Abstract
An endogenous natriuretic and vasoconstrictor Na/K-ATPase inhibitor, marinobufagenin (MBG), is implicated in NaCl-induced hypertension and in ethanol addiction. In rats, MBG suppresses voluntary alcohol intake, while immunization against MBG induces alcohol-seeking behavior. Since alcohol withdrawal is associated with elevation of blood pressure (BP) and renal sodium retention, we hypothesized that MBG mediates pressor response to ethanol withdrawal. In male Sprague-Dawley rats, forced ethanol intake (20% v/v, 2.8±0.2 g/day for 7 days) did not affect BP and MBG excretion. Ethanol withdrawal was associated with a 21 mm Hg increase in BP, a 10% decrease in hematocrit, and a three-fold increase in renal MBG excretion. In vivo administration of anti-MBG antibody to rats prevented withdrawal-induced BP elevation. Therefore, MBG mediates pressor response to ethanol withdrawal, and may link mechanisms of ethanol dependence and hypertension.
1. INTRODUCTION
Chronic excessive alcohol consumption is a risk factor for hypertension (Di Gennaro et al., 2002). Endogenous digitalis-like inhibitors of the Na/K-ATPase (cardiotonic steroids - CTS) are implicated in the mechanisms of NaCl-sensitive hypertension (Haddy, 2006) and in ethanol tolerance (Bagrov et al, 2002). In the hypertensives, levels of CTS increase with an adaptive role to induce natriuresis via inhibition of the sodium pump in renal tubuli (Haddy, 2006). In addition, CTS inhibit the Na/K-ATPase in the vascular smooth muscle and potentiate vasoconstriction (Haddy, 2006). Marinobufagenin (MBG), a CTS belonging to a class of bufadienolides, exhibits high affinity to ouabain-resistant α-1 Na/K-ATPase isoform, the main sodium pump isoform in vascular sarcolemma and an exclusive sodium pump isoform in renal epithelium (Fedorova et al, 2002, 2005). Accordingly, in vivo MBG acts as a natriuretic and as a vasoconstrictor (Fedorova et al, 2002, 2005).
MBG is also implicated in the development of ethanol addiction in rats. In our previous experiments, administration of MBG to rats reduced voluntary ethanol consumption (Kashkin et al., 2002), while active immunization of rats against MBG facilitated ethanol-seeking behavior (Bagrov et al., 1999).
In alcoholics, ethanol withdrawal is associated with a pressor response and renal sodium retention (Di Gennaro et al., 2002). Since MBG is a natriuretic and a vasoconstrictor (Fedorova et al., 2002; 2005), and since this hormone exhibits anti-addictive properties (Bagrov et al., 2002), we hypothesized that MBG may mediate ethanol withdrawal-induced pressor response.
METHODS
The protocol of the study has been approved by the Animal Care and Use Committee of the National Institute on Aging, NIH. Thirty male Sprague-Dawley rats (251±2 grams) (Charles River Laboratories, Inc., Wilmington, MA) were housed in individual cages with free access to standard chow and drinking water and with a 12:12-hour light/dark cycle (t = 21C).
Ethanol withdrawal was induced as reported previously in detail (Spanagel et al., 1996). After a seven-day period of acclimation, 30 rats were divided into two groups. Six rats from the control group received drinking water ad libitum during the 10 days of the experiment. In 24 rats, water was replaced by a 20% (v/v) alcohol solution, which was given as the sole drinking fluid. Animals that drank less than 10 mL of 20% ethanol per day were excluded from the study. Six rats drank 20% ethanol for 9 days (ethanol group; 2.8±0.2 g/day), and in 18 animals, the ethanol solution was for two days replaced by water (withdrawal group) at day 7. Six rats from withdrawal group were observed for 2 days, and the remaining 12 rats from “withdrawal group” were administered vehicle (n=6) or anti-MBG antibody (a-MBG-P)(n=6) intraperitoneally 1 hour prior to withdrawal, and observed for 3 hours. The a-MBG-P was used at concentration, which in vivo blocks 75% of circulating steroid (Fedorova et al, 2002). Systolic blood pressure was measured via tail plethysmography before ethanol administration (baseline), at days 1, 3, 5, 7 and 9 during ethanol intake, and at 48 hours following withdrawal.
In a subset of rats from the withdrawal group, treated by a-MBG-P (n=6) or vehicle (n=6), blood pressure was measured hourly for two hours prior to and three hours following ethanol withdrawal. At the end of the experiment, rats were anesthetized with ketamine (100 mg/kg) and sacrificed by exsanguinations from abdominal aorta.
Urinary concentrations of MBG and endogenous ouabain were measured using DELFIA fluoroimmunoassays, as reported recently in detail (Fedorova et al., 2002).
Results were analyzed statistically using one-way and repeated measures ANOVA followed by Newman-Keuls multiple comparisons tests (GraphPad Prism 4, GraphPad Software Inc., San Diego, CA).
RESULTS
As presented in Figure 1A, systolic blood pressure did not exhibit significant changes in both “control” and “ethanol” groups during 9 days of observation, while in rats from “withdrawal” groups, arterial pressure rose by 21 mmHg within 48 hours of replacement of 20% ethanol with water.
FIGURE 1.
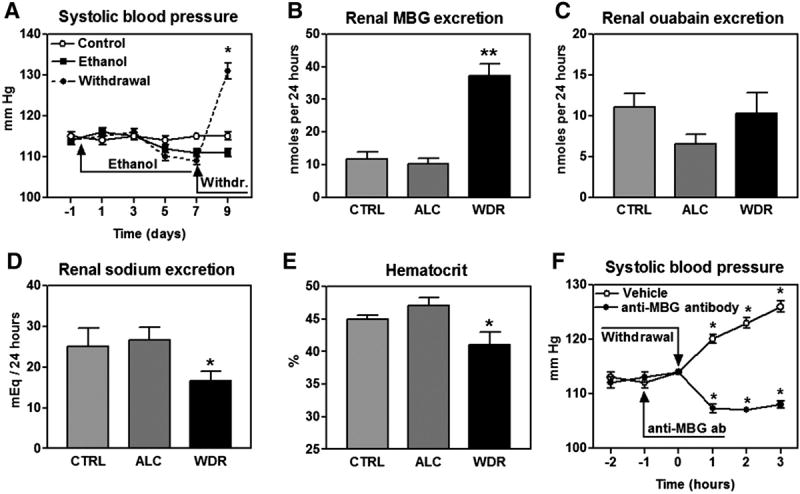
A – times course of changes of systolic blood pressure in rats from control, ethanol and withdrawal groups. By repeated measures ANOVA and Neuman-Keuls test: P<0.01 for withdrawal group vs. control and ethanol groups. * - P<0.01 vs. blood pressure in withdrawal group at days -1 and 7 of the experiment (one way ANOVA followed by Newman-Keuls multiple comparisons test).
Levels of renal excretion of MBG (B), endogenous ouabain (C), and sodium (D), and hematocrit (E) in rats from control (CTRL), ethanol (ALC) and withdrawal (WDR) groups on day 9 of the experiment. Means ± SEM from 6 observations. By one-way ANOVA followed by Neuman-Keuls test: (*) – P<0.05 and (**) – P<0.01. vs. ALC.
F - acute changes in systolic blood pressure in rats from the withdrawal group administered anti-MBG antibody or vehicle. By repeated measures ANOVA and Neuman-Keuls test: P<0.01 for antibody treatment vs. vehicle. * - P<0.01 vs. blood pressure prior to withdrawal (one way ANOVA followed by Newman-Keuls multiple comparisons test).
Levels of renal excretion of MBG, endogenous ouabain and sodium, and hematocrit assessed at day 9 of the experiment are illustrated in Figure 1B-E. Forced ethanol ingestion did not significantly affect any of the parameters studied. Ethanol withdrawal did not affect renal excretion of endogenous ouabain, but was associated with a substantial increase in MBG excretion, accompanied by reduction in renal sodium excretion and drop in hematocrit.
Changes in systolic blood pressure prior to and following 3 hours of ethanol withdrawal are presented in Figure 1F. As demonstrated in the Figure, in vehicle-treated rats, blood pressure elevation began within 1 hour following withdrawal, and in 3 hours blood pressure rose by 12 mm Hg. In rats pretreated with anti-MBG antibody, during withdrawal blood pressure not only did not increase, but exhibited a 6 mm Hg decrease.
DISCUSSION
The main observation of the present experiment is that in rats, acute ethanol withdrawal is associated with a rise in arterial pressure, with renal sodium retention, volume expansion, and a drop in hematocrit, and with an increase in renal excretion of MBG, an endogenous bufadienolide with natriuretic, vasopressor and anti-addictive properties.
The fact that, in a subset of rats, withdrawal-induced pressor response was prevented by in vivo administration of anti-MBG antibody, indicates that MBG is not only a marker, but a likely mediator of abstinence-induced hypertension. Recently, the same antibody was reported to reduce blood pressure in experimental NaCl-sensitive hypertension (Fedorova et al., 2002, 2005). Renal excretion of another CTS, endogenous ouabain, did not change following ethanol withdrawal, which agrees with the data, indicating that this hormone is not sensitive to plasma volume expansion (Haddy, 2006).
Ethanol withdrawal induces behavioral stress and is associated with anxiety and depression (Wetterling, Junghanns, 2006). Likewise, an increasing body of evidence demonstrates that changes in the activity and expression of brain Na/K-ATPase and in the levels of its endogenous ligands, CTS, are implicated in behavioral stress, mood control, and depression (Goldstein et al., 2006, Grider et al, 1999, Mynett-Johnson et al., 1998, de Vasconcellos et al, 2006). Since MBG exhibits anti-addictive properties (Bagrov et al., 1999, 2002, Kashkin et al., 2002), enhanced elaboration of this hormone would be anticipated during ethanol withdrawal. Remain to be understood are the mechanisms linking effects of MBG to the complex chain of central neurochemical events implicated in ethanol withdrawal.
Previously, NaCl sensitivity was considered a factor contributing to the susceptibility of alcoholics to cardiovascular diseases (Klatsky, 1995). Recently, Di Gennaro et al. (2002) demonstrated that in human subjects early alcohol withdrawal is associated with a positive sodium balance, increased NaCl-sensitivity of arterial pressure, and suppressed plasma renin activity, i.e., features which alcohol withdrawal shares with NaCl-sensitive hypertension (Weinberger et al., 1986: Weinberger, 1986). Our unpublished data (Kashkin, 2002, PhD Thesis) demonstrate that, in detoxified alcoholics, plasma levels of MBG exhibit a 2.5-fold increase. Since levels of MBG, a vasoconstrictor and a natriuretic, are increased in hypertensive humans, in experimental NaCl-sensitive hypertension, and during ethanol withdrawal, this hormone may be one of mediators linking ethanol withdrawal with the development of hypertension.
Acknowledgments
The experiments described in this paper were supported by the National Institute on Aging, National Institutes of Health Intramural Research Program and by Russian Foundation for Fundamental Science (grant 06-04-48956). The authors gratefully acknowledge excellent editorial assistance by Marinella Macri, and excellent technical support by Chad Boily BA, and Danielle Joseph, AA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bagrov AY, Bagrov YY, Fedorova OV, Kashkin VA, Patkina NA, Zvartau EE. Endogenous digitalis-like ligands of the sodium pump: possible involvement in mood control and ethanol addiction. Eur Neuropsychopharmacol. 2002;12:1–12. doi: 10.1016/s0924-977x(01)00127-4. [DOI] [PubMed] [Google Scholar]
- Bagrov YY, Dmitrieva NI, Manusova NB, Zvartau EE, Patkina NA, Bagrov AY. Involvement of endogenous digitalis-like factors in voluntary selection of alcohol by rats. Life Sci. 1999;64:219–225. doi: 10.1016/s0024-3205(99)00131-9. [DOI] [PubMed] [Google Scholar]
- Di Gennaro C, Vescovi PP, Barilli AL, Giuffredi C, Delsignore R, Montanari A. Sodium sensitivity as a main determinant of blood pressure changes during early withdrawal in heavy alcoholics. Alcohol Clin Exp Res. 2002;26:1810–1815. doi: 10.1097/01.ALC.0000042010.30185.31. [DOI] [PubMed] [Google Scholar]
- Goldstein I, Levy T, Galili D, Ovadia H, Yirmiya R, Rosen H, Lichtstein D. Involvement of Na,K-ATPase and endogenous digitalis-like compounds in depressive disorders. Biol Psychiatry. 2006;60:491–499. doi: 10.1016/j.biopsych.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Grider G, El-Mallakh RS, Huff MO, Buss TJ, Miller J, Valdes R., Jr Endogenous digoxin-like immunoreactive factor (DLIF) serum concentrations are decreased in manic bipolar patients compared to normal controls. J Affect Disord. 1999;54:261–267. doi: 10.1016/s0165-0327(98)00208-0. [DOI] [PubMed] [Google Scholar]
- Fedorova OV, Agalakova NI, Talan MI, Lakatta EG, Bagrov AY. Brain ouabain stimulates peripheral marinobufagenin via angiotensin II signalling in NaCl-loaded Dahl-S rats. J Hypertens. 2005;23:1515–1523. doi: 10.1097/01.hjh.0000174969.79836.8b. [DOI] [PubMed] [Google Scholar]
- Fedorova OV, Talan MI, Agalakova NI, Lakatta EG, Bagrov AY. Endogenous ligand of alpha(1) sodium pump, marinobufagenin, is a novel mediator of sodium chloride-dependent hypertension. Circulation. 2002;105:1122–1127. doi: 10.1161/hc0902.104710. [DOI] [PubMed] [Google Scholar]
- Haddy FJ. Role of dietary salt in hypertension. Life Sci. 2006;79:1585–1592. doi: 10.1016/j.lfs.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Kashkin VA, Bagrov AY, Fedorova OV, Bagrov YY, Agalakova NI, Patkina NA, Zvartau EE. Marinobufagenin (MBG) suppression of ethanol-seeking behavior is associated with inhibition of brain cortex Na/K-ATPase in mice. Eur Neuropsychopharmacol. 2002;12:217–223. doi: 10.1016/s0924-977x(02)00026-3. [DOI] [PubMed] [Google Scholar]
- Klatsky AL. Blood pressure and alcohol intake. In: Laragh JH, Brenner BM, editors. Hypertension: Pathophysiology, Diagnosis, and Management. 2nd. New York: Raven Press; 1995. pp. 2649–2667. [Google Scholar]
- Mynett-Johnson L, Murphy V, McCormack J, Shields DC, Claffey E, Manley P, McKeon P. Evidence for an allelic association between bipolar disorder and a Na+, K+ adenosine triphosphatase alpha subunit gene (ATP1A3) Biol Psychiatry. 1998;44:47–51. doi: 10.1016/s0006-3223(97)00343-0. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Holter SM, Allingham K, Landgraf R, Zieglgansberger W. Acamprosate and alcohol: I Effects on alcohol intake following alcohol deprivation in the rat. Eur J Pharmacol. 1996;305:39–44. doi: 10.1016/0014-2999(96)00174-4. [DOI] [PubMed] [Google Scholar]
- de Vasconcellos AP, Zugno AI, Dos Santos AH, Nietto FB, Crema LM, Goncalves M, Franzon R, de Souza Wyse AT, da Rocha ER, Dalmaz C. Na+,K(+)-ATPase activity is reduced in hippocampus of rats submitted to an experimental model of depression: effect of chronic lithium treatment and possible involvement in learning deficits. Neurobiol Learn Mem. 2006;84:102–110. doi: 10.1016/j.nlm.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Weinberger MH. Salt sensitivity of blood pressure. Hypertension (Part 2) 1996;27:481–490. doi: 10.1161/01.hyp.27.3.481. [DOI] [PubMed] [Google Scholar]
- Weinberger MH, Miller JZ, Luft FC, Grim CE, Fineberg NS. Definitions and characteristics of sodium sensitivity and blood pressure resistance. Hypertension. 1986;8 2:127–134. doi: 10.1161/01.hyp.8.6_pt_2.ii127. [DOI] [PubMed] [Google Scholar]
- Wetterling T, Junghanns K. Psychopathology of alcoholics during withdrawal and early abstinence. Eur Psychiatry. 2006;15:483–488. doi: 10.1016/s0924-9338(00)00519-8. [DOI] [PubMed] [Google Scholar]


