Abstract
Leptin regulates energy balance through its actions in the brain on appetite and energy expenditure and also shares properties with cytokines such as IL-1. We report here that leptin, injected into rats intracerebroventricularly or peripherally, induces significant dose-dependent increases in core body temperature as well as suppression of appetite. Leptin failed to affect food intake or body temperature in obese (fa/fa) Zucker rats, which posses a defective leptin receptor. Furthermore, injection of leptin increased levels of the proinflammatory cytokine IL-1β in the hypothalamus of normal Sprague–Dawley rats. Central injection of IL-1 receptor antagonist (IL-1ra) inhibited the suppression of food intake caused by central or peripheral injection of leptin (60 and 84%, respectively) and abolished the leptin-induced increase in body temperature in both cases. Mice lacking (gene knockout) the main IL-1 receptor (80 kDa, R1) responsible for IL-1 actions showed no reduction in food intake in response to leptin. These data indicate that leptin actions in the brain depend on IL-1, and we show further that the effect of leptin on fever, but not food intake, is abolished by a cyclooxygenase inhibitor. Thus, we propose that in addition to its role in body weight regulation, leptin may mediate neuroimmune responses via actions in the brain dependent on release of IL-1 and prostaglandins.
Leptin, the 16-kDa protein product of the ob gene (1), is an important regulator of energy balance through its actions in the brain on appetite and energy expenditure (2–4). Leptin is synthesized mainly by adipose tissue in proportion to body fat mass (5) and released into circulation to act both peripherally and in the brain (6). Leptin enters the brain via a saturable transport mechanism (7) and is believed to act primarily on hypothalamic targets involved in satiety and the regulation of energy balance. The evidence that leptin acts in the brain is derived from observations that much lower doses of leptin are required to elicit responses when injected centrally rather than peripherally (2), and lesions of the ventromedial hypothalamus induce obesity and increase ob gene expression (8). Furthermore, the leptin receptor, which shares sequence homology and functional similarity with members of the class I cytokine receptors (9–11), is expressed primarily in the hypothalamus (12, 13).
Infection, injury, and inflammation are associated with negative energy balance, characterized by reduced food intake, weight loss, increased thermogenesis, and fever. Administration of bacterial lipopolysaccharide (LPS) in rodents, to generate a host defense response, up-regulates leptin gene expression and serum protein levels (14, 15). LPS is a potent inducer of cytokines both in vivo and in vitro (16), and induction of leptin in response to LPS appears to be mediated via release of the cytokines IL-1 and tumor necrosis factor α (TNF-α) (15, 17, 18). Indeed, both IL-1 and TNF-α have been shown to directly increase ob mRNA expression and serum leptin concentration in rodents (14, 15), and LPS fails to increase leptin levels in mice lacking (gene knockout) IL-1β (17). Conversely, exogenous leptin has been demonstrated to up-regulate LPS-induced phagocytosis and proinflammatory cytokine expression (TNF-α, IL-6, IL-12) in ex vivo macrophages from mice (19). In addition, leptin-deficient mice (ob/ob) and rats that possess a defective leptin receptor (fa/fa) exhibit attenuated levels of serum TNF-α and IL-6 in response to LPS administration (19).
The results of these studies suggest that leptin may interact with cytokines to influence responses to infection, such as anorexia and cachexia. In the present study we investigated the interactions between leptin and the cytokine IL-1. Here we present evidence that leptin directly induces release of IL-1 within the brains of normal rats. We also demonstrate that leptin mimics the actions of this cytokine in the central nervous system and that effects of leptin on food intake and body temperature are mediated by IL-1.
METHODS
Animals and Injections.
All studies were conducted in young adult male Sprague–Dawley (SD) rats (250–300 g, Charles River Breeding Laboratories) unless stated otherwise. Additional experiments were performed by using male, genetically obese Zucker rats (fa/fa, 500–600 g, Harlan Olac, Bichester, U.K.), and their lean controls (Fa/?, 350–400 g) and C57BL/6 mice or mice lacking the IL-1RI receptor [Immunex (20)].
Animals were exposed to an ambient temperature of 22°C and a 12-h light/12-h dark cycle (8:00 a.m. to 8:00 p.m.). Indwelling guide cannulae were stereotaxically implanted in the right lateral cerebral ventricle [intracerebroventricularly (ICV)] of rats under halothane anesthesia, at least 7 days before experiments. During the same procedure, temperature-sensitive radiotransmitters (Datasciences, Minneapolis) were implanted into the peritoneum.
ICV injections (2 μl) administered via indwelling guide cannulae or peripheral (i.p.) injections were performed at 10:00 a.m. in free-moving animals (n = 5–6). Core body temperatures were monitored by remote radiotelemetry for 10 h after injection. Food intake (pelleted rat chow; Beekay International, Hull, U.K.) and body weights were measured more than 22 h after injection, until the beginning of the subsequent light phase.
Drugs.
Recombinant murine leptin, obtained from Insight Biotechnology (Wembley, U.K.) or kindly donated by Brian Holloway, Zeneca Pharmaceuticals, Alderley Edge, U.K. (endotoxin level <0.1 ng/μg of leptin, i.e., below the detection limit of endotoxin assay) was dissolved in sterile water for injection and administered at doses of 0.4, 1, or 4 μg/rat (ICV) or 3.5 mg/kg (≈1 mg/rat, i.p.) at the 0-h time point (10:00 a.m.). Recombinant human IL-1 receptor antagonist (IL-1ra, 200 μg/rat; PeproTech, Rocky Hill, NJ) was administered ICV in saline vehicle at 0 and 1 h. The cyclooxygenase inhibitor flurbiprofen (Knoll, Liestal, Switzerland) was dissolved in 1% sodium bicarbonate and 0.9% sterile saline and administered i.p. (1 mg/kg) at 0 h. Prostaglandin E2 (500 ng/rat; Sigma) was dissolved in 0.9% saline and injected ICV at 0 h.
ELISA.
Immunoassays were performed on hypothalamic samples (average mass, 40 mg) from animals (n = 6) injected ICV with either leptin (4 μg) or vehicle, by using ELISA assays specific for rat IL-1β (kindly provided by Steve Poole, National Institute of Biological Standards and Controls, U.K.). Four hours after injection, blood plasma was sampled, the animals were killed by cervical dislocation, and the hypothalami were removed. The hypothalami were placed in PBS containing protease inhibitors, homogenized, and centrifuged (6,000 rpm for 30 min at 4°C). Both hypothalamic supernatant and blood plasma were diluted in high-performance buffer (HPE; Central Laboratory for Blood Transfusion, Amsterdam) and assayed for immunoreactive IL-1β by ELISA, using immunoaffinity-purified polyclonal antibodies (2 μg/ml) from sheep anti-rat IL-1β (S1002BH), recombinant rat IL-1β (1.9–2,000 pg/ml) as reference standards; biotinylated, immunoaffinity-purified polyclonal antibodies from sheep anti-rat IL-1β serum (1/1,000), avidin-horseradish peroxidase (1/5,000), and color reagent (O-phenylenediamine). We have established that brain homogenates do not influence detection of IL-1 in the HPE buffer. The optical densities of the wells were read at 490 nm by using an MRX Microplate reader (Dynatech). Data were corrected for tissue protein concentration (Bio-Rad).
Data Analysis.
All data are reported as mean values ± SEM. Comparisons of data from two treatment groups were performed by using an unpaired t test. Data from more than two treatment groups were analyzed by ANOVA, followed by Newman–Keuls multiple comparisons test. Body temperatures of animals were compared over the time course of the experiment by MANOVA, which allows multiple analysis of variance of several groups with time. Where MANOVA revealed significant difference, individual time points of interest were analyzed further by using ANOVA. Statistical analyses revealing values less than 5% were deemed significant.
RESULTS
Effects of Leptin on Food Intake and Core Body Temperature.
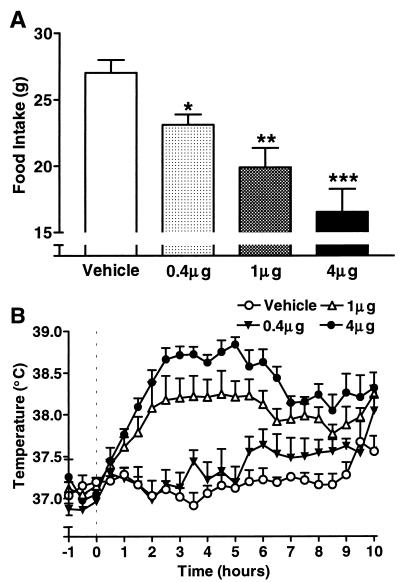
Vehicle-treated animals consumed 27.0 ± 1.0 g food over the 22 h after injection at 10:00 a.m. (Fig. 1A). All rats injected (ICV) with leptin exhibited significantly attenuated food intake compared with the control group (ANOVA). Administration of 0.4 μg leptin inhibited food intake by 15% (P < 0.05), whereas 1 and 4 μg attenuated consumption by 26% (P < 0.01) and 40% (P < 0.001), respectively, compared with vehicle-treated rats.
Figure 1.
Effects of leptin on food intake and core body temperature. (A) Leptin (0.4, 1, and 4 μg/rat) injected ICV (at 10:00 a.m.) induced dose-dependent reduction in food intake over 22 h in SD rats (ANOVA; ∗, P < 0.05, ∗∗, P < 0.01, ∗∗∗, P < 0.001 vs. vehicle, n = 5). (B) ICV injection (at 10:00 a.m. = 0 h) of leptin (0.4, 1, and 4 μg/rat) elicited dose-dependent increases in core body temperature in SD rats (MANOVA, 0.4 μg, not significant; 1 μg, P < 0.01; 4 μg, P < 0.001 vs. vehicle, n = 5).
Core body temperatures of vehicle-treated rats remained between 36.9 and 37.3°C for 9 h after injection, before rising to 37.7°C at the 9.5-h time point at the beginning of the dark phase (Fig. 1B). Data were similar for vehicle injections in all subsequent experiments. Animals injected with the lowest dose of leptin (0.4 μg) exhibited body temperatures that, although elevated after the 5-h time point, did not deviate significantly from animals receiving vehicle [multiple ANOVA (MANOVA)]. Injection of 1 μg leptin induced a significant increase in core body temperature (MANOVA, P < 0.01 vs. vehicle) that began to rise 1 h after injection and remained constant between the 2.5- and 5.5-h time points, 1.0°C above temperatures of vehicle-treated animals (ANOVA, P < 0.01). The highest dose of leptin (4 μg) also elicited a significant increase in core body temperature (MANOVA, P < 0.001 vs. vehicle) that began to rise 1 h after injection and peaked at the 5-h time point (1.7°C above control; ANOVA, P < 0.001).
To test whether the observed effect of leptin on core body temperature was caused by endotoxin contamination, a number of control experiments were conducted. First, leptin was heat-treated (98°C for 30 min) to denature the protein. Injection of this solution into normal rats failed to affect food intake or body temperature (data not shown). Second, leptin (4 μg) was injected (ICV) in lean (Fa/?) and obese (fa/fa) Zucker rats (which possess a defective leptin receptor). Lean animals exhibited reductions in food intake (attenuated by 50%; ANOVA, P < 0.001 vs. lean vehicle) and temperature responses (MANOVA, P < 0.01 vs. lean vehicle) similar to those observed in normal SD rats in response to ICV injection of leptin. Conversely, we observed no effects of leptin on food intake or body temperature in obese Zucker rats. In contrast, ICV injection of the proinflammatory molecule prostaglandin E2 (a mediator of IL-1 action) elicited similar reductions in food intake (17% in lean and 22% in obese Zucker rats; ANOVA, P < 0.05 vs. vehicle) and increases in body temperature (MANOVA, P < 0.001 vs. vehicle) in both lean and obese rats.
Leptin Increases Hypothalamic IL-1β.
Leptin has been reported to exert many of its actions in the brain by acting on its receptors in the hypothalamus—a region associated with energy balance (21) and neuroimmune responses involving cytokines such as IL-1β (22). Administration of leptin (4 μg, ICV) elicited a significant increase (t test; P < 0.001, n = 6) in the hypothalamic levels of immunoreactive IL-1β (1,800 ± 30 pg/mg protein) compared with vehicle-treated animals (300 ± 100 pg/mg of protein) measured by ELISA 4 h after injection. Peripheral administration of leptin (1 mg/rat, i.p.) also induced a rise in hypothalamic IL-1β 4 h after injection (58 ± 4 pg/mg of protein) that was significantly higher than IL-1β levels of vehicle-treated animals, which were below the detection limit of this assay (22 pg/mg of protein). Levels of IL-1β in plasma and hypothalami taken from uncannulated animals were below the detection limit of the assay (data not shown).
IL-1ra Inhibits Actions of Leptin.
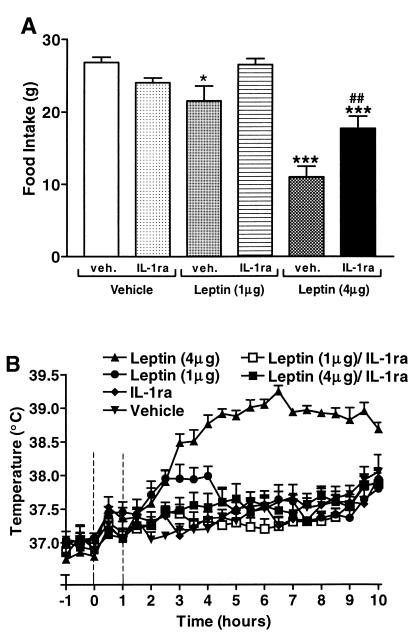
Food intake (Fig. 2A) was reduced significantly by 55% (ANOVA, P < 0.001) over 22 h in normal rats in response to ICV injection of 4 μg leptin compared with vehicle-treated animals (26.8 ± 0.1 g). Central injection (ICV) of IL-1ra (200 μg) at 0 and 1 h alone did not significantly affect food intake (ANOVA). Coadministration of leptin and IL-1ra, however, attenuated (by 60%) the suppression of food intake observed in leptin-treated rats (ANOVA, P < 0.001), so that food intake was returned to within 70% of respective controls (ANOVA, P < 0.001). In a different set of animals, a lower dose of leptin (1 μg) was injected and, as with the higher dose (4 μg), caused a significant inhibition of food intake (28%; ANOVA, P < 0.05 vs. vehicle). This effect was partially but not significantly attenuated by coadministration of IL-ra (200 μg, ICV; Fig. 2A).
Figure 2.
IL-1ra attenuates responses to central injection of leptin. (A) Food intake of SD rats, measured over 22 h, was reduced significantly (ANOVA; ∗, P < 0.05 and ∗∗∗, P < 0.001 vs. vehicle) by ICV injection of leptin (1 μg/rat or 4 μg/rat, at 10:00 a.m. = 0 h). IL-1ra (200 μg/rat, ICV, at 0 and 1 h) attenuated this suppression of appetite in the case of the 4-μg dose (##, P < 0.01), although values remained significantly lower than food intake of vehicle-treated rats (∗∗∗, P < 0.001, n = 5–6). (B) The increase in core temperature (MANOVA; P < 0.001 vs. vehicle) elicited by injection (ICV, at 10:00 a.m. = 0 h) of leptin (1 and 4 μg/rat) in SD rats was abolished (MANOVA; P < 0.001) by injection (ICV, at 0 and 1 h) of IL-1ra (200 μg/rat, n = 5–6).
Central administration (ICV) of IL-1ra (200 μg, 0 and 1 h) did not significantly affect core body temperature (Fig. 2B) compared with vehicle-treated rats. Injection (ICV) of leptin (4 μg) induced a significant increase in core body temperature (MANOVA, P < 0.001 vs. vehicle). Temperatures of leptin-treated animals rose after the 1.5-h time point and peaked (38.9 ± 0.1°C) 4.5 h after injection (ANOVA, P < 0.001 vs. vehicle). Body temperatures returned to control levels at the 10-h time point. This leptin-induced response was abolished completely (MANOVA, P < 0.001) by coadministration of IL-1ra. A different group of animals was treated with a lower dose of leptin (1 μg/rat ICV) in the presence and absence of the same dose of IL-1ra. At this dose, leptin induced an increase in body temperature that peaked at 3 h postinjection (38.1 ± 0.1°C) and was abolished by coadministration of IL-1ra.
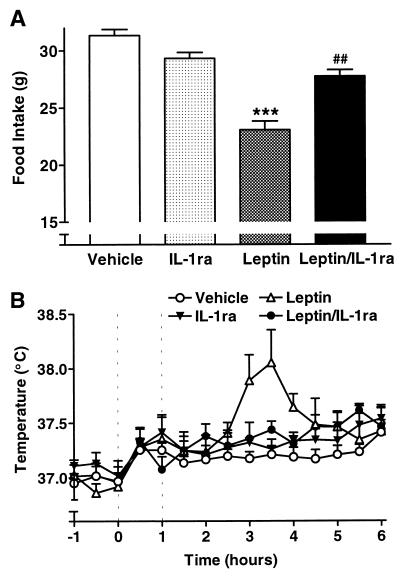
Peripheral (i.p.) administration of leptin (1 mg/rat) also significantly reduced food intake (Fig. 3A) by 26% over 22 h after injection (ANOVA, P < 0.001) compared with vehicle-treated animals (31.5 ± 0.5 g). Central injection (ICV) of IL-1ra (200 μg, 0 and 1 h) alone did not significantly affect food intake. However, coadministration of IL-1ra significantly (ANOVA, P < 0.01) attenuated (by 60%) the reduced consumption induced by leptin, such that food intake was not significantly different from that of vehicle-treated animal control levels. In a separate experiment, a 10-fold-higher dose of leptin (10 mg/rat, i.p.) had a similar effect on body temperature and food intake to the 1 mg/rat dose, indicating that the latter was maximal (data not shown). Leptin (1 mg/rat, i.p.) also induced a significant increase in core body temperature (MANOVA, P < 0.01 vs. vehicle) after the 2.5-h time point. Temperatures peaked 3.5 h (38.0 ± 0.3°C; ANOVA, P < 0.01 compared with controls) after injection before returning to control temperatures at the 5.5-h time point. Coadministration of IL-1ra abolished this increase in core body temperature (MANOVA, P < 0.05) to control levels.
Figure 3.
IL-1ra attenuates responses to peripheral injection of leptin. (A) Food intake was reduced significantly (ANOVA; ∗∗∗, P < 0.001 vs. vehicle) over 22 h in SD rats by i.p. injection (at 10:00 a.m. = 0 h) of leptin (3.5 mg/kg). This response was abolished completely by administration of IL-1ra (200 μg/rat, ICV, at 0 and 1 h) (ANOVA; ##, P < 0.01, n = 5). (B) Peripheral injection of leptin (3.5 mg/kg, i.p., at 10:00 a.m. = 0 h) induced a significant rise in core body temperature in SD rats (MANOVA; P < 0.01 vs. vehicle). This rise in body temperature was abolished (##, P < 0.01) by coadministration (ICV, at 0 and 1 h) of IL-1ra (200 μg/rat, n = 5).
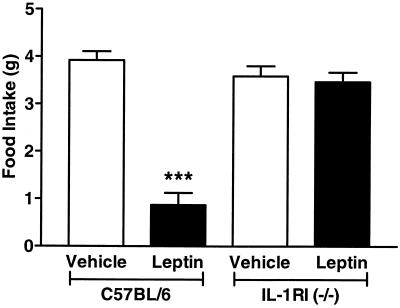
To confirm further the involvement of IL-1 in the actions of leptin, studies were conducted in mice lacking the IL-1RI receptor, which is responsible for all known actions of IL-1 (23). Normal, C57BL/6 mice injected with 4 μg leptin ICV showed a 77% reduction in food intake over a 22-h period compared with injection of vehicle. In contrast, in RI knockout mice, leptin had no effect on food intake (Fig. 4). Levels of food intake were similar for both groups of animals injected with vehicle (C57BL/6 3.9 ± 0.2 g and IL-1RI 3.6 ± 0.2 g; Fig. 4).
Figure 4.
Leptin fails to induce suppression of food intake in IL-1RI−/− mice. Leptin (4 μg/mouse, ICV) caused a highly significant (∗∗∗, P < 0.001 vs. vehicle) reduction in food intake over 22 h in C57BL/6 mice but had no effect in IL-1RI−/− mice that consumed a similar amount of food as the vehicle-treated animals (n = 6).
Influence of a Cyclooxygenase Inhibitor on Leptin Actions.
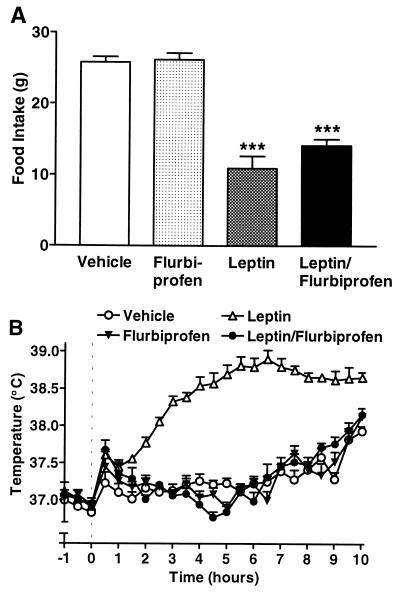
Vehicle-treated animals consumed 25.8 ± 0.8 g food over 22 h after injection (Fig. 5A) which was similar (26.2 ± 0.9 g) in animals injected with flurbiprofen (1 mg/kg, i.p.). Leptin administration (4 μg, ICV) induced a significant reduction (by 58%) in food intake (ANOVA, P < 0.001 vs. vehicle). Animals coinjected with leptin and flurbiprofen exhibited food intake (14.1 ± 0.9 g) that was not significantly different from those treated with leptin alone (10.9 ± 1.7 g, ANOVA), but nevertheless was attenuated significantly (by 45%) compared with food intake of vehicle-treated rats (ANOVA, P < 0.001).
Figure 5.
Cyclooxygenase inhibitor attenuates leptin-induced increase in body temperature, but not appetite suppression. (A) Injection (i.p., at 10:00 a.m. = 0 h) of flurbiprofen (1 mg/kg) had no effect on the suppression of food intake (ANOVA; ∗∗∗, P < 0.001 vs. vehicle) measured over 22 h in SD rats in response to injection (ICV, at 0 h) of leptin (4 μg/rat, n = 6). (B) ICV injection (at 10:00 a.m. = 0 h) of leptin (4 μg/rat) elicited an increase in core temperature (MANOVA; P < 0.001 vs. vehicle) in SD rats that was abolished (MANOVA; P < 0.001) by i.p. injection (at 0 h) of flurbiprofen (1 mg/kg, n = 6).
Injection of leptin induced a significant increase in core body temperature (MANOVA, P < 0.001 vs. vehicle) that began to rise 1.5 h after injection and reached a maximal value of 38.9 ± 0.1°C (Fig. 5B). Coadministration of flurbiprofen completely abolished the increase in core body temperature elicited by injection of leptin (MANOVA, P < 0.001) such that temperatures were not significantly different from those exhibited by vehicle-treated animals (MANOVA).
In another series of experiments, flurbiprofen (25 μg/rat) was coadministered ICV with leptin. As in the previous experiment, injection of flurbiprofen (ICV) abolished the febrile response to leptin but failed to significantly reverse the effects of leptin on food intake (vehicle + vehicle, 25.0 ± 2.6 g; vehicle + flurbiprofen, 25.1 ± 1.8 g; leptin + vehicle, 16.8 ± 1.2 g, P < 0.05 vs. vehicle; leptin + flurbiprofen, 19.2 ± 2.1 g, P < 0.05 vs. vehicle; n = 5–6 in all experiments).
DISCUSSION
The results of this study show that injection of leptin, at doses that inhibit food intake, causes a significant increase in core body temperature. Earlier studies have reported that leptin normalizes body temperature of obese ob/ob mice (4, 24), which are deficient in endogenous leptin, but did not report acute effects of leptin on body temperature. These experiments were performed at an ambient temperature (26°C) that is not optimal for measuring pyrogenic responses in mice (25), so any increases in temperature may have been masked. A separate study has reported modest increases in body temperature in response to injection (i.p.) of a pool of 20-aa ob peptide fragments (1–15 mg/kg each peptide). However, the high basal temperatures (>38°C) reported in this study (26) and the timing of injections (just before the increase in nocturnal activity) may have limited any temperature changes.
Administration of leptin at a dose similar to that used in previous studies (4 μg; refs. 13, 27, and 28) induced significant effects on body temperature and food intake (Fig. 1). Similarly, systemic (i.p.) administrations of leptin (1 mg/rat) elicited febrile responses and reduced food intake but of a slightly lower magnitude than the ICV route (Fig. 3). Increasing the dose of peripheral leptin to 10 mg/rat failed to increase the effects of leptin on either food intake or body temperature (data not shown), indicating that a single injection of 1 mg elicits a maximal response, though it is possible that prolonged infusion may have a greater effect.
The changes in food intake and body temperature induced by leptin are comparable to those elicited by other inflammatory stimuli such as LPS (29) or the proinflammatory cytokine IL-1 (30, 31). There are structural similarities between the size of the leptin molecule (1) and that of many cytokines (32); the leptin receptor is a member of the class I cytokine receptor family (9–11) and is expressed in areas where cytokines mediate effects on energy balance (12, 13). There is also growing evidence to suggest that cytokines are primary mediators of leptin synthesis and release from peripheral tissues in response to infection (14, 15, 17–19). Therefore, we hypothesized that leptin may depend on cytokine action in the brain.
These observations prompted us to investigate whether leptin induces IL-1β in the brain and whether its actions are dependent on IL-1. Hypothalamic immunoreactive IL-1β was up-regulated significantly (6-fold) in the hypothalamus of leptin-treated rats. Because levels of IL-1 in circulation were below the detection limit of the assay in both leptin and vehicle-treated rats (data not shown), brain IL-1 could not have been derived from the circulation. Immunoreactive IL-1β also was up-regulated to a lesser extent (2.5-fold over detection limit) by peripheral injection of leptin (1 mg/rat) and correlated with the changes in food intake and body temperature. The magnitude of the increase of hypothalamic IL-1β in response to leptin is similar to those induced by a pyrogenic dose of LPS (33).
The results described here, together with the reported relationship between cytokines and leptin in the periphery (14, 15, 17–19), led us to propose that actions of leptin on food intake and body temperature could be associated with, or mediated by, release of IL-1 in the brain. This hypothesis is supported by our demonstration that administration of the highly selective IL-1ra, at a dose that prevents febrile responses to cytokines (but is not effective peripherally) (34), significantly inhibited the hypophagia (60%) (Fig. 2A) and completely abolished the pyrogenic responses to central injection of leptin (Fig. 2B). Failure of IL-1ra to completely abolish the effect of leptin on food intake indicates that IL-1 may act in conjunction with other mediators such as melanocortin, which has been implicated in appetite suppression in leptin-treated rats (35). In separate experiments, (i.p.) injection of leptin at a dose within the range used in previous studies (2–4, 21, 24) caused an increase in body temperature in addition to a reduction in food intake (Fig. 3). The febrile responses to this or a higher dose of leptin (10 mg/rat, i.p.; data not shown) were completely abolished by IL-1ra (ICV), whereas the effect of leptin on food intake was inhibited only partially but significantly. These data are supported by the observation that mice lacking the RI receptor (responsible for IL-1 signaling) fail to suppress food intake in response to leptin (Fig. 4). The IL-1 receptor-deficient mice (RI−/−) used in this study are a hybrid C57BL6 crossed with 129Jsv and, in the present investigation, were compared with C57BL/6 mice that are not genetically identical. Furthermore, only a single dose of leptin was used. However, this dose of leptin (4 μg) markedly inhibited food intake in normal rats (SD and lean Zucker) and mice; thus, although IL-1RI-deficient mice may have responded to a higher dose of leptin, they show a clear change in responsiveness to it.
Leptin is synthesized and released into circulation from peripheral adipose tissue (5, 6). Therefore, the pyrogenic and anorexic effects observed in response to peripheral injection of leptin strongly reinforce the suggestion that the responses to central injection of leptin described here are relevant to pathophysiological conditions. That these responses to i.p. injection of leptin also were both abolished totally by coinjection of IL-1ra (ICV) indicates further that central release of IL-1 mediates actions of leptin on food intake and body temperature.
Separate experiments indicated that distinct pathways mediate the effects of leptin on food intake and core body temperature. Cyclooxygenase products (most notably prostaglandins) have been implicated in actions of IL-1 (16, 36–39). Injection of the cyclooxygenase inhibitor flurbiprofen (40) (1 mg/kg, i.p., Fig. 5) completely abolished the increase in temperature induced by injection (ICV) of leptin, but failed to modify significantly its effect on food intake or body weight. These results suggest that the changes in temperature but not food intake elicited by leptin are mediated by prostaglandin-dependent mechanisms. The possibility that the target for the two processes (fever and food intake) is differentially accessible to flurbiprofen cannot be ruled out. To address this possibility, flurbiprofen (25 μg/rat) was coinjected ICV with leptin. The results from this experiment demonstrate that, as with the peripheral injection, the febrile response was abolished whereas the effect on food intake suppression was not inhibited significantly (data not shown). Previous studies have reported that whereas a cyclooxygenase inhibitor administered systemically attenuates anorexic effects of IL-1, ICV injection of the same inhibitor fails to affect responses to IL-1 (39).
The results presented here indicate that leptin causes release, within the brain, of the proinflammatory cytokine (IL-1) that is involved in effects of leptin on food intake and body temperature. However, actions of leptin on appetite and temperature appear to depend on separate mechanisms, with only the latter involving cyclooxygenase products. Thus, we propose that leptin may act as an important mediator of neuroimmune actions and could serve as a major circulating afferent signal for activation of responses to disease such as fever and loss of appetite. Indeed, there is recent evidence that leptin regulates immune responses (phagocytosis) as well as cytokine expression (19). Furthermore, a recent study (41) suggests that the brain also could be a major source of circulating leptin. We are aware of no published data on effects of leptin on body temperature in humans. Nevertheless, our findings have implications for the understanding of host defense responses to disease, as well as for the use of leptin or its analogues in the treatment of obesity.
Acknowledgments
We are grateful to the Ministry of Agriculture, Fisheries, and Food, the Wellcome Trust, and the Medical Research Council for financial support; Dr. Tammy Cartmell for technical support; and Dr. Steve Poole (National Institute of Biological Standards and Controls) and the European Union concerted action program (Biomed 1, Cytokines in the brain PL96) for supply of rat IL-1β and ELISA reagents. We are also grateful to Immunex for the supply of the IL-1RI−/− mice.
ABBREVIATIONS
- LPS
bacterial lipopolysaccharide
- ICV
intracerebroventricular(ly)
- SD
Sprague–Dawley
- MANOVA
multiple ANOVA
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman J M. Nature (London) 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 2.Campfield L A, Smith F J, Guisez Y, Devos R, Burn P. Science. 1995;269:546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- 3.Halaas J L, Gajiwala K S, Maffei M, Cohen S L, Chait B T, Rabinowitz D, Lallone R L, Burley S K, Friedman J M. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 4.Pelleymounter M A, Cullen M J, Baker M B, Hecht R, Winters D, Boone T, Collins F. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 5.Frederich R C, Hamann A, Anderson S, Lollmann B, Lowell B B, Flier J S. Nat Med. 1995;1:1311–1314. doi: 10.1038/nm1295-1311. [DOI] [PubMed] [Google Scholar]
- 6.Auwerx J, Staels B. Lancet. 1998;351:737–742. doi: 10.1016/S0140-6736(97)06348-4. [DOI] [PubMed] [Google Scholar]
- 7.Banks W A, Kastin A J, Huang W, Jaspan J B, Maness L M. Peptides. 1996;17:305–311. doi: 10.1016/0196-9781(96)00025-3. [DOI] [PubMed] [Google Scholar]
- 8.Funahashi T, Shimomura I, Hiraoka H, Arai T, Takahashi M, Nakamura T, Nozaki S, Yamashita S, Takemura K, Tokunaga K. Biochem Biophys Res Commun. 1995;211:469–475. doi: 10.1006/bbrc.1995.1837. [DOI] [PubMed] [Google Scholar]
- 9.Nakashima K, Narazaki M, Taga T. FEBS Lett. 1997;401:49–52. doi: 10.1016/s0014-5793(96)01430-5. [DOI] [PubMed] [Google Scholar]
- 10.Baumann H, Morella K K, White D W, Dembski M, Bailon P S, Kim H, Lai C F, Tartaglia L A. Proc Natl Acad Sci USA. 1996;93:8374–8378. doi: 10.1073/pnas.93.16.8374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White D W, Tartaglia L A. Cytokine Growth Factor Rev. 1996;7:303–309. doi: 10.1016/s1359-6101(96)00040-8. [DOI] [PubMed] [Google Scholar]
- 12.Tartaglia L A, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards G J, Campfield L A, Clark F T, Deeds J. Cell. 1995;83:1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz M W, Seeley R J, Campfield L A, Burn P, Baskin D G. J Clin Invest. 1996;98:1101–1106. doi: 10.1172/JCI118891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grunfeld C, Zhao C, Fuller J, Pollack A, Moser A, Friedman J, Feingold K R. J Clin Invest. 1996;97:2152–2157. doi: 10.1172/JCI118653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarraf P, Frederich R C, Turner E M, Ma G, Jaskowiak N T, Rivet D J, Flier J S, Lowell B B, Fraker D L, Alexander H R. J Exp Med. 1997;185:171–175. doi: 10.1084/jem.185.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rothwell N J, Hopkins S J. Trends Neurosci. 1995;18:130–136. doi: 10.1016/0166-2236(95)93890-a. [DOI] [PubMed] [Google Scholar]
- 17.Faggioni R, Fantuzzi G, Fuller J, Dinarello C A, Feingold K R, Grunfeld C. Am J Physiol. 1998;274:R204–R208. doi: 10.1152/ajpregu.1998.274.1.R204. [DOI] [PubMed] [Google Scholar]
- 18.Finck B N, Kelley K W, Dantzer R, Johnson R W. Endocrinology. 1998;139:2278–2283. doi: 10.1210/endo.139.5.6012. [DOI] [PubMed] [Google Scholar]
- 19.Loffreda S, Yang S Q, Lin H Z, Karp C L, Brengman M L, Wang D J, Klein A S, Bulkley G B, Bao C, Noble P W, et al. FASEB J. 1998;12:57–65. [PubMed] [Google Scholar]
- 20.Glaccum M B, Stocking K L, Charrier K, Smith J L, Willis C R, Maliszewski C, Livingston D J, Peschon J J, Morrissey P J. J Immunol. 1997;159:3364–3371. [PubMed] [Google Scholar]
- 21.Campfield L A, Smith F J, Burn P. Horm Metab Res. 1996;28:619–632. doi: 10.1055/s-2007-979867. [DOI] [PubMed] [Google Scholar]
- 22.Rothwell N J. Crit Rev Neurobiol. 1994;8:1–10. [PubMed] [Google Scholar]
- 23.Sims J E, Gayle M A, Slack J L, Alderson M R, Bird T A, Giri J G, Colotta F, Re F, Mantovani A, Shanebeck K. Proc Natl Acad Sci USA. 1993;90:6155–6159. doi: 10.1073/pnas.90.13.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris R B, Zhou J, Redmann S M, Jr, Smagin G N, Smith S R, Rodgers E, Zachwieja J J. Endocrinology. 1998;139:8–19. doi: 10.1210/endo.139.1.5675. [DOI] [PubMed] [Google Scholar]
- 25.Kozak W, Conn C A, Kluger M J. Am J Physiol. 1994;266:R125–R135. doi: 10.1152/ajpregu.1994.266.1.R125. [DOI] [PubMed] [Google Scholar]
- 26.Fruhbeck G, Garcia-Granero M, Martinez J A. Regul Pept. 1998;73:83–87. doi: 10.1016/s0167-0115(97)01061-6. [DOI] [PubMed] [Google Scholar]
- 27.Seeley R J, van Dijk G, Campfield L A, Smith F J, Burn P, Nelligan J A, Bell S M, Baskin D G, Woods S C, Schwartz M W. Horm Metab Res. 1996;28:664–668. doi: 10.1055/s-2007-979874. [DOI] [PubMed] [Google Scholar]
- 28.Gardner J D, Rothwell N J, Luheshi G N. Nat Neurosci. 1998;1:103. doi: 10.1038/353. [DOI] [PubMed] [Google Scholar]
- 29.McCarthy D O, Kluger M J, Vander A J. Physiol Behav. 1986;36:745–749. doi: 10.1016/0031-9384(86)90363-x. [DOI] [PubMed] [Google Scholar]
- 30.Mrosovsky N, Molony L A, Conn C A, Kluger M J. Am J Physiol. 1989;257:R1315–R1321. doi: 10.1152/ajpregu.1989.257.6.R1315. [DOI] [PubMed] [Google Scholar]
- 31.Uehara A, Sekiya C, Takasugi Y, Namiki M, Arimura A. Am J Physiol. 1989;257:R613–R617. doi: 10.1152/ajpregu.1989.257.3.R613. [DOI] [PubMed] [Google Scholar]
- 32.Hopkins S J, Rothwell N J. Trends Neurosci. 1995;18:83–88. [PubMed] [Google Scholar]
- 33.Hagan P, Poole S, Bristow A F. J Mol Endocrinol. 1993;11:31–36. doi: 10.1677/jme.0.0110031. [DOI] [PubMed] [Google Scholar]
- 34.Luheshi G, Miller A J, Brouwer S, Dascombe M J, Rothwell N J, Hopkins S J. Am J Physiol. 1996;270:E91–E95. doi: 10.1152/ajpendo.1996.270.1.E91. [DOI] [PubMed] [Google Scholar]
- 35.Seeley R J, Yagaloff K A, Fisher S L, Burn P, Thiele T E, van Dijk G, Baskin D G, Schwartz M W. Nature (London) 1997;390:349–349. doi: 10.1038/37016. [DOI] [PubMed] [Google Scholar]
- 36.Hellerstein M K, Meydani S N, Meydani M, Wu K, Dinarello C A. J Clin Invest. 1989;84:228–235. doi: 10.1172/JCI114145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kluger M J. Physiol Rev. 1991;71:93–127. doi: 10.1152/physrev.1991.71.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rothwell N J, Luheshi G, Toulmond S. Pharmacol Ther. 1996;69:85–95. doi: 10.1016/0163-7258(95)02033-0. [DOI] [PubMed] [Google Scholar]
- 39.Shimizu H, Uehara Y, Shimomura Y, Kobayashi I. Eur J Pharmacol. 1991;195:281–284. doi: 10.1016/0014-2999(91)90547-4. [DOI] [PubMed] [Google Scholar]
- 40.Rothwell N J, Busbridge N J, Leleuvre R A, Hardwick A J, Gauldie J, Hopkins S J. Can J Physiol Pharmacol. 1991;69:1465–1469. doi: 10.1139/y91-219. [DOI] [PubMed] [Google Scholar]
- 41.Esler M, Vaz M, Collier G, Nestel P, Jennings G, Kaye D, Seals D, Lambert G. Lancet. 1998;351:879. doi: 10.1016/S0140-6736(05)70289-0. [DOI] [PubMed] [Google Scholar]