Abstract
Stimulation of cardiac β1-adrenergic receptors is the main mechanism that increases heart rate and contractility. Consequently, several pharmacological and gene transfer strategies for the prevention of heart failure aim at improving the function of the cardiac β-adrenergic receptor system, whereas current clinical treatment favors a reduction of cardiac stimulation. To address this controversy, we have generated mice with heart-specific overexpression of β1-adrenergic receptors. Their cardiac function was investigated in organ bath experiments as well as in vivo by cardiac catheterization and by time-resolved NMR imaging. The transgenic mice had increased cardiac contractility at a young age but also developed marked myocyte hypertrophy (3.5-fold increase in myocyte area). This increase was followed by progressive heart failure with functional and histological deficits typical for humans with heart failure. Contractility was reduced by ≈50% in 35-week-old mice, and ejection fraction was reduced down to a minimum of ≈20%. We conclude that overexpression of β1-adrenergic receptors in the heart may lead to a short-lived improvement of cardiac function, but that increased β1-adrenergic receptor signalling is ultimately detrimental.
Among the many mechanisms that control cardiac function, the stimulation of the frequency and force of contraction by epinephrine and norepinephrine via specific β-adrenergic receptors is the most prominent and, functionally, the most relevant pathway (1). The majority of cardiac β-adrenergic receptors are of the β1-subtype, and the minority are of the β2-subtype (2). In chronic heart failure, these receptors are reduced in number (3, 4) and in function (1, 4–6). Reductions in receptor number seem to occur early in the development of heart failure (7) and to correlate with the severity of the disease (8, 9). However, it is still a matter of debate as to whether these receptor alterations contribute to the development of heart failure by depriving the heart of its major physiological stimulus or whether they represent a protective mechanism preventing overstimulation by the increased levels of catecholamines occurring in heart failure (10).
The advent of transgenic techniques has provided a tool with which to address such questions. Over the last few years, several transgenic models have been developed to understand the role of diverse proteins in cardiac function (11). Transgenic expression of the β2-adrenergic receptor under the control of the α-myosin heavy chain promoter has been achieved in atria as well as in ventricles. When the receptors were overexpressed 200-fold, the basal production of cAMP in cardiac membranes was enhanced, and an increased left ventricular contractility was observed in vivo (12). However, more moderate overexpression of the wild-type or even of a constitutively active mutant of the β2-adrenergic receptor showed no such phenotype (13). More recently, it has been shown that contractility in single myocytes prepared from failing rabbit hearts can be restored via adenoviral gene transfer of β2-adrenergic receptors, and it has been proposed that this approach might become a strategy in the treatment of heart failure (14).
It has been suggested recently that—aside from activating adenylyl cyclase—the signaling pathways of β1- and β2-adrenergic receptors might be divergent (15). Subtype-specificity is also apparent from the observation that, in cardiac disease, the β1-subtype undergoes much more down-regulation than the β2-subtype (1, 4). Furthermore, given that, physiologically, the β1-subtype is the predominant or even exclusive subtype on cardiac myocytes (2), transgenic alterations of this subtype might help to identify the pathophysiological consequences of changes in the activity of the cardiac β-adrenergic receptor system.
Therefore, we set out to generate such a transgenic model by using the α-myosin heavy chain promoter. This promoter is active in the developing and adult atria and also in the adult ventricles but not in the developing ventricle or other muscular tissues (16, 17). Thus, expression under this promoter allows heart-specific expression, not only in the atria, but also in the ventricles without the likelihood of causing defects in heart-ventricle development. We found that transgenic expression of the β1-adrenergic receptor under this promoter resulted not only in an expected increased sensitivity toward catecholamines but also in alterations in basal cardiac function and morphology, which, taken together, indicate cardiac hypertrophy, remodeling, and early cardiac failure.
MATERIALS AND METHODS
Generation of Transgenic Mice.
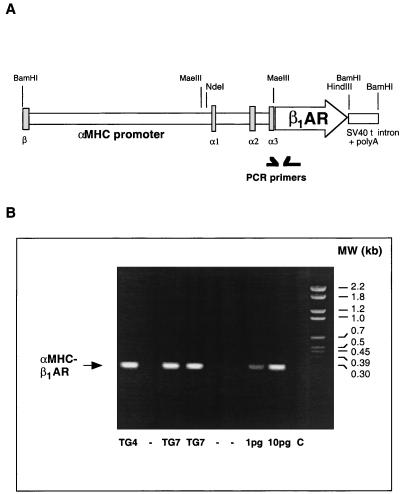
From the plasmid depicted in Fig. 1A, the segment containing murine α-myosin heavy chain promoter and human β1-adrenergic receptor cDNA (18) was isolated. The purified linear DNA (1 ng/μl) was injected into fertilized oocytes from superovulated FVB/N mice according to standard procedures (19). The injected oocytes were transferred to the oviducts of pseudopregnant CD-1 mice. All mice were kept in a specific-pathogen-free facility. Generation and investigation of these mice was approved by the responsible government authorities. The F0 generation was screened for integration of the transgene by PCR with a sense primer, 5′-AGG ACT TCA CAT AGA AGC CTA G, located in the α-myosin heavy chain promoter and an antisense primer, 5′-TGT CCA CTG CTG AGA CAG CG, located in the β1-receptor coding sequence.
Figure 1.
Generation and identification of mice transgenic for the β1-adrenergic receptor. (A) Transgenic vector. The complete intergenic region between the β- and α-myosin heavy chain genes (α-myosin heavy chain promoter) was ligated 5′ to the coding region of the human β1-adrenergic receptor. At the 3′ end of the receptor cDNA, the intron and poly(A)-containing sites of the simian virus 40 T antigen were added. β, α1, α2, and α3 denote exons of the β- and α-myosin heavy chain genes. The positions of the PCR primers used to detect the transgene (B) are indicated. (B) Detection of the transgene in animals of the lines β1TG7 and β1TG4 by PCR with the primers indicated in A. The lanes at the right side contained 1 and 10 pg of the transgene vector in the PCR as standards and a negative control (C) without genomic DNA. αMHC, α-myosin heavy chain.
Radioligand Binding Studies.
Cell membranes were prepared by homogenization of myocardia of 6-week-old mice in 5 mM Tris⋅HCl/5 mM EDTA, pH 7.4, with centrifugation at 1,000 × g for 10 min (4°C) and centrifugation of the supernatants at 50,000 × g for 15 min (4°C). The pellets were resuspended in 75 mM Tris⋅HCl/12.5 mM MgCl2/1 mM EDTA, pH 7.4. For radioligand binding assays, 20 μg of membrane protein was incubated for 1 h at 37°C with various concentrations of [125I]cyanopindolol (up to 300 pM) by using 10 μM (−)propranolol to define nonspecific binding. Incubations were terminated by filtration through Whatman GF/C filters.
Organ Bath Experiments.
Hearts were rapidly excised and placed in carbogenated modified Tyrode’s solution (119 mM NaCl/5.4 mM KCl/1.2 mM CaCl2/1 mM MgCl2/22.6 mM NaHCO3/0.42 mM NaH2PO4/0.025 mM EDTA/10 mM glucose/0.2 mM ascorbic acid, pH 7.4). Right atria of 8- to 12-week-old mice were dissected, tied with two 6-0 silk sutures, and placed in a carbogenated 37°C tissue bath with modified Tyrode’s solution. The atria were allowed to contract spontaneously. Signals from isometric force transducers were fed via a bridge amplifier to a PowerLab system (A. D. Instruments, Castle Hill, Australia).
Histological Analysis.
Cross sections from the heart equator (2-mm thick) were fixed with 4% paraformaldehyde, dehydrated with alcohol, embedded in paraffin, and cut into 5-μm slices, which were stained with hematoxylin/eosin. For morphometrical analysis, photographs of 20 ventricular sections from wild-type and β1TG4 mice were taken at ×320 magnification (Zeiss IM-35), and myocyte cross-sectional areas were determined by digitizing the images and computerized pixel counting. Only nucleated myocytes from areas with transversely cut muscle fibers were evaluated.
Left Ventricular Catheterization.
Mice were anaesthetized with tribromoethanol (13 μl of 2.5% solution per g of body weight) and placed on a 37°C table. The left jugular vein was cannulated with a custom-fashioned polyethylene tubing connected to a microinfusion pump (Braun, Melsungen, Germany) for drug application. A 1.8 French high-fidelity catheter-tip micromanometer (Millar Instruments, Houston, TX) was inserted into the aorta via the right carotid artery and advanced into the left ventricle under continuous monitoring of the pressure waveform. Pressure signals were digitized at a sampling rate of 4,000 Hz and recorded with a PowerLab system.
NMR Imaging.
Images of mice were taken with a 7.05 T BIOSPEC 70/20 scanner (flip angle of 40°; echo time = 1.5 ms; in-plane pixel resolution = 117 μm2; slice thickness = 1 mm; Brucker, Billerica, MA; ref. 20). Mice were anaesthetized with isoflurane [2.0% (vol/vol) isoflurane in 1 liter/min oxygen flow] via a nose cone and kept normothermic with a warming pad. Ejection fractions were calculated from endsystolic and enddiastolic slice volumes (20).
RESULTS
To investigate the long-term consequences of alterations in cardiac β-adrenergic receptor numbers, we generated transgenic mice with moderate heart-specific overexpression of the β1-adrenergic receptor. The transgenic construct is shown in Fig. 1A; the coding sequence of the human β1-adrenergic receptor (18) was placed under the control of the α-myosin heavy chain promoter, which directs expression in adult murine atria and ventricles (16). The fragment containing promoter and receptor DNA was injected into the pronucleus of fertilized FVB/N oocytes. The transgene was detected by PCR in two founder mice. Breeding of these founder mice with wild-type FVB/N mice gave two independent lines, designated β1TG4 and β1TG7, with the expected 50% transgenic offspring (Fig. 1B). The F1 generation of both lines was investigated. Identification of the DNA by Southern blotting of genomic DNA from these animals indicated the integration of several copies of receptor cDNA into the genome (not shown).
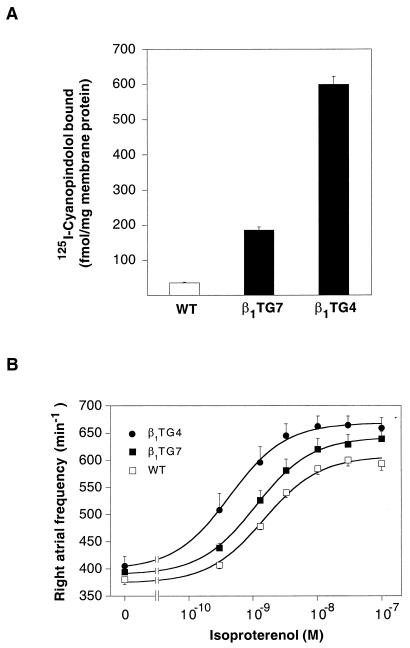
Expression of transgenic β1-adrenergic receptors in the heart was verified in binding experiments with the specific radioligand [125I]cyanopindolol (Fig. 2A), which showed 5-fold (β1TG7) and 15-fold (β1TG4) increases in receptor numbers. Competition with subtype-selective antagonists confirmed that the overexpressed receptors were of the β1-subtype (not shown).
Figure 2.
Expression and function of β1-adrenergic receptors in transgenic animals. (A) Detection of β-adrenergic receptors in left ventricular myocardial membranes prepared from wild-type mice (WT) and mice from the two transgenic lines β1TG7 and β1TG4. β-Adrenergic receptors were quantified by saturation with the radioligand [125I]cyanopindolol. The data shown are means ± SEM of four experiments. (B) Frequency responses in organ bath experiments. Right atria from 8- to 12-week-old wild-type mice (▫) and mice from the two transgenic lines β1TG7 (▪) and β1TG4 (●) were mounted in an organ bath. Spontaneous contraction frequencies were recorded under basal conditions and in the presence of increasing concentrations of (−)isoproterenol. EC50 values were 1.5 ± 0.2 nM for wild-type mice, 1.1 ± 0.1 nM for β1TG7, and 0.4 ± 0.05 nM for β1TG4, and maximal effects were 233 ± 9, 253 ± 4, and 270 ± 8 beats per min, respectively. The data shown are means ± SEM of nine (WT), six (β1TG7), and four (β1TG4) atria.
The functional consequences of this overexpression were characterized initially in organ bath experiments. Spontaneously beating wild-type right atria had basal frequencies of 380 ± 9 beats per min, and these were elevated only slightly in atria from either transgenic line (Fig. 2B). Isoproterenol caused concentration-dependent increases in the frequency of contraction. This response was of higher sensitivity and larger amplitude in the transgenic lines, indicating functionality of the receptors expressed from the transgene.
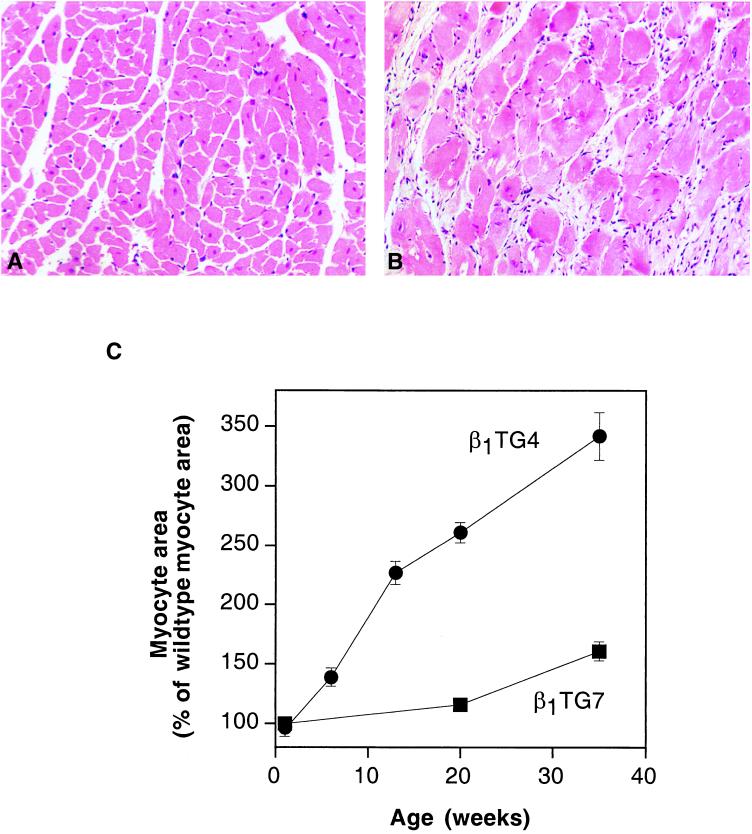
Microscopic examination identified marked myocyte hypertrophy in the transgenic hearts, accompanied by fibrosis (Fig. 3). In addition, transgenic hearts contained large pleomorphic nuclei, which were never found in wild-type hearts. Computerized morphometric analysis of the myocyte cross-sectional areas identified a progressive myocyte hypertrophy that was not present at birth but developed after a few weeks in the β1TG4 line, and later—and to a lesser extent—in the β1TG7 line (Fig. 3C). In spite of marked myocyte hypertrophy, the overall weight of the transgenic hearts was increased by only 10% (not shown), compatible with cell death and replacement by fibrous tissue (Fig. 3B).
Figure 3.
Histological alterations in transgenic hearts. (A and B) Hematoxylin/eosin-stained 5-μm sections of paraffin-embedded left ventricular myocardium from wild-type (A) and β1TG4 mice (B). (C) Morphometrical analysis of myocyte cross-sectional areas. Myocyte cross-sectional areas were determined from hematoxylin/eosin stained sections from β1TG4 (●), β1TG7 (▪), and wild-type left ventricles. Data are expressed as percentage of the respective wild-type value and represent means ± SEM of several sections from three animals at each age group.
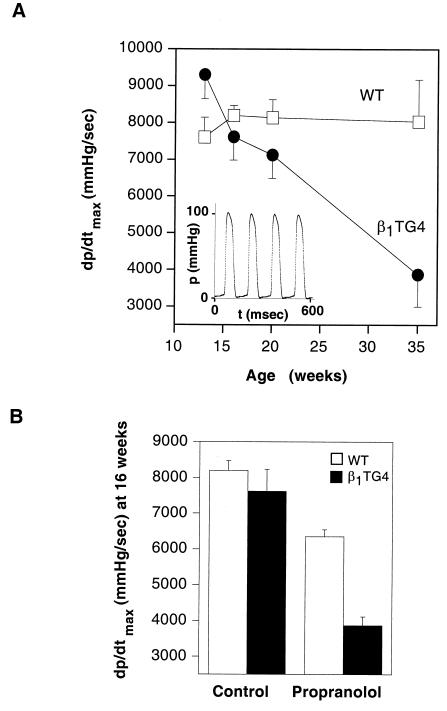
In vivo cardiac function in wild-type and β1TG4 mice was investigated by left ventricular catheterization. Fig. 4A shows the maximal contractility for wild-type and β1TG4 mice at different ages. Although the transgenic animals showed an increased contractility at a young age, this difference was lost at 16 weeks and contractility continued to decrease thereafter, going to below 50% of the wild-type value at 35 weeks. Loss of contractility was already apparent at 16 weeks when the stimulation of cardiac β-adrenergic receptors by endogenous catecholamines was blocked by administration of propranolol (Fig. 4B). In line with the larger numbers of cardiac β1-adrenergic receptors, propranolol caused a greater drop in contractility in the transgenic animals and revealed an impaired systolic function.
Figure 4.
In vivo determination of left ventricular contractility in β1TG4 and wild-type mice. Left ventricular pressures in anaesthetized mice were measured with a Millar 1.8 French catheter. (A) Left ventricular maximal contractility (dp/dtmax) was measured at different ages in wild-type (▫) and β1TG4 (●) mice. Data are from four mice for each age group. The Inset shows an original pressure tracing. (B) Effects of β-adrenergic receptor blockade by 1.5 μg of propranolol per g of body weight on dp/dtmax in wild-type and β1TG4 transgenic animals at 16 weeks of age. mmHg, millimeters of mercury.
Spontaneous cardiac frequency in these investigations was constantly elevated in the transgenic animals at all ages, with 462 ± 8 beats per min in the β1TG4 animals vs. 395 ± 9 beats per min in wild-type animals. In the presence of propranolol, this difference was abolished (315 ± 9 beats per min in β1TG4 vs. 333 ± 9 beats per min in wild-type mice). These data indicate that the β1-adrenergic receptors remained functional at all ages.
Additional evidence that the transgenic receptors themselves were functional was provided by i.v. application of dobutamine to increase cardiac contractility. The sensitivity of the contractile responses to dobutamine was higher in transgenic animals and was in line with the respective receptor levels: the EC50 values were 6.2, 3.0, and 1.7 nM for the wild-type, β1TG7, and β1TG4 mice, respectively (not shown).
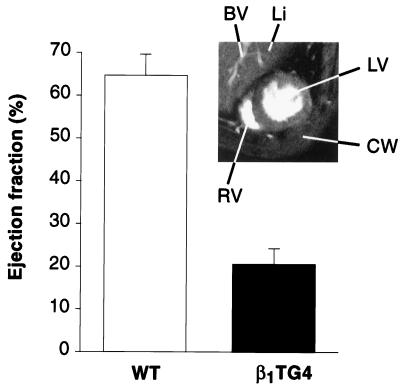
The loss of contractility in the 35-week-old transgenic animals resulted in a severe reduction of left ventricular function, as determined by NMR imaging, again in the presence of propranolol (Fig. 5, Inset). Comparison of the images at maximal diastole (Fig. 5, Inset) and systole indicated a markedly reduced function in the β1TG4 mice, with a calculated ejection fraction of only 21%. In addition to the dysfunction indicated by NMR, several transgenic mice showed clinical signs of heart failure such as ascites and death before the age of 14 months (not shown).
Figure 5.
Detection of heart failure in β1TG4 transgenic mice by NMR imaging. ECG-triggered series of NMR images of 35-week-old transgenic and wild-type (WT) mice were recorded with a 7.05 T BIOSPEC 70/20 scanner (20). The Inset shows an image in maximal diastole. LV, left ventricle; RV, right ventricle; CW, chest wall; Li, liver; BV, blood vessel (of portal circulation). Ejection fractions were calculated from images in maximal diastole and systole (20).
DISCUSSION
There are four findings that are remarkable about this transgenic model. First, it resulted in the expected increased sensitivity of the transgenic hearts to catecholamines, as indicated by a leftward shift of the frequency and contractility concentration-response curves to β-adrenergic receptor agonists both in vitro and in vivo. Second, there was an increased heart rate in the intact animal but no difference in isolated atria, suggesting that the increased heart rate in vivo was due to stimulation by catecholamines and not to constitutive activity of the transgenic receptors. Third, the hearts were structurally altered, evincing progressive hypertrophy and later fibrosis. Finally, the β1TG4 mice developed progressive heart failure in a manner very similar to that seen in patients with heart failure.
These results are quite different from those reported with heart-specific overexpression of the β2-adrenergic receptor (12). In that model, increased sensitivity to receptor stimulation was seen when the receptors were overexpressed ≈200-fold (12), but this level of receptor expression also resulted in marked increases in basal heart rate in vitro, indicative of constitutive activity of the transgenic receptors, which was not seen in our model (see above). It is surprising that lower levels of cardiac β1- than of β2-receptors are required to produce increased sensitivity to agonists, given that, in most cell lines, the β2-subtype couples better than the β1-receptor to adenylyl cyclase (21, 22). This discrepancy may be in line with recent proposals of distinct signaling pathways for β1- and β2-adrenergic receptors (15, 23). It is also in line with the observation that the disruption of the β1-receptor gene essentially abolished cardiac responses to isoproterenol (24).
The most remarkable finding regarding the β1-receptor transgenic animals was that their hearts displayed major morphological and functional alterations. Myocyte hypertrophy together with replacement by fibrous tissue indicated remodeling, which is also typical for cardiac failure in humans. Hypertrophic responses have been described for the Gq pathway (25), and transgenic overexpression of Gαq in the heart promotes the development of pressure-induced heart failure (26). Histological changes have long been known to occur in hearts in response to chronic treatment with isoproterenol (27) but have not been reported in the animals with ≈200-fold overexpression of the β2-adrenergic receptor (12). Again, this result is compatible with the hypothesis that the β1-subtype is better coupled to cardiac responses. β-Adrenergic receptors recently have been observed to couple to the growth-promoting mitogen-activated protein kinase pathway, in addition to the classical cAMP pathway (23). However, compared with other receptors, these growth responses are modest, and the relevance of this signaling pathway in vivo is uncertain. Our data indicate that growth responses to β-adrenergic receptor stimulation do occur in vivo, but it remains to be established whether they are a direct consequence of βadrenergic receptor signaling or whether they are caused by the increased work load due to a higher basal cardiac performance.
Increased responsiveness to ambient catecholamines, together with myocyte hypertrophy, is most likely responsible for the increased basal cardiac contractility seen in β1TG4 mice at young ages. However, even at an age when this suffices to maintain apparently normal basal cardiac contractility, blockade of β-adrenergic receptors with propranolol revealed a functional deficit, and basal cardiac contractility continued to decline thereafter. We conclude that the loss of β1-adrenergic receptor number and function in heart failure is presumably protective, and, although this loss acutely decreases cardiac performance, it may in the long run help to preserve cardiac function; attempts to block this down-regulation will most likely prove to be ultimately detrimental.
Recently, various transgenic models have been shown to have impaired cardiac function, including overexpression of signaling proteins as well as gene knockouts disrupting myocyte architecture. The large number of such models suggests that cardiac hypertrophy and eventually failure represent a common final pathway in response to multiple disruptions of cardiac function. Down-regulation of β1-adrenergic receptors is a general feature of these final steps and apparently an appropriate adaptive response. Transgenic mice overexpressing cardiac β1-adrenergic receptors may imitate the detrimental sympathetic stimulation seen in chronic heart failure and thus reflect a common final pathway in the development of this important disease.
Acknowledgments
We thank Eva Schmitteckert for help in setting up the generation of transgenic mice, Stefan Neubauer, Jan Ruff, and Axel Haase for support in the NMR studies, and Thomas Eschenhagen for advice on in vitro functional studies. These studies were supported by Deutsche Forschungsgemeinschaft Grant SFB 355 and by grants from the European Commission and the Fonds der Chemischen Industrie.
References
- 1.Brodde O E. Pharmacol Rev. 1991;43:203–242. [PubMed] [Google Scholar]
- 2.Buxton I L, Brunton L L. Circ Res. 1985;56:126–132. doi: 10.1161/01.res.56.1.126. [DOI] [PubMed] [Google Scholar]
- 3.Bristow M R, Ginsburg R, Minobe W, Cubiciotti R S, Sageman W S, Lurie K, Billingham M E, Harrison D E, Stinson E B. N Engl J Med. 1982;307:205–211. doi: 10.1056/NEJM198207223070401. [DOI] [PubMed] [Google Scholar]
- 4.Ungerer M, Böhm M, Elce J S, Erdmann E, Lohse M J. Circulation. 1993;87:454–463. doi: 10.1161/01.cir.87.2.454. [DOI] [PubMed] [Google Scholar]
- 5.Lohse M J. Biochim Biophys Acta. 1993;1179:171–188. doi: 10.1016/0167-4889(93)90139-g. [DOI] [PubMed] [Google Scholar]
- 6.Böhm M, Beukelmann D, Brown L, Feiler G, Lorenz B, Näbauer M, Kemkes B, Erdmann E. Eur Heart J. 1988;9:844–852. doi: 10.1093/oxfordjournals.eurheartj.a062577. [DOI] [PubMed] [Google Scholar]
- 7.Kiuchi K, Shannon R P, Komamura K, Cohen D J, Bianchi C, Homcy C J, Vatner S F, Vatner D E. J Clin Invest. 1993;91:907–914. doi: 10.1172/JCI116312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brodde O E, Zerkowski H R, Doetsch N, Motomura S, Khamssi M, Michel M C. J Am Coll Cardiol. 1989;14:323–331. doi: 10.1016/0735-1097(89)90181-2. [DOI] [PubMed] [Google Scholar]
- 9.Engelhardt S, Böhm M, Erdmann E, Lohse M J. J Am Coll Cardiol. 1996;27:146–154. doi: 10.1016/0735-1097(95)00425-4. [DOI] [PubMed] [Google Scholar]
- 10.Packer M. Circulation. 1988;77:721–730. doi: 10.1161/01.cir.77.4.721. [DOI] [PubMed] [Google Scholar]
- 11.Franz W M, Mueller O J, Hartong R, Frey N, Katus H A. J Mol Med. 1997;75:115–129. doi: 10.1007/s001090050096. [DOI] [PubMed] [Google Scholar]
- 12.Milano C A, Allen L F, Rockman H A, Dolber P C, McMinn T R, Chien K R, Johnson T D, Bond R A, Lefkowitz R J. Science. 1994;264:582–586. doi: 10.1126/science.8160017. [DOI] [PubMed] [Google Scholar]
- 13.Samama P, Bond R A, Rockman H A, Milano C A, Lefkowitz R J. Proc Natl Acad Sci USA. 1997;94:137–141. doi: 10.1073/pnas.94.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akhter S A, Skaer C A, Kypson A P, McDonald P H, Peppel K C, Glower D D, Lefkowitz R J, Koch W J. Proc Natl Acad Sci USA. 1997;94:12100–12105. doi: 10.1073/pnas.94.22.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao R P, Hohl C, Altschuld R, Jones L, Livingston B, Ziman B, Tantini B, Lakatta E G. J Biol Chem. 1994;269:19151–19156. [PubMed] [Google Scholar]
- 16.Ng W A, Grupp I L, Subramaniam A, Robbins J. Circ Res. 1991;69:1745–1750. doi: 10.1161/01.res.68.6.1742. [DOI] [PubMed] [Google Scholar]
- 17.Palermo J, Gulick J, Colbert M, Fewell J, Robbins J. Circ Res. 1995;78:504–509. doi: 10.1161/01.res.78.3.504. [DOI] [PubMed] [Google Scholar]
- 18.Frielle T, Collins S, Daniel K W, Caron M G, Lefkowitz R J, Kobilka B K. Proc Natl Acad Sci USA. 1987;84:7920–7924. doi: 10.1073/pnas.84.22.7920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hein L, Stevens M E, Barsh G S, Pratt R E, Kobilka B K, Dzau V J. Proc Natl Acad Sci USA. 1997;94:6391–6396. doi: 10.1073/pnas.94.12.6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruff J, Wiesmann F, Hiller K H, Voll S, von Kienlin M, Bauer W R, Rommel E, Neubauer S, Haase A. Magn Reson Med. 1998;40:43–48. doi: 10.1002/mrm.1910400106. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki T, Nguyen C T, Nantel F, Bonin H, Valiquette M, Frielle T, Bouvier M. Mol Pharmacol. 1992;41:542–548. [PubMed] [Google Scholar]
- 22.Levy F O, Zhu X, Kaumann A J, Birnbaumer L. Proc Natl Acad Sci USA. 1993;90:10798–10802. doi: 10.1073/pnas.90.22.10798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Biesen T, Lutrell L M, Hawes B E, Lefkowitz R J. Endocrine Rev. 1996;17:698–714. doi: 10.1210/edrv-17-6-698. [DOI] [PubMed] [Google Scholar]
- 24.Rohrer D K, Desai K H, Jasper J R, Stevens M E, Regula D P, Jr, Barsh G S, Bernstein D, Kobilka B K. Proc Natl Acad Sci USA. 1996;93:7375–7380. doi: 10.1073/pnas.93.14.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milano C A, Dolber P C, Rockman H A, Bond R A, Venable M E, Allen L F, Lefkowitz R J. Proc Natl Acad Sci USA. 1994;91:10109–10113. doi: 10.1073/pnas.91.21.10109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakata Y, Hoit B D, Liggett S B, Walsh R A, Dorn G W, II. Circulation. 1998;97:1488–1495. doi: 10.1161/01.cir.97.15.1488. [DOI] [PubMed] [Google Scholar]
- 27.Rona G, Chappel G I, Balazs T, Gaudry R. Arch Pathol. 1959;67:99–111. [PubMed] [Google Scholar]