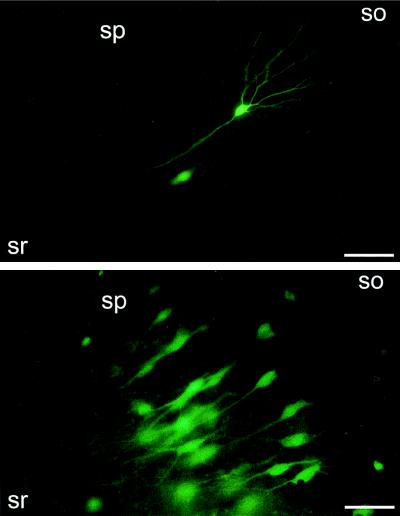
Because the spatial resolution of X-Gal staining is compromised by the precipitation of its chromogenic reaction product at some distance from its enzyme (6), we studied transgene expression of infected cells in living hippocampal slices by using the GFP reporter gene. We injected a total of 11 slices with two different concentrations of SFV encoding GFP under the direct control of the subgenomic RNA promoter (SFV–GFP; 3 × 106–3 × 107 infectious particles per ml). Infected cells could be detected as early as 14 h postinfection (the earliest time point tested). The GFP fluorescence was increased at 30 h postinfection and allowed the unambiguous identification of CA1 and CA3 pyramidal cells as well as interneurons. At this and subsequent time points, GFP fluorescence could easily be detected in very distal and thin dendrites and even spines (Fig. 3). On injection of 500–1,000 infectious SFV–GFP particles, 215 ± 58 cells in the injected areas per slice (n = 4) expressed GFP fluorescence at 30 h postinfection. Compared with the LacZ experiments, more GFP-positive cells (>90%) exhibited neuronal morphology. Neurons could be analyzed in living cultures at various time points (14, 30, and 51 h) postinfection, and no spreading of GFP fluorescence emerging from single GFP-positive cells was detected.
Figure 3.
Expression of GFP after infection with recombinant SFV. Fluorescence illuminations of SFV–GFP-injected, living hippocampal slice cultures. Slices at 11 (A) or 48 (B) days in culture and 2 (A) or 5 (B) days postinfection (≈5 × 107 infectious particles per ml); (C and D) CA1 pyramidal cells at 5 days postinfection. Abbreviations are as in Fig. 1. [Bar = 330 μm (A and B), 80 μm (C), 25 μm (D).]
Results similar to the SFV–GFP data were obtained in 19 slices that had been injected with SIN particles encoding GFP (SIN–GFP). At 20 h to 8 days postinfection, GFP fluorescence could easily be detected in very distal and thin dendrites of up to fourth order (Fig. 4 Upper). There was a total of 194 GFP-fluorescent cells in the pyramidal cell layer of nine slices, and 97% exhibited neuronal morphology. GFP fluorescence levels at 1–5 days postinfection were comparable in SIN–GFP- and SFV–GFP-infected neurons. These data are consistent with the preferential infection of neurons vs. glia by SFV and SIN vectors in hippocampal slice cultures. The efficient infection of neurons by SFV and SIN vectors contrasts with data obtained on injection of recombinant adenovirus expressing GFP under the control of a cytomegalovirus promoter (25) into the pyramidal cell layer of hippocampal slice cultures (unpublished results).
Figure 4.
Expression of GFP by using SIN (Upper) and SFV (Lower) vectors. Fluorescence micrographs of CA1 regions from hippocampal slice cultures 46 and 66 h after injection of SIN–GFP and SFV–IRES–GFP (106 infectious particles per ml), respectively. Abbreviations are as in Fig. 1. (Bars = 85 μm.)
Detection of transfected cells in living slices is crucial in many experimental situations (e.g., confocal imaging or electrophysiological recording). It is thus useful to cotransfect GFP as either the separate protein or a fusion construct together with the gene to be studied. As a first step in developing this approach, we tested SFV particles encoding GFP under the control of an IRES rather than the subgenomic RNA promoter. SFV–IRES–GFP was injected into 14 slice cultures at three different concentrations (105–107 infectious particles per ml). At 20 h postinfection, significant GFP expression was detected in the cell body and proximal dendrites of infected pyramidal cells. GFP fluorescence continued to increase over the following 24–72 h and was then detected in distal dendrites (Fig. 4 Lower). At all virus concentrations tested, single fluorescent cells could be discerned. Over 90% of the cells with detectable GFP fluorescence in the pyramidal cell layer were morphologically identified as neurons. The results obtained with SFV–IRES–GFP were therefore comparable with the data for SFV and SIN particles encoding GFP under the direct control of the subgenomic RNA promoter. Compared with this promoter, however, IRES-dependent gene expression is ≈10-fold weaker and resulted in much lower GFP fluorescence levels at 1 and 2 days postinfection.
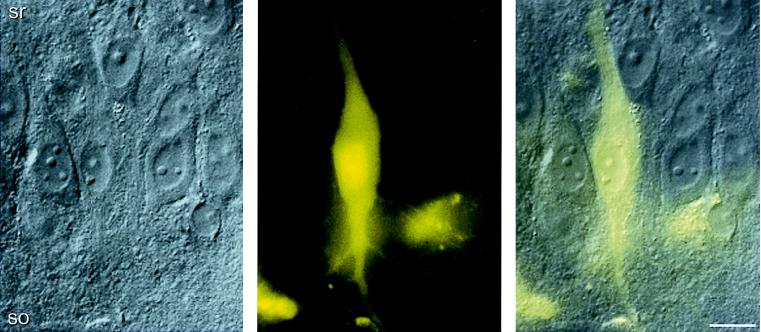
Infected pyramidal cells expressing GFP were identified at higher magnification by comparing differential interference contrast and fluorescence images from stratum pyramidale, as illustrated for CA3 neurons (Fig. 5). Again, fine dendritic processes could be resolved. The cell body, nucleus, and dendrites of infected cells appeared normal at ≤ 5 days after infection (Figs. 3–5), indicating that both SFV and SIN particles do not cause morphological defects up to 5 days postinfection.
Figure 5.
Pyramidal cell infected by recombinant SFV expressing GFP. Differential interference contrast (Left) and GFP fluorescence micrograph (Center) of CA3 pyramidal cells 72 h postinfection. The GFP micrograph was recorded as a grayscale image and later transformed into a false color image. Overlay (Right) demonstrates the location of the GFP-positive cell in stratum pyramidale. Abbreviations are as in Fig. 1. (Bar = 20 μm.)
Viability of Infected Cells.
For both SFV and SIN, single GFP-positive neurons appearing morphologically intact could be recognized and tracked at increasing intervals (up to 8 days) postinfection. At the same time, the GFP fluorescence did not spread from these isolated neurons onto neighboring cells (Fig. 4). Moreover, the number of GFP-positive neurons per slice was roughly comparable at 1–5 days after injection of similar amounts of SFV–GFP (Fig. 3 A vs. B) and SIN–GFP. Any reduction in the number of GFP-positive cells was paralleled by a concomitant death of nonfluorescent cells in the slice culture, presumably because of excitotoxic effects associated with the injection procedure (see Materials and Methods). Taken together, our results indicate that (i) neurons can tolerate infection by SFV and SIN for several days and (ii) the replicons do not propagate in the slice cultures.
We additionally examined cell survival by assaying for exclusion of propidium iodide. At 5 days postinfection with SIN–GFP, 93% of all GFP-positive pyramidal cells (total of 105 cells analyzed) excluded propidium iodide. This result suggests that neurons infected by SFV and SIN vectors in hippocampal slice cultures remain viable up to 5 days postinfection. At later time points (9–10 days) postinfection, by contrast, almost all fluorescent cells appeared to have died, because they were round and without processes (data not shown). Normal function of the infected neurons within the hippocampal slices at ≤5 days postinfection remains to be demonstrated.
DISCUSSION
Preferential Infection of Neurons.
In this study we have demonstrated that (i) both SFV and SIN vectors are useful for the efficient transfer of genes into neurons in hippocampal slices, and (ii) infected neurons appear morphologically normal and viable up to 5 days postinfection. Although successful infection of neurons in slices has most recently been observed for SIN particles (26), this has not been previously demonstrated for SFV vectors. We injected the SFV and SIN particles into the pyramidal cell layer, i.e., an area known to contain large numbers of glia and microglia in hippocampal slice cultures (27, 28). The resulting infection patterns (neurons vs. nonneuronal cells) are similar for both vectors (Figs. 1–4). This result is not surprising because the wild-type viruses are closely related (8). In each case, ≈60% and >90% of the cells expressing LacZ and GFP, respectively, showed neuronal morphology. Wild-type SFV and SIN have a broad host range and also infect glial cells in situ (10, 29, 30); however, neurons are the predominant neural cell type infected (9, 10, 13, 15, 26, 31). Our finding that the neuronal preference of both vectors was more obvious for GFP than LacZ was unexpected. X-Gal staining requires prior fixation of the slices. One explanation is that our fixation conditions inactivated LacZ in certain dendrites of cells, thus hindering their identification as neurons. Identification of infected neurons in living slices is likely to be a more valuable approach, and our results with GFP have clearly demonstrated preferred infection of neurons by SFV and SIN vectors in cultured hippocampal slices. Favored infection of neurons by both replication-defective vectors has previously been observed (13, 15, 26) and can be used as an alternative method to using neuron-specific promoters (32) for directing transgene expression in vitro and in vivo.
Viability of Infected Cells.
In cultures of dissociated hippocampal cells, SFV vectors can cause visible neuronal damage within short time periods (<24 h; ref. 14). However, we recently found that cytotoxic effects in dissociated neurons are reduced when SFV particles are resuspended in neuronal culture medium before infection (K.L., unpublished data). Therefore, the reported damage in dissociated hippocampal neurons (14) may have partially been because of the presence of neurotoxic compounds in the supernatant of BHK-21 cells. During our infection procedure, the viral supernatants were diluted from 1:100 to 1:10,000 before injection, and the slices were washed twice thereafter. Thus, in our slice cultures, the infected pyramidal cells appeared healthy even 5 days postinfection. It is also possible that the matrix surrounding the neurons makes these cells more resistant to possible deleterious effects of infection with SFV and SIN expression vectors. Nevertheless, cytopathic effects are likely to occur after long time periods because alphavirus vectors normally inhibit host-cell protein synthesis (11).
The SFV and SIN particles used in the present study are replication-deficient suicide vectors that normally complete only one infection cycle. In addition, the SFV particles used herein provide the supplementary safety feature of three point mutations in the viral p62 precursor, resulting in conditionally infectious SFV particles (18). Our observation that, on infection, the GFP fluorescence does not spread further supports the suicidal nature of the SFV and SIN vectors used. Data from studies in mice with replication-competent SFV (33) and SIN (9) have indicated that neuronal survival and neurotropism are determined by both the viral strain and the age of the injected animals. It has been demonstrated that a single amino acid substitution in the E2 spike protein of SFV can dramatically change the virulence (34). SIN strains also show remarkable differences in neurovirulence based on one or very few amino acid changes in the E2 protein (35, 36). Less virulent forms of these expression vectors may allow even better neuron survival.
Visualization of Infected Neurons in Living Slice Cultures.
Although viral infection of hippocampal neurons in slices and in vivo has previously been demonstrated (1–3, 13, 37), visualization of living transfected cells is often difficult in these preparations, and detection of the reporter gene product is restricted to the cell body and proximal dendrites (3). In contrast, living transfected neurons can easily be identified in hippocampal slice cultures, and reporter gene expression is visible even in distal dendrites (Figs. 3 and 4).
Hippocampal slices cultured on a porous membrane have also been infected with viral vectors (2, 26, 38). Both the membrane and the thickness of the preparation (100–150 μm; ref. 16), however, can interfere with certain types of microscopic analysis. By using the novel combination of (i) viral vectors that efficiently infect neurons, (ii) the GFP reporter, and (iii) 50- to 100-μm thick (16, 21) organotypic slices on glass coverslips, we now show that living transfected neurons can readily be distinguished with standard microscopy. It should also be noted that both SFV and SIN vectors can be quickly produced (within 2 days), and no further purification or concentration is necessary. This approach, therefore, will be advantageous for studies where physiological analysis of transfected neurons, e.g., by electrophysiological recording or intracellular calcium imaging, is required.
In conclusion, we have demonstrated that SFV and SIN expression vectors preferentially infect pyramidal cells, interneurons, and granule cells in rat hippocampal slice cultures. By using GFP as a reporter, infected neurons were easily identified in living slices. They appeared morphologically normal and viable for up to at least 5 days postinfection. The excellent visualization of GFP-tagged neurons in living organotypic slices by conventional microscopy will facilitate the physiological analysis of selected gene products in transfected neurons.
Acknowledgments
We thank Dr. K. Monastyrskaia for constructing pSFV2 gen–IRES–GFP, L. Rietschin and L. Heeb for preparing the slice cultures, R. Schöb for help with the photographs, Dr. R.A. McKinney for helpful discussions, and Dr. U. Gerber and Dr. M. Lanzrein for comments on the manuscript. This work was supported by a grant of the Betty and David Koetser Foundation for Brain Research.
ABBREVIATIONS
- BHK
baby hamster kidney
- GFP
green fluorescent protein
- LacZ
β-galactosidase
- IRES
cap-independent ribosomal entry site
- SFV
Semliki Forest virus
- SIN
Sindbis virus
- X-Gal
5-bromo-4-chloro-3-indolyl β-d-galactoside
References
- 1.Le Gal La Salle G, Robert J J, Berrard S, Ridoux V, Stratford-Perricaudet L D, Perricaudet M, Mallet J. Science. 1993;259:988–990. doi: 10.1126/science.8382374. [DOI] [PubMed] [Google Scholar]
- 2.Casaccia-Bonnefil P, Benedikz E, Shen H, Stelzer A, Edelstein D, Geschwind M, Brownlee M, Federoff H J, Bergold P J. J Neurosci Methods. 1993;50:341–351. doi: 10.1016/0165-0270(93)90040-x. [DOI] [PubMed] [Google Scholar]
- 3.Pettit D L, Perlman S, Malinow R. Science. 1994;266:1881–1885. doi: 10.1126/science.7997883. [DOI] [PubMed] [Google Scholar]
- 4.Xiao X, Li J, McCown T J, Samulski R J. Exp Neurol. 1997;144:113–124. doi: 10.1006/exnr.1996.6396. [DOI] [PubMed] [Google Scholar]
- 5.Naldini L, Blömer U, Gallay P, Ory D, Mulligan R, Gage F H, Verma I M, Trono D. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 6.Lo D C, McAllister A K, Katz L C. Neuron. 1994;13:1263–1268. doi: 10.1016/0896-6273(94)90412-x. [DOI] [PubMed] [Google Scholar]
- 7.Johnson P A, Yoshida K, Gage F H, Friedmann T. Mol Brain Res. 1992;12:95–102. doi: 10.1016/0169-328x(92)90072-j. [DOI] [PubMed] [Google Scholar]
- 8.Strauss J H, Strauss E G. Microbiol Rev. 1994;58:491–562. doi: 10.1128/mr.58.3.491-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffin D E. Neurosci Biobehav Rev. 1998;22:721–723. doi: 10.1016/s0149-7634(98)00010-4. [DOI] [PubMed] [Google Scholar]
- 10.Fazakerley J K, Pathak S, Scallan M, Amor S, Dyson H. Virology. 1993;195:627–637. doi: 10.1006/viro.1993.1414. [DOI] [PubMed] [Google Scholar]
- 11.Schlesinger S. Trends Biotechnol. 1993;11:18–22. doi: 10.1016/0167-7799(93)90070-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liljeström P, Garoff H. Biotechnology. 1991;9:1356–1361. doi: 10.1038/nbt1291-1356. [DOI] [PubMed] [Google Scholar]
- 13.Gwag B J, Kim E Y, Ryu B R, Won S J, Ko H W, Oh Y J, Cho Y-G, Ha S J, Sung Y C. Mol Brain Res. 1998;63:53–61. doi: 10.1016/s0169-328x(98)00251-4. [DOI] [PubMed] [Google Scholar]
- 14.Olkkonen V M, Liljeström P, Garoff H, Simons K, Dotti C G. J Neurosci Res. 1993;35:445–451. doi: 10.1002/jnr.490350412. [DOI] [PubMed] [Google Scholar]
- 15.Lundstrom K, Richards J G, Pink J R, Jenck F. Gene Ther Mol Biol. 1999;3:15–23. [Google Scholar]
- 16.Gähwiler B H, Capogna M, Debanne D, McKinney R A, Thompson S M. Trends Neurosci. 1997;20:471–477. doi: 10.1016/s0166-2236(97)01122-3. [DOI] [PubMed] [Google Scholar]
- 17.Lundstrom K, Mills A, Buell G, Allet E, Adami N, Liljeström P. Eur J Biochem. 1994;224:917–921. doi: 10.1111/j.1432-1033.1994.00917.x. [DOI] [PubMed] [Google Scholar]
- 18.Berglund P, Sjöberg M, Garoff H, Atkins G J, Sheahan B J, Liljeström P. Biotechnology. 1993;11:916–920. doi: 10.1038/nbt0893-916. [DOI] [PubMed] [Google Scholar]
- 19.Bredenbeek P J, Frolov I, Rice C M, Schlesinger S. J Virol. 1993;67:6439–6446. doi: 10.1128/jvi.67.11.6439-6446.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frolov I, Schlesinger S. J Virol. 1994;68:1721–1727. doi: 10.1128/jvi.68.3.1721-1727.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gähwiler B H. J Neurosci Methods. 1981;4:329–342. doi: 10.1016/0165-0270(81)90003-0. [DOI] [PubMed] [Google Scholar]
- 22.Sanes J R, Rubenstein J L R, Nicolas J-F. EMBO J. 1986;5:3133–3142. doi: 10.1002/j.1460-2075.1986.tb04620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ankarcrona M, Dypbukt J M, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton S A, Nicotera P. Neuron. 1995;15:961–973. doi: 10.1016/0896-6273(95)90186-8. [DOI] [PubMed] [Google Scholar]
- 24.Poncer J-C, McKinney R A, Gähwiler B H, Thompson S M. Neuron. 1997;18:463–472. doi: 10.1016/s0896-6273(00)81246-5. [DOI] [PubMed] [Google Scholar]
- 25.Ehrengruber M U, Lanzrein M, Xu Y, Jasek M C, Kantor D B, Schuman E M, Lester H A, Davidson N. Methods Enzymol. 1998;293:483–503. doi: 10.1016/s0076-6879(98)93030-0. [DOI] [PubMed] [Google Scholar]
- 26.Maletic-Savatic M, Malinow R, Svoboda K. Science. 1999;283:1923–1927. doi: 10.1126/science.283.5409.1923. [DOI] [PubMed] [Google Scholar]
- 27.del Rio J A, Heimrich B, Soriano E, Schwegler H, Frotscher M. Neuroscience. 1991;43:335–347. doi: 10.1016/0306-4522(91)90298-3. [DOI] [PubMed] [Google Scholar]
- 28.Hailer N P, Järhult J D, Nitsch R. Glia. 1996;18:319–331. doi: 10.1002/(sici)1098-1136(199612)18:4<319::aid-glia6>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 29.Shahar A, Lustig S, Akov Y, David Y, Schneider P, Levin R. J Neurosci Res. 1990;25:345–352. doi: 10.1002/jnr.490250311. [DOI] [PubMed] [Google Scholar]
- 30.Balluz I M, Glasgow G M, Killen H M, Mabruk M J M E F, Sheahan B J, Atkins G J. Neuropath Appl Neurobiol. 1993;19:233–239. doi: 10.1111/j.1365-2990.1993.tb00433.x. [DOI] [PubMed] [Google Scholar]
- 31.Jackson A C, Moench T R, Trapp B D, Griffin D E. Lab Invest. 1988;58:503–509. [PubMed] [Google Scholar]
- 32.Forss-Petter S, Danielson P E, Catsicas S, Battenberg E, Price J, Nerenberg M, Sutcliffe J G. Neuron. 1990;5:187–197. doi: 10.1016/0896-6273(90)90308-3. [DOI] [PubMed] [Google Scholar]
- 33.Oliver K R, Scallan M F, Dyson H, Fazakerley J K. J Neurovirol. 1997;3:38–48. doi: 10.3109/13550289709015791. [DOI] [PubMed] [Google Scholar]
- 34.Glasgow G M, Killen H M, Liljeström P, Sheahan B J, Atkins G J. J Gen Virol. 1994;75:663–668. doi: 10.1099/0022-1317-75-3-663. [DOI] [PubMed] [Google Scholar]
- 35.Davis N L, Fuller F J, Dougherty W G, Olmsted R A, Johnston R E. Proc Natl Acad Sci USA. 1986;83:6771–6775. doi: 10.1073/pnas.83.18.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lustig S, Jackson A C, Hahn C S, Griffin D E, Strauss E G, Strauss J H. J Virol. 1988;62:2329–2336. doi: 10.1128/jvi.62.7.2329-2336.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kantor D B, Lanzrein M, Stary S J, Sandoval G M, Smith W B, Sullivan B M, Davidson N, Schuman E M. Science. 1996;274:1744–1748. doi: 10.1126/science.274.5293.1744. [DOI] [PubMed] [Google Scholar]
- 38.Ridoux V, Robert J-J, Perricaudet M, Mallet J, Le Gal La Salle G. Neurobiol Dis. 1995;2:49–54. doi: 10.1006/nbdi.1995.0005. [DOI] [PubMed] [Google Scholar]