Abstract
Rad51 requires a number of other proteins, including the Rad51 paralogs, for efficient recombination in vivo. Current evidence suggests that the yeast Rad51 paralogs, Rad55 and Rad57, are important in formation or stabilization of the Rad51 nucleoprotein filament. To gain further insights into the function of the Rad51 paralogs, reporters were designed to measure spontaneous or double-strand break (DSB)-induced sister or nonsister recombination. Spontaneous sister chromatid recombination (SCR) was reduced 6000-fold in the rad57 mutant, significantly more than in the rad51 mutant. Although the DSB-induced recombination defect of rad57 was suppressed by overexpression of Rad51, elevated temperature, or expression of both mating-type alleles, the rad57 defect in spontaneous SCR was not strongly suppressed by these same factors. In addition, the UV sensitivity of the rad57 mutant was not strongly suppressed by MAT heterozygosity, even though Rad51 foci were restored under these conditions. This lack of suppression suggests that Rad55 and Rad57 have different roles in the recombinational repair of stalled replication forks compared with DSB repair. Furthermore, these data suggest that most spontaneous SCR initiates from single-stranded gaps formed at stalled replication forks rather than DSBs.
HOMOLOGOUS recombination is an important DNA repair mechanism to maintain genome integrity. Central to the process of homologous recombination is the pairing of DNA molecules and exchange of single strands to form heteroduplex DNA, a reaction catalyzed by members of the RecA/Rad51 family of proteins. Yeast and humans encode two RecA homologs, Rad51 and Dmc1, as well as Rad51-related proteins, referred to as Rad51 paralogs (Gasior et al. 2001; Thompson and Schild 2001). Yeast RAD51 is required for resistance to ionizing radiation, for spontaneous and induced mitotic recombination, and for meiotic recombination (Symington 2002). The Rad51 paralogs of Saccharomyces cerevisiae are encoded by the RAD55 and RAD57 genes and are determined by genetic studies to function in the same pathway for DNA repair and recombination as RAD51 (Kans and Mortimer 1991; Lovett 1994; Rattray and Symington 1995). The vertebrate Rad51 paralogs are encoded by the RAD51B, RAD51C, RAD51D, XRCC2, and XRCC3 genes (Thompson and Schild 2001). Mutation of any of these genes in the chicken DT40 cell line results in high sensitivity to DNA cross-linking agents, decreased frequencies of gene targeting, and increased frequencies of spontaneous chromosome aberrations (Takata et al. 2001).
Purified Rad51 forms right-handed helical filaments on single-stranded (ss) and double-stranded (ds) DNA (Ogawa et al. 1993; Sung and Robberson 1995). The Rad51-ssDNA nucleoprotein filament is active for homologous pairing and strand exchange with dsDNA. Formation of filaments on ssDNA is stimulated in the presence of the replication protein A (RPA) (Sung and Robberson 1995; Sugiyama et al. 1997), which is thought to allow the formation of continuous filaments by removal of secondary structures from ssDNA (Sugiyama et al. 1997). However, addition of RPA prior to or simultaneously with Rad51 is inhibitory to DNA binding and strand exchange by Rad51.
The Rad55 and Rad57 proteins, which form a stable heterodimer, can overcome the inhibition to Rad51-promoted strand exchange imposed by RPA, but the mechanism of mediation is unknown (Sung 1997). Consistent with a role in Rad51 recruitment, Rad51 foci are not observed in rad55 or rad57 mutants during meiosis (Gasior et al. 1998). However, Rad51 is still able to associate with double-strand breaks (DSBs) in rad55 mutants during vegetative growth although recruitment of Rad51 is slower and less extensive in rad55 mutants than in wild type (Sugawara et al. 2003; Lisby et al. 2004; Fung et al. 2006). The role of the Rad51 paralogs as accessory proteins for Rad51 is also supported by the observation that overexpression of RAD51 partially suppresses the radiation or mitomycin C sensitivity of cell lines with mutations in any of the Rad51 paralog-encoding genes (Hays et al. 1995; Johnson and Symington 1995; Takata et al. 2001). Furthermore, gain-of-function alleles of yeast RAD51 that encode proteins with higher affinity for DNA than wild-type Rad51 partially suppress the ionizing radiation (IR) sensitivity of rad55 or rad57 mutants (Fortin and Symington 2002). The IR sensitivity of rad55 or rad57 mutants is also suppressed by expression of both mating-type alleles in haploids. It has been suggested that this suppression acts at the level of Rad51 activity because MAT heterozygosity, deletion of SRS2, or overexpression of Rad51 also suppresses other mutants known to have mediator defects, such as rad51-K191R (Fung et al. 2006) and rad52-20 (Schild 1995), but does not suppress rad51 or rad52 null mutants. In budding and fission yeasts, rad55 or rad57 null mutants exhibit cold sensitivity for DSB repair (DSBR) (Symington 2002). Cold sensitivity is a property often associated with proteins composed of multiple subunits or large multiprotein complexes (Scheraga et al. 1962), consistent with a role for the Rad51 paralogs in stabilizing Rad51 nucleoprotein filaments.
While the biochemical and cytological studies support a role for the Rad51 paralogs in promoting assembly or stability of the Rad51 nucleoprotein filament (Gasior et al. 1998; Van Veelen et al. 2005), recent studies suggest the possibility of an additional late function in recombination. Rad51B and the BCDX2 complex have been shown to preferentially bind synthetic Holliday junctions (HJs) over other types of DNA substrates (Yokoyama et al. 2004). Furthermore, extracts made from XRCC3−/− or RAD51C−/− hamster cells lacked normal levels of HJ resolvase activity, suggesting that the Rad51C-Xrcc3 complex may contribute to the resolution of recombination intermediates (Liu et al. 2004). Increased evidence for this postulate comes from the report that Rad51C localizes to paired bivalents during the late stages of prophase during meiosis I when crossovers are thought to occur (Liu et al. 2007).
Mammalian XRCC3−/− or RAD51C−/− cell lines, and Schizosaccharomyces pombe rad57 mutants, which show reduced frequencies of DSB-induced recombination, also show alterations in the products recovered with an increase in long-tract gene conversion events (Brenneman et al. 2002; Nagaraju et al. 2006; Akamatsu et al. 2007; Hope et al. 2007). The long-tract events could be due to altered processing of recombination intermediates, perhaps in displacement of the invading strand.
Understanding the role of the Rad51 paralogs in homologous recombination in yeast has been difficult because the rad55 and rad57 mutants show minimal phenotypes in assays that measure spontaneous mitotic recombination between heteroalleles located on homologous chromosomes in diploids or positioned on nonhomologous chromosomes in haploids (Lovett and Mortimer 1987; Freedman and Jinks-Robertson 2002). This weak phenotype in recombination assays is in contrast to the high IR sensitivity displayed by rad55 and rad57 mutants (Symington 2002). A common feature of the recombination assays is selection for spontaneous recombination between nonsister chromatids, whereas IR-induced damage (DSBs) is thought to be repaired primarily by sister chromatid recombination (Kadyk and Hartwell 1992). In addition, rad55 or rad57 mutants that express both MATa and MATα alleles, as is typically the case in diploids, show much higher resistance to IR, suggesting that the failure to detect a recombination defect in diploids could be a consequence of suppression by MAT heterozygosity.
To determine whether the weak recombination defect of rad55 and rad57 mutants relates to MAT heterozygosity, the type of recombination reporter used, or the source of DNA damage, we designed two recombination systems to measure spontaneous or DSB-induced gene conversion. We show that rad57 mutants are highly defective for spontaneous gene conversion between direct repeats and that this defect is not suppressed by factors that suppress the IR sensitivity of the rad57 mutants, suggesting a specialized function for Rad55-Rad57 in the repair of spontaneous lesions. In addition, these studies suggest that the substrate for spontaneous sister chromatid recombination is more likely to be a ssDNA gap (SSG) formed at a stalled replication fork than a DSB.
MATERIALS AND METHODS
Media, growth conditions, and genetic methods:
Rich medium (yeast extract–peptone–dextrose, YPD), synthetic complete (SC) medium lacking the appropriate amino acids or nucleic acid bases, sporulation medium, and genetic methods were as described previously (Sherman et al. 1986). Synthetic minimal medium containing 2% lactate (pH 5.5) and supplemented with adenine, uracil, and leucine was used for the galactose induction of I-SceI in the direct-repeat double-strand break-induced recombination assays. Minimal medium containing 2% lactate (pH 5.5) and supplemented with adenine, uracil, leucine, and tryptophan was used for the galactose induction of I-SceI in the heteroallelic double-strand break-induced recombination assays. Transformation of yeast cells was performed by the lithium acetate method (Ito et al. 1983).
Yeast strains and plasmids:
S. cerevisiae strains used in this study are listed in Table 1. All strains are in the RAD5-corrected W303 background (his3-11, 15 leu2-3, 112 trp1-1 ura3-1 ade2-1 can1-100 RAD5) except those listed specifically as rad5-535 and BY4742. To create a haploid strain with the ade2-n∷TRP1∷ade2-I direct repeat, pLS189 was digested with BglII, which cuts between the NdeI site mutation and the I-SceI site insertion within ade2, to target integration at the ADE2 locus of LSY697. Trp+ transformants that were red (ade2) were analyzed by Southern hybridization to determine the structure of the integrated plasmid. LSY1429#2 contains a direct repeat of ade2-nde− and ade2-I-SceI+ alleles separated by vector sequences and TRP1 (referred to as ade2-n∷TRP1∷ade2-I). Strain LSY1430 was made by the same method to create the ade2-n∷TRP1∷ade2-I reporter in the exo1 background. Strain LSY1309 was made by gene replacement of the MATα locus with URA3 using pFP19. Transformants deleted for the MAT locus become “a fakers” and are competent for mating with a MATα test strain. Haploid strains expressing both mating-type alleles were made by transforming MATa haploids with pRS414-MATα or by deleting SIR4 using a PCR fragment containing homologous 5′- and 3′-flanking sequences from BY4742 sir4∷KanMX4, resulting in the integration of the KanMX4 marker and the loss of the wild-type SIR4 allele. LSY1788 was made by the same method, replacing the RAD57 locus in LSY1519-1D with a PCR fragment from LSY536, resulting in integration of the URA3 marker and loss of the native RAD57 allele. LSY1518 was made by one-step gene replacement of the RAD55 locus in W1588-4C with a PCR fragment containing 50 bp of homologous 5′ and 3′ sequences flanking the His3MX6 module amplified from pFA6a-His3MX6 (Longtine et al. 1998). To construct LSY1567, LSY1568, LSY1700, and LSY1722, corresponding strains LSY1421-2A, LSY1422-3A, LSY1421-5B, and LSY1721-2D were patched onto synthetic medium containing 5-fluoroorotic acid (5-FOA) to select for pop-out events (Boeke et al. 1987), and then red colonies were screened by PCR of the ADE2 locus followed by restriction enzyme analysis to obtain clones with the desired ade2 allele. To construct LSY1566, LSY1569, LSY1661, and LSY1703, respective strains LSY1516-10C, LSY1519-1D, LSY1538-8B, and LSY1693-3A were patched onto synthetic medium containing 5-fluoroanthranilic acid (5-FAA) to select for pop-out events (Toyn et al. 2000), and red colonies were then screened by PCR and restriction enzyme analysis to find clones with the desired ade2 allele. Most other haploid strains were made by mating appropriate haploid strains, sporulating the resulting diploids, and screening the haploid segregants for the desired genotype. Diploids used for recombination assays were made by crossing the appropriate haploid strains.
TABLE 1.
Yeast strains
| Strain | Genotype | Source or reference |
|---|---|---|
| W1588-4C | MATa | R. Rothstein |
| W1588-4A | MATα | R. Rothstein |
| W3770-4D | MATaleu2ΔEcoRI∷URA3-HO∷leu2ΔBstEII | Smith and Rothstein (1999) |
| W4121-20D | MATaADE2 bar1∷LEU2 YFP-RAD51 | Lisby et al. (2004) |
| W5857-15A | MATaADE2 bar1∷LEU2 YFP-RAD51 RAD54-CFP rad55 | Lisby et al. (2004) |
| B366-6A | MATaade2-n rad5-535 | Bai and Symington (1996) |
| yKH12α | MATα ade2-a∷URA3∷ade2-n rad5-535 | Huang and Symington (1994) |
| LSY383 | MATarad51∷LEU2 rad5-535 | Mcdonald and Rothstein (1994) |
| LSY401 | MATα rad51∷LEU2 | H. Klein |
| LSY408 | MATα rad57∷LEU2 | H. Klein |
| LSY410-1 | MATarad51∷URA3 rad5-535 | Rattray and Symington (1994) |
| LSY536 | MATα rad57∷URA3 rad5-535 | Johnson and Symington (1995) |
| LSY697 | MATamet17-sna ADE2 | Bartsch et al. (2000) |
| LSY1309-1 | MATΔ∷URA3 ade2-n rad5-535 | Fung et al. (2006) |
| LSY1390 | MATα rad55∷LEU2 srs2∷HIS3 | Fung et al. (2006) |
| LSY1392 | MATα rad57∷LEU2 srs2∷HIS3 | Fung et al. (2006) |
| LSY1421-2A | MATaade2-n∷URA3∷ade2-a rad55∷LEU2 | This study |
| LSY1421-5B | MATα ade2-n∷URA3∷ade2-a rad55∷LEU2 rad5-535 | This study |
| LSY1422-3A | MATaade2-n∷URA3∷ade2-a rad57∷LEU2 rad5-535 | This study |
| LSY1430 | MATaade2-n∷TRP1∷ade2-I exo1∷HIS3 | This study |
| LSY1429#2 | MATaade2-n∷TRP1∷ade2-I | This study |
| LSY1516-10C | MATα ade2-n∷TRP1∷ade2-I rad57∷URA3 rad5-535 | This study |
| LSY1518 | MATarad55∷His3MX6 | This study |
| LSY1519-1D | MATα ade2-n∷TRP1∷ade2-I | This study |
| LSY1538-8B | MATα ade2-n∷TRP1∷ade2-I rad55∷His3MX6 | This study |
| LSY1566 | MATα ade2-n rad57∷LEU2 rad5-535 | This study |
| LSY1567 | MATaade2-n rad55∷LEU2 | This study |
| LSY1568 | MATaade2-n rad57∷LEU2 rad5-535 | This study |
| LSY1569 LSY1739-6B | MATα ade2-I | This study |
| LSY1661 | MATα ade2-I rad55∷His3MX6 | This study |
| LSY1667 | MATa/MATα ade2-n/ade2-I RAD5/rad5-535 | This study |
| LSY1668 | MATa/MATα ade2-n/ade2-I rad55∷LEU2/rad55∷ His3MX6 | This study |
| LSY1693-3A | MATα ade2-n∷TRP1∷ade2-I rad51∷URA3 | This study |
| LSY1699 | MATΔ∷URA3 ade2-I rad55∷His3MX6 rad5-535 | This study |
| LSY1700 | MATα ade2-n rad55∷LEU2 rad5-535 | This study |
| LSY1703 | MATα ade2-I rad51∷URA3 rad5-535 | This study |
| LSY1708-5C | MATα ade2-n∷TRP1∷ade2-I rad51∷LEU2 | This study |
| LSY1721-2D | MATaade2-n∷URA3∷ade2-I rad5-535 | This study |
| LSY1722 | MATaade2-n rad51∷LEU2 rad5-535 | This study |
| LSY1734 | MATα ade2-n∷TRP1∷ade2-I sir4∷KanMX4 | This study |
| LSY1736 | MATα ade2-n∷TRP1∷ade2-I rad51∷LEU2 sir4∷KanMX4 | This study |
| LSY1740-1D | MATΔ∷URA3 ade2-n rad51∷LEU2 | This study |
| LSY1759 | MATΔ∷URA3/MATα ade2-n/ade2-I RAD5/rad5-535 | This study |
| LSY1761-2D | MATΔ∷URA3 ade2-n rad57∷LEU2 rad5-535 | This study |
| LSY1767 | MATΔ∷URA3/MATα ade2-n/ade2-I rad51∷URA3/rad51∷LEU2 | This study |
| LSY1784 | MATa ade2-I dnl4∷KanMX4 | |
| LSY1785 | MATΔ∷URA3 ade2-n dnl4∷KanMX4 | |
| LSY1788 | MATα ade2-n∷TRP1∷ade2-I rad57∷URA3 | This study |
| LSY1789 | MATα ade2-n∷TRP1∷ade2-I rad57∷URA3 sir4∷KanMX4 | This study |
| LSY1790 | MATΔ∷URA3/MATα ade2-n/ade2-I dnl4∷kanMX4/ dnl4∷kanMX4 | This study |
| LSY1876 | MATα ade2-I rad57∷URA3 | This study |
| LSY1877 | MATα ade2-n rad55∷LEU2 | This study |
| LSY1878 | MATα ade2-I rad51∷URA3 | This study |
| LSY1881 | MATa/MATα ade2-n/ade2-I rad57∷LEU2/rad57∷URA3 RAD5/rad5-535 | This study |
| LSY1882 | MATa/MATα ade2-n/ade2-I rad51∷URA3/rad51∷LEU2 RAD5/rad5-535 | This study |
| LSY1883 | MATΔ∷URA3/MATα ade2-n/ade2-I rad55∷LEU2/rad55∷ His3MX6 RAD5/rad5-535 | This study |
| LSY1884 | MATΔ∷URA3/MATα ade2-n/ade2-I rad57∷LEU2/rad57∷ URA3 RAD5/rad5-535 | This study |
| LSY1973-1B | MATaleu2ΔEcoRI∷URA3-HO∷leu2ΔBstEII rad51∷HIS3 | This study |
| LSY1974 | MATaleu2ΔEcoRI∷URA3-HO∷leu2ΔBstEII rad55∷HIS3 | This study |
| BY4742 sir4∷KanMX4 | MATα sir4∷KanMX4 his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 | Winzeler et al. (1999) |
To create an ade2 allele with an I-SceI site, two 29-mer oligonucleotides containing the I-SceI recognition sequence were annealed and ligated to AatII-digested pAL78, which contains the ade2-n allele (Rattray and Symington 1994), creating pLS188. A 3.6-kb BamHI fragment from pLS188 containing the ade2-n, I-SceI allele was cloned into the multiple-cloning site of pRS404 to generate pLS189. The plasmid for expression of I-SceI (p373) was a gift from S. Marcand (Frank-Vaillant and Marcand 2001), pFP19 was a gift from J. Haber, and pRS414-MATα was a gift from R. Rothstein. The high-copy-number plasmid expressing RAD51 from the native promoter, YEp24∷RAD51, was described previously (Bai and Symington 1996).
Determination of mitotic recombination frequencies and rates:
Mitotic recombination rates were determined by the method of the median (Lea and Coulson 1948). Yeast strains were grown on YPD plates for 2–3 days at 30° or for 3–4 days at 23°, nine independent colonies were inoculated into 5 ml of YPD, and cultures were grown overnight at either 30° or 23°. Cells were pelleted and resupended in 1 ml of sterile H2O. Aliquots of appropriate dilutions were plated onto SC medium to determine the number of viable cells in each culture and onto SC medium minus adenine and tryptophan for the direct-repeat assay, or SC −Ade for interhomolog, to determine the total number of recombinants in each culture. Plates were incubated for 3–5 days, after which colonies were counted. For each strain, recombination rates were measured three times on independent isolates and the mean values are presented. t-Tests were used to determine the statistical significance of differences in recombination rates between given strains.
To determine I-SceI-induced recombination frequencies, strains with genetic recombination reporters were transformed with the HIS3-containing I-SceI expression plasmid (p373) and were grown to saturation in selective medium at either 30° or 23°. Cultures were diluted 1:100 into minimal medium containing 2% lactate (pH 5.5) supplemented with the appropriate amino or nucleic acids and cultured overnight at either 30° or 23° to a cell density of 3 × 107 cells/ml. Galactose was added to a final concentration of 2% (w/v). For strains containing the ade2 direct-repeat reporter, tryptophan was also added upon galactose induction. Cells were removed prior to and 3 hr after galactose induction, and aliquots (50 μl) of appropriate dilutions were plated onto SC medium to determine the number of viable cells in each culture and onto SC −Ade (for ade2 heteroallelic reporter) or SC −Ade −Trp (for ade2 direct-repeat reporter) to determine the total number of recombinants in each culture. Plates were incubated for 3 or 5 days at 30° or 23°, respectively, after which colonies were counted. For each strain, recombination frequencies were measured three times on independent His+ transformants and the mean values are presented.
Clastogen sensitivity tests:
For ultraviolent (UV) and γ-irradiation (IR) sensitivity tests, cells were grown in liquid YPD medium at 30° to midlog. The cultures were serially diluted and aliquots of each dilution were spotted onto YPD plates. The plates were irradiated either in a Gammacell-220 irradiator containing 60Co or in a Stratagene (La Jolla, CA) UV Stratalinker 2400 and incubated at 30° for 3 days. For camptothecin (CPT) sensitivity tests, cells were grown in YPD overnight at 30°. Strains were diluted to a concentration of 0.7 × 107 cells/ml, and five additional 10-fold serial dilutions were made. Aliquots of each dilution were spotted onto the indicated media and incubated at 30° for 3 days. Strains were spotted onto YPD plates containing 0.5 or 1 μg/ml CPT and 2% dimethyl sulfoxide (DMSO). Control plates contained 2% DMSO.
Microscopy:
Cells were grown in SC medium or SC media minus tryptophan to an optical density at 600 nm (OD600) of 0.2, at which time the liquid cultures were exposed to IR or UV radiation or were left unirradiated. For IR, 1 ml of cells was placed in an Eppendorf tube and exposed to defined doses of γ-rays in a Gammacell-220 60Co irradiator. For UV irradiation, 1 ml of cells was briefly centrifuged and resuspended in 20 μl of media, and 10 μl of the concentrated cell culture was spotted onto a microscope slide, left uncovered, and irradiated at 20 J/m2 in a Stratagene Stratalinker 2400. Aliquots of the cultures were processed immediately for imaging as described previously (Lisby et al. 2004). Yellow fluorescent protein (YFP) fluorescence was acquired using Openlab software (Improvision).
RESULTS
Experimental design:
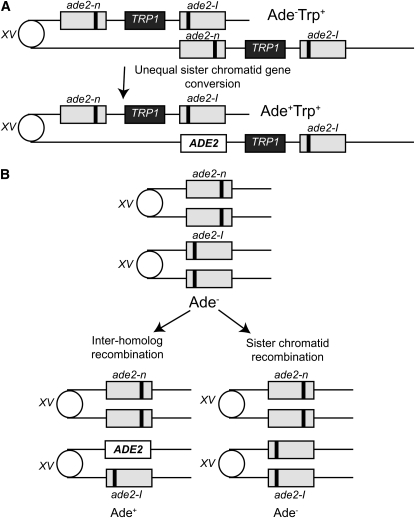
To measure sister and intrachromatid recombination events, we constructed a substrate that contains a direct repeat of alleles of the ade2 gene separated by plasmid sequences and a copy of the TRP1 gene integrated at the ADE2 locus on chromosome XV (Figure 1A). One allele contains a 2-bp fill-in mutation of the NdeI site resulting in a frameshift (Huang and Symington 1994); the other allele contains a 29-bp insertion of the I-SceI recognition site at the AatII site. Recombination between the ade2 repeats to generate a wild-type copy of the ADE2 gene results in two phenotypic classes. Events that retain the duplication are Ade+ Trp+; these could occur by intrachromatid or unequal sister chromatid gene conversion. Recombination events that result in the loss of one of the repeats and the TRP1 marker are referred to as pop-outs. These could occur by any one of an array of mechanisms, including intrachromatid crossing over, unequal sister chromatid exchange, unequal sister chromatid conversion, single-strand annealing, or replication mispairing (Symington 2002). Recombination between the ade2 alleles could occur spontaneously or be induced following induction of the I-SceI nuclease, which makes a DSB at the artificially inserted cut site within the ade2-I allele. This DSB will induce recombination with the ade2-n repeat.
Figure 1.—
Recombination substrates and products. (A) The direct-repeat recombination substrate contains 3.6-kb repeats with different ade2 alleles integrated at the endogenous locus on chromosome XV separated by plasmid sequences and the TRP1 gene. One allele contains a 2-bp fill-in mutation of the NdeI site resulting in a frameshift while the other allele has an I-SceI cut site insertion disrupting the wild-type AatII site. Unequal sister chromatid or intrachromatid gene conversion between the two ade2 repeats can generate Ade+ Trp+ recombinants that retain the duplication. Either allele could be converted; only one type of conversion is shown here. (B) The heteroallelic recombination substrate contains the different ade2 alleles at the native chromosomal loci in diploid strains. Interhomolog recombination between the mutant alleles can generate Ade+ recombinants. Sister chromatid recombination is phenotypically silent in this assay.
To measure mitotic interchromosomal recombination events, we introduced the two different ade2 alleles in diploid strains (Figure 1B). Analogous to the direct-repeat recombination substrate, recombination between the ade2 heteroalleles can occur spontaneously or be induced by a site-specific DSB. Using these assay systems with the same pair of heteroalleles, spontaneous or DSB-induced direct-repeat or interchromosomal recombination can be compared in various mutant strains.
Spontaneous gene conversion between direct repeats requires RAD55 and RAD57:
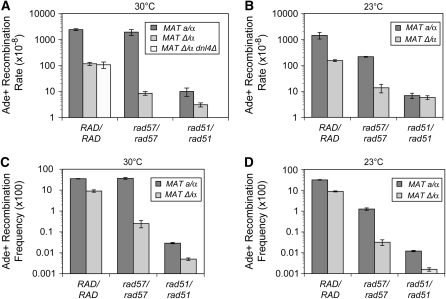
To determine the role of RAD57 in spontaneous sister chromatid recombination (SCR), we measured the rate of Ade+ prototroph formation in rad57 and wild-type strains using the direct-repeat substrate (Figure 1A). Consistent with previous reports (McDonald and Rothstein 1994; Liefshitz et al. 1995), the rate of direct-repeat recombination was the same for wild-type and rad57 strains, but closer analysis revealed that the classes of recombinants recovered from the two strains were markedly different. Eighty-five percent of the Ade+ recombinants in the wild-type strain were also Trp+, whereas only 0.2% of the Ade+ recombinants in the rad57 mutant were Trp+ (supplemental Figure 1 at http://www.genetics.org/supplemental/). Thus most of the Ade+ recombinants in the wild-type strain occur by gene conversion, whereas pop-outs account for most of the events in the rad57 mutant. The pop-out events most likely occur by single-strand annealing because this mechanism is known to be independent of RAD51 and RAD57 (Ivanov et al. 1996). These results suggest that RAD57 is important for gene conversion between direct repeats and consequently all further assays with this substrate measured formation of Ade+ Trp+ recombinants.
The rate of spontaneous Ade+ Trp+ recombinants in the wild-type strain, 1.48 × 10−3/cell/generation, is artificially high due to the slight toxicity of the red pigment that accumulates in ade2 mutants, resulting in a selective advantage for Ade+ recombinants. Because of the low rates of recombination observed for some mutants it was necessary to expand the cultures for an additional day, resulting in the wild-type rates being higher than reported previously using similar substrates (Huang and Symington 1994; Rattray and Symington 1994). The rad57 mutant was severely defective in this assay system, showing a 6000-fold reduction in the rate of recombination compared to wild type (Figure 2A). Because isogenic strains containing a disruption of the RAD55 gene behaved equivalently to rad57 mutants in all of the recombination and cell survival assays (data not shown), for brevity only data from the rad57 mutant are shown. Restriction enzyme mapping and Southern blot analysis of DNA from 20 independently derived Ade+ Trp+ recombinants from wild type and the rad57 mutant confirmed retention of the duplication, as expected for a simple gene conversion event (data not shown). Unexpectedly, the rad51 mutant had a recombination rate 4-fold higher than that of the rad57 mutant (P < 0.01) and the rad51 rad57 double mutant behaved the same as the rad57 mutant (Figure 2A). Although the wild-type rate in this system may be artificially high, the rad55 mutant showed a 50-fold reduction in the rate of spontaneous gene conversion using a leu2 direct-repeat construct, similar to the rate observed for the rad51 mutant (supplemental Figure 2 at http://www.genetics.org/supplemental/). In contrast to the ade2 assay, there was no significant difference in the rate of Leu+ Ura+ recombinants between rad51 and rad55 mutants. This could be due to the greater range observed with the ade2 assay, allowing detection of small, but significant differences between mutants. These findings support the notion that Rad55 and Rad57 are extremely important in the loading of Rad51 onto single-stranded DNA in the context of the replication fork.
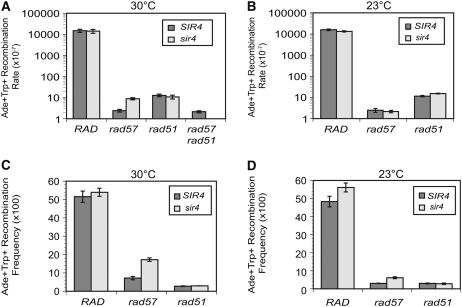
Figure 2.—
RAD57 is required for spontaneous sister chromatid recombination. (A) Spontaneous sister chromatid recombination rates at 30°. SIR4 strains used were LSY1519-1D (Rad+), LSY1788 (rad57∷URA3), LSY1708-5C (rad51∷LEU2), and LSY1933-5C (rad57∷URA3 rad51∷LEU2); sir4 strains used were LSY1734 (Rad+), LSY1789 (rad57∷URA3), and LSY1736 (rad51∷LEU2). (B) Spontaneous sister chromatid recombination rates at 23°. (C) DSB-induced recombination frequencies at 30°. (D) DSB-induced recombination frequencies at 23°.
The spontaneous direct-repeat gene conversion defect conferred by rad57 is not suppressed by MAT heterozygosity and is temperature independent:
It has been reported previously that MAT heterozygosity, overexpressing Rad51, or high temperature suppress the ionizing radiation sensitivity and DSB-induced recombination defects of rad55 and rad57 mutants (Lovett and Mortimer 1987; Hays et al. 1995; Johnson and Symington 1995). Therefore, it was of interest to know if these suppressors of Rad51 mediator defects would suppress the defect in spontaneous direct-repeat recombination observed for the rad57 mutant.
To make haploid strains express both mating-type alleles, we made a sir4 mutation in these strains. The SIR genes are required for transcriptional silencing of the HMRa and HMLα loci (Rine and Herskowitz 1987). The sir4 mutation suppressed the spontaneous recombination defect of the rad57 mutant by only 4-fold compared to the rad57 single mutant. The rad57 sir4 double mutant still showed a 1500-fold decrease in the recombination rate compared to the Rad+ sir4 strain and had the same recombination rate as a rad51mutant (Figure 2A). As described below, the IR sensitivity of the rad57 mutant was suppressed >100-fold by the sir4 mutation. To ensure that the sir4 mutation is equivalent to simply expressing both MAT alleles, we transformed a rad57 MATa haploid with a plasmid expressing the MATα allele and determined recombination rates. The rate was 4-fold higher than that of the rad57 strain expressing only one MAT allele (data not shown), consistent with the sir4 effect being due to MAT heterozygosity. In accordance with MAT heterozygosity upregulating Rad51 filament formation or function, MAT heterozygosity does not suppress the spontaneous recombination defect of a rad51 mutant (Figure 2A). It was also found that overexpressing Rad51 suppresses the spontaneous direct-repeat recombination defect of the rad55 mutant by only 4-fold (supplemental Figure 3 at http://www.genetics.org/supplemental/). When recombination rates were determined at 23°, the rad57 mutant did not display a further decrease (P > 0.5) (Figure 2B). This was surprising since rad55 and rad57 mutants have been reported to exhibit cold sensitivity to ionizing radiation and for DSB-induced recombination (Lovett and Mortimer 1987; Hays et al. 1995; Johnson and Symington 1995).
The DSB-induced direct-repeat gene conversion defect of the rad57 mutant is suppressed by MAT heterozygosity and is temperature dependent:
Earlier studies have reported that rad55 and rad57 haploid strains are defective for DSB-induced recombination, but are not as defective as a rad51 mutant (Firmenich et al. 1995). It has also been shown that the DSBR defect of rad55 or rad57 mutants can be suppressed 25-fold by overexpression of Rad51 (Hays et al. 1995). We wanted to verify these past results with the ade2 direct-repeat reporter and investigate whether other suppressors of Rad51 filament formation, specifically MAT heterozygosity and temperature, suppress the rad57 DSB-induced recombination defect. At 30° the rad57 mutant showed a 6-fold reduction in DSB-induced direct-repeat gene conversion compared to wild type (P < 0.001), whereas recombination was decreased by 18-fold in the rad51 mutant, significantly less than in wild-type (P < 0.0005) or rad57 (P < 0.01) strains (Figure 2C). This differs from what was observed in the spontaneous recombination assay, in which rad57 had a more severe phenotype than the rad51 mutant. This distinction between spontaneous and DSB-induced recombination is furthered by the observation that MAT heterozygosity effectively suppressed the rad57 mutant; DSB-induced recombination in the rad57 sir4 double mutant was significantly increased compared with rad57 (P < 0.005) and the double mutant now had only a 3-fold defect in relation to wild type. More support for the incongruity in RAD57's role between spontaneous and DSB-induced recombination was garnered by the observation that the DSB-induced gene conversion defect of the rad57 mutant is cold sensitive. At 23°, the rad57 mutant showed a 2.3-fold reduced recombination frequency compared to rad57 at 30° (P = 0.01) and was as defective as rad51. Suppression by MAT heterozygosity also appears to be temperature dependent because the rad57 sir4 double-mutant recombination frequency was reduced 2.8-fold at 23° compared with rad57 sir4 at 30° (P = 0.005) (Figure 2D). The strong suppression of the rad57 DSB-induced recombination defect by suppressors of Rad51 filament formation suggests that the function of Rad55-Rad57 in DSBR is primarily in mediation of the Rad51 filament. This is in contrast to the role of Rad55-Rad57 in the repair of spontaneous lesions, which is not cold sensitive and not strongly suppressed by MAT heterozygosity or by overexpression of Rad51.
MAT heterozygosity strongly suppresses the sensitivity of the rad57 mutant to genotoxic agents that cause DSBs but not single-stranded gaps:
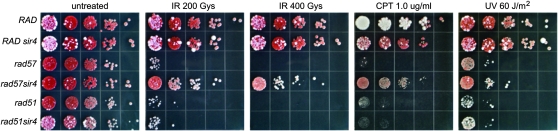
A prediction from the above findings is that MAT heterozygosity should suppress the sensitivity of the rad57 mutant to agents that create DSBs. The sir4 mutation (MAT heterozygous) suppressed the IR sensitivity of the rad57 mutant by >100-fold to 200 Gy IR (Figure 3). As described previously, the suppression by MAT heterozygosity is not observed for the rad51 mutant. CPT stabilizes the covalent DNA-Top1 intermediate that forms during the catalytic DNA nicking-closing cycle of Top1 and these stable nicks can then be converted into recombinogenic DSBs during replication (Hsiang et al. 1989). Similar to the response to IR, the rad57 mutant is extremely sensitive to CPT and this sensitivity is strongly suppressed by MAT heterozygosity (Figure 3). Thus the rad57 defect in repair of DSBs made in the context of the replication fork is the same as DSBs made by IR.
Figure 3.—
MAT heterozygosity strongly suppresses the sensitivity of rad57 to DSB-inducing genotoxic agents but not those forming single-stranded gaps. Tenfold serial dilutions of log-phase cultures of the strains were spotted onto YPD plates and left unirradiated or irradiated with 200 or 400 Gy or were UV irradiated at 75 J/m2. Survival was assessed following growth for 3 days at 30°. Serial dilutions of saturated cultures of the same strains as above were spotted onto YPD only or YPD containing 2% DMSO and 1.0 μg/ml camptothecin (CPT). Survival was assessed following growth for 3 days at 30°. Strains used were LSY1519-1D (RAD), LSY1734 (RAD sir4∷kanMX6), LSY1788 (rad57∷URA3), LSY1789 (rad57∷URA3 sir4∷kanMX6), LSY1708-5C (rad51∷LEU2), and LSY1736 (rad51∷LEU2 sir4∷kanMX6).
UV irradiation is thought to cause replication fork stalling and consequently, single-stranded gaps (Lopes et al. 2006). The rad57 mutant was 1000-fold more UV sensitive than wild type at a UV dose of 60 J/m2 and had a sensitivity that closely resembled that of the rad51 mutant. Significantly, the rad57 sir4 double mutant showed only a 3- to 10-fold increase in UV survival compared to the rad57 single mutant (Figure 3). Similarly, overexpression of Rad51 suppressed the UV sensitivity of the rad55 mutant only slightly while sensitivity to CPT was strongly suppressed (supplemental Figure 3 at http://www.genetics.org/supplemental/). The weak suppression of the UV sensitivity and spontaneous recombination defect of the rad57 mutant by MAT heterozygosity and overexpression of Rad51, in contrast to the strong suppression seen in assays for DSBR, suggests that SSGs are the primary lesion initiating spontaneous SCR.
MAT heterozygosity suppresses the defect of rad57 in the formation of YFP-Rad51 foci following UV-induced damage:
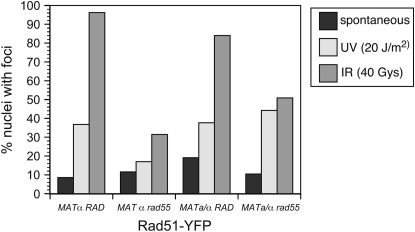
The lack of suppression of the UV sensitivity of rad55 and rad57 mutants by known suppressors of Rad51 filament formation could be interpreted as evidence for a late role for Rad55-Rad57 in recombination after Rad51 filament formation or that loading of Rad51 onto single-stranded DNA during UV-induced recombination is qualitatively different from filament formation in DSB-induced recombination. To differentiate between these two possibilities we monitored Rad51 recruitment to DNA-damaged sites by epifluorescence microscopy, using a fusion of YFP to Rad51 (Lisby et al. 2004) (Figure 4). Although we assume Rad51 foci formation corresponds to recruitment and filament formation by Rad51 at sites of DNA damage, the foci could also represent Rad51-mediated joint molecules. As expected, the MAT homozygous rad55 mutant was defective in forming YFP-Rad51 foci after UV or IR treatment (P < 0.001). In agreement with the clastogen spot assays, MAT heterozygosity partially suppressed the rad55 defect in YFP-Rad51 focus formation following treatment with IR (P < 0.005). However, in contrast to the weak suppression of the UV sensitivity of the rad55 mutant by MAT heterozygosity, expression of both mating-type alleles in the rad55 mutant rescued YFP-Rad51 formation in response to UV (P = 0.0001). As reported previously, the Rad51 foci formed in response to UV or IR in the rad55 mutant were less bright than those observed in wild-type cells (Fung et al. 2006). The dimmer foci suggest Rad51 is still able to nucleate in the absence of the Rad51 paralogs, but is unable to form extensive filaments or the filaments are less stable.
Figure 4.—
The UV sensitivity of the rad55 mutant is caused by defects independent of Rad51 recruitment. YFP fusions were made with Rad51 in RAD and rad55 backgrounds (LSY1575 and W5857-15A, respectively). MATa haploids were transformed with pRS414-MATα to express both mating-type alleles. Log-phase cultures of the strains were exposed to no irradiation, 20 J/m2 of UV irradiation, or 40 Gy IR, followed by microscopy to monitor focus formation. For each strain between 77 and 183 cells were counted.
MAT heterozygosity and temperature suppress the spontaneous and DSB-induced interhomolog recombination defects of rad57Δ/rad57Δ diploids:
In previous studies, rad55 homozygous diploids were shown to have wild-type frequencies of recombination at 30° and a 10-fold decrease in recombination frequencies at 23° (Lovett and Mortimer 1987; Signon et al. 2001). We confirmed that at 30° MAT heterozygous rad57 or rad55 diploids do not have a defect in spontaneous interhomolog recombination yet the rad51 mutant shows a 240-fold reduction compared to wild type (P < 0.0005) (Figure 5A, rad55 data not shown). Akin to the spontaneous interhomolog results, the MAT heterozygous rad57 diploid did not display a DSB-induced recombination defect. This is in contrast to the rad51 diploid, which showed a 1000-fold reduction in DSB-induced recombination compared with wild type (P < 0.0005) (Figure 5C).
Figure 5.—
MAT heterozygosity and temperature suppress the interhomolog recombination defect of the rad57 diploid. (A) Spontaneous interhomolog recombination rates at 30°. MAT heterozygous (MATa/α) strains used were LSY1667 (RAD/RAD), LSY1881 (rad57∷LEU2/rad57∷URA3), and LSY1882 (rad51∷URA3/rad51∷LEU2). MAT homozygous (MATΔ/α) strains used were LSY1759 (RAD/RAD), LSY1884 (rad57∷URA3/rad57∷LEU2), and LSY1767 (rad51∷URA3/rad51∷LEU2). (B) Spontaneous interhomolog recombination rates at 23°. (C) DSB-induced interhomolog recombination frequencies at 30°. (D) DSB-induced interhomolog recombination frequencies at 23°.
These results suggest either that RAD55 and RAD57 are not important for interhomolog recombination or that temperature and/or MAT heterozygosity suppress the phenotype of rad55 and rad57 mutants in this assay. To distinguish between these possibilities, we assayed spontaneous and DSB-induced recombination at 23°. At 23°, the MAT heterozygous rad57 mutant had a 9-fold decrease in the rate of spontaneous interhomolog recombination compared to rad57 at 30° (P < 0.05) (Figure 5B). The problem of MAT heterozygosity in the diploid reporter strains was circumvented by deleting one of the MAT alleles. As reported previously, wild-type strains expressing only one MAT allele showed a reduced rate of spontaneous interhomolog recombination (Friis and Roman 1968). At 30° recombination was reduced 17-fold in the MATα rad57 diploid compared to a MATα Rad+ diploid (P = 0.001) (Figure 5A). Both of these results are in contrast to the MAT heterozygous rad57 mutant that displayed wild-type levels of spontaneous interhomolog recombination at 30°. The MATα rad57 mutant did not display a further defect in spontaneous recombination at 23° (Figure 5B). The same trends were seen in the DSB-induced interhomolog assay. MAT heterozygosity and temperature suppressed the DSB-induced recombination defect of the rad57 mutant (Figure 5, C and D). DSB-induced interhomolog recombination was also reduced in the MATα rad51 diploid compared with the MAT heterozygous rad51 diploid. One possible explanation is that nonhomologous end joining (NHEJ) is active in MATα diploids and could compete for repair of the I-SceI-induced break without production of Ade+ recombinants (Frank-Vaillant and Marcand 2001; Valencia et al. 2001). Activation of NHEJ could explain the difference between MATα and MATa/α diploids for DSB-induced recombination, but is not responsible for the difference in spontaneous recombination (Figure 5A). These findings support the idea that MAT heterozygosity suppresses the role of RAD55 and RAD57 in interhomolog recombination and this suppression appears to be temperature dependent. Interestingly, the increased need for RAD57 over RAD51 found in the spontaneous direct-repeat recombination assay is not seen in the interhomolog assay. The rad51 mutant had a more severe defect than the rad57 mutant under all conditions tested.
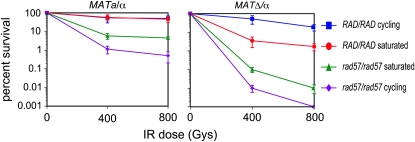
As anticipated the MATα rad57 diploid was extremely sensitive to IR, similar to a MATα rad57 haploid strain (Figure 6). To determine whether the increased resistance of the MAT heterozygous rad57 diploid is due to increased interhomolog or SCR, we compared survival to IR for exponential and stationary phase cultures. Growing cells will preferentially repair IR-induced lesions from sister chromatids whereas stationary phase cultures are predominantly made up of G1 cells that have only a homolog to template repair. The RAD MAT heterozygous strains showed high radiation resistance for both exponential and stationary phase cultures (Figure 6). In contrast, G1-phase MAT heterozygous rad57 diploids were 10-fold more resistant to IR than their cycling counterparts. These data augment the hypothesis that MAT heterozygosity efficiently suppresses the defect of rad55 and rad57 mutants in DSB-induced recombination and that this suppression is more effective for interhomolog recombination than SCR.
Figure 6.—
Rad55 and Rad57 are more necessary for sister chromatid recombination than for interhomolog recombination. Strains were either grown to log phase (cycling) or grown 4 days to saturation. Serial dilutions were plated onto solid YPD medium and were unirradiated or exposed to 400 or 800 Gy of γ-irradiation; surviving colonies were counted after 3 days. MAT heterozygous (MATa/α) strains used were LSY1667 (RAD/RAD) and LSY1881 (rad57∷URA3/rad57∷LEU2); MATΔ/α strains used were LSY1759 (RAD/RAD) and LSY1884 (rad57∷URA3/rad57∷LEU2).
DISCUSSION
Loss of resistance to IR is generally coupled with decreased proficiency in recombination. Thus the strong IR sensitivity of rad55 and rad57 mutants is paradoxical in light of their minimal defects in spontaneous mitotic recombination as noted in earlier studies. IR sensitivity of haploid yeast in active growth results from failure to repair DSBs by sister chromatid recombination. One possible explanation is that previous studies focused on the behavior of rad55 and rad57 mutants in nonsister recombination events or direct-repeat recombination assays that select for pop-outs (Lovett and Mortimer 1987; Mcdonald and Rothstein 1994; Freedman and Jinks-Robertson 2002). Alternatively, Rad55-Rad57 could have a more important role in DSBR than in the repair of spontaneous lesions. Further complications in interpretation arise from the observation that MAT heterozygosity and temperature suppress the IR sensitivity of rad55 and rad57 mutants and these suppressive effects could obviate any phenotype seen in diploids grown at 30° (Lovett and Mortimer 1987). To elucidate the role of the yeast Rad51 paralogs in homologous recombination two recombination reporters were designed to measure recombination proficiency in regard to template choice (sister vs. homolog), source of DNA damage (spontaneous or DSB induced), and mating type (MAT homozygous vs. MAT heterozygous).
Spontaneous sister chromatid recombination requires the Rad51 paralogs and known suppressors of Rad51 filament formation do not strongly suppress the mutant defects:
The rad57 haploid mutant displayed a 6000-fold decrease in the rate of spontaneous gene conversion between direct repeats compared to wild type (Figure 2A). These events are expected to arise by unequal gene conversion between sister chromatids or by intrachromatid gene conversion. Although we cannot distinguish between these mechanisms, previous studies suggest most events are the result of SCR. The similar phenotype conferred by rad57 in the ade2 repeat assay and by sensitivity to UV is also consistent with the notion that the Ade+ Trp+ recombinants are generated by SCR. An unexpected finding was that the rad51 mutant was not as defective as rad57 in the direct-repeat recombination assay. Furthermore, a haploid rad57 mutant expressing both mating-type alleles or a rad55 mutant overexpressing Rad51 still showed a 1500-fold reduced recombination rate compared to wild type. The partially suppressed recombination rates of the mutants were equivalent to the rate observed for the rad51 mutant, whereas in all the other assays the recombination rates of the MAT heterozygous rad57 mutant were significantly higher than that of rad51. We also found that the rad57 mutant was not cold sensitive in the spontaneous direct-repeat assay, in contrast to the phenotype in DSBR. These findings suggest that Rad55 and Rad57 have a unique role in spontaneous SCR that is distinct from their role in DSBR.
The phenotype of the rad57 mutant in spontaneous recombination between direct repeats of ade2 is much more severe by comparison than when the alleles are in different configurations. Gene conversion between ade2 repeats oriented as an inverted repeat was previously shown to be decreased 20- to 30-fold in rad55 and rad57 mutants and the mutant defects were temperature dependent. It is possible that events initiate or are resolved by different mechanisms during recombination between inverted repeats, compared with direct repeats. DSBs have been detected at some inverted repeats in yeast and may form at low frequency within the ade2-inverted repeat (Lobachev et al. 2002; Lemoine et al. 2005). In an assay to measure spontaneous unequal sister chromatid exchange, rad51, rad55, and rad57 mutants were found to have the same rate as wild type (Dong and Fasullo 2003). The failure to detect Rad51-dependent events in this system could be due to the selection for exchange events, the short homology between the repeats (0.3 kb), or use of an alternate postreplication repair pathway, such as template switching, to generate recombinants.
Repair of a single HO-induced DSB by ectopic recombination in haploid cells requires RAD55 and RAD57, even at 30° (Hays et al. 1995; Sugawara et al. 1995; Aylon et al. 2003). As measured with the direct-repeat reporter, the rad57 mutant was moderately defective in DSB-induced recombination compared to wild type, although the phenotype was not as severe as the rad51 mutant (Figure 2C) or as reported previously for DSB-induced ectopic recombination. Additionally, MAT heterozygosity, overexpression of Rad51, or elevated temperature effectively suppressed the DSBR defect of the rad57 mutant (Figures 2 and 3). The cold sensitivity and the strong suppression by factors acting in Rad51 filament formation of the rad57 mutant phenotype in DSBR contrast with the behavior of the rad57 mutant in the spontaneous direct-repeat recombination or UV-sensitivity assays.
Initiation of spontaneous sister chromatid recombination at single-stranded DNA gaps?
The phenotype of rad55 and rad57 mutants in the spontaneous SCR assay was remarkably similar to that observed for the repair of UV-induced lesions in that both defects were only weakly suppressed by MAT heterozygosity or overexpression of Rad51. In excision repair-deficient yeast cells, treatment with UV leads to the uncoupling of leading- and lagging-strand synthesis and results in long stretches of single-stranded DNA specifically on the leading strand as well as smaller single-stranded DNA gaps on both strands (Lopes et al. 2006). Therefore, it seems likely that most spontaneous recombination events initiate at SSGs in the context of the replication fork. In support of this idea, the mammalian Rad51 paralogs, Xrcc2 and Xrcc3, were shown to be required for replication fork slowing following treatment with cisplatin or UV, presumably because homologous recombination is necessary for bypass of the lesions and this process is slower than lesion bypass by other mechanisms (Henry-Mowatt et al. 2003). These data implicate the Rad51 paralogs in homologous recombination in the context of the replication fork. Lettier et al. (2006) characterized an unusual class of rad52 mutants that are defective for DSBR, but show normal rates of spontaneous and UV-induced interhomolog recombination, providing further support for the proposal that spontaneous recombination initiates at lesions other than DSBs. The postreplication repair (PRR) pathway provides alternate mechanisms to bypass damage at stalled replication forks. Rad57 mutants show increased spontaneous mutagenesis that is REV3 dependent (Rattray et al. 2002), consistent with competition between homologous recombination and PRR at SSGs.
There are some parallels between the Rad51 paralogs in eukaryotes and RecF, RecO, and RecR (RecFOR) functions in bacteria. RecFOR are important for recombinational repair in response to UV and it has been suggested that RecFOR function during the recovery of replication after UV-induced DNA damage (Courcelle et al. 1997; Morimatsu and Kowalczykowski 2003). The UV sensitivity of recFOR mutants can be suppressed by overexpression of recA or by gain-of-function recA alleles that encode proteins with higher DNA affinity than wild-type RecA (Wang et al. 1993; Kowalczykowski et al. 1994). Thus, Rad55 and Rad57 appear to behave somewhat analogously to RecFOR in the repair of single-stranded gaps in the context of DNA replication. In contrast to rad55 and rad57, recFOR mutants are not sensitive to agents that create DSBs unless the sbcB and sbcCD nucleases are inactivated in the recBC background (Kowalczykowski et al. 1994).
Role of the Rad51 paralogs in single-strand gap repair:
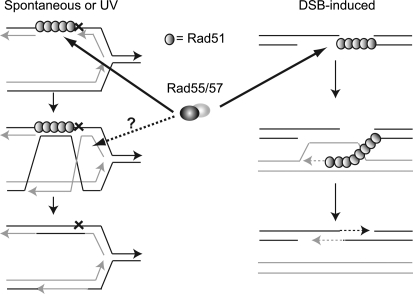
On the basis of the weak suppression of the rad57 defect in spontaneous SCR by suppressors of the DSBR defect, we suggest the role for Rad55-Rad57 in the repair of SSGs formed at stalled replication forks is distinct from its role in DSBR. The increased need for Rad55-Rad57 in the repair of SSGs compared to DSBs could be due to one of two possibilities that are not mutually exclusive: either there is a greater need for the paralogs in Rad51 filament extension or stabilization in SSG repair or the paralogs are needed for a subsequent step in the repair of SSGs that is not essential during DSBR (Figure 7). Both types of DNA repair appear to require the mediator function of the Rad51 paralogs because Rad51 recruitment in response to UV or IR is reduced in the rad55 mutant (Figure 4). However, Rad51 recruitment to UV- or IR-induced lesions in the rad55 mutant was partially suppressed by MAT heterozygosity even though expression of both mating-type alleles only weakly suppressed cell survival after UV irradiation. Although Rad51 foci formation is partially suppressed by MAT heterozygosity it seems likely that the filaments formed are not fully functional to account for the incomplete suppression of the DSBR defects and these partial filaments formed at SSGs are unable to promote pairing with a sister chromatid. One difference between SSG repair (SSGR) and DSBR is that pairing between ssDNA formed at a gap on the template strand and the sister chromatid creates a topological problem compared with invasion of a ssDNA tail formed at a DSB, which has a free end and presumably no restraint on rotation. It is possible that Rad55-Rad57 is required at this step. A defect in pairing with duplex DNA is consistent with the failure to recombine despite partial suppression of Rad51 recruitment by MAT heterozygosity. Finally, the branched structures formed during SSGR may require junction-processing activities. In this regard it is notable that a resolvase activity has been identified that is associated with the human Rad51 paralogs, Rad51C and Xrcc3 (Liu et al. 2004, 2007).
Figure 7.—
The role of Rad55-Rad57 in spontaneous vs. DSB-induced recombination. The Rad51 paralogs are important for mediation of the Rad51 filament in the repair of single-stranded gaps as well as DSBs. In SSGR the Rad51 paralogs may have an additional function subsequent to Rad51 filament formation. Possible functions include pairing of topologically constrained ssDNA with dsDNA or the processing of branched recombination intermediates formed in the repair of single-stranded gaps but not DSBs.
Temperature and MAT heterozygosity suppress the role of RAD55 and RAD57 in interhomolog recombination:
Consistent with previous reports (Lovett and Mortimer 1987; Signon et al. 2001), we found that MAT heterozygous rad57/rad57 diploids displayed no defect in spontaneous or DSB-induced interhomolog recombination at 30° (Figure 5). However, a defect in interhomolog recombination was observed for the rad57 diploid expressing only one MAT allele and/or at 23°. The full suppression observed by MAT heterozygosity and temperature in the spontaneous interhomolog recombination assay, but not in the spontaneous direct-repeat system, suggests the primary role for Rad55-Rad57 in interhomolog recombination is in mediating Rad51 filament formation whereas in SCR the paralogs might have ancillary role(s) apart from Rad51 mediation. Furthermore, the similar behavior of the rad57 diploid for both spontaneous and DSB-induced events raises the possibility that spontaneous lesions channeled to the homolog are DSBs. Spontaneous interhomolog recombination is unlikely to occur in the context of the replication fork and Rad55-Rad57 appear more important for sister chromatid than nonsister recombination as evidenced by the diploid IR survival data (Figure 6). The MAT heterozygous rad57 mutant was more proficient in repair when cultures were not cycling, presumably due to interhomolog recombination in the G1 phase, than in repair during exponential growth. In contrast, Rad+ diploids grown to exponential or stationary phase show equivalent sensitivity to IR. The increased survival of rad57 diploids in stationary phase is unlikely to be due to nonhomologous end joining because this pathway is suppressed by mating-type heterozygosity (Frank-Vaillant and Marcand 2001; Valencia et al. 2001) and supports the hypothesis that Rad55-Rad57 are more important for sister chromatid than nonsister recombination.
The sporulation and spore viability defects of MATa/α rad57/rad57 diploids seem at odds with the suppression of the mitotic DSBR defect of rad57 mutants by MAT heterozygosity. Meiotic DSBs persist and are hyperresected in rad55 and rad57 mutant diploids, but recombinant products are still detected by RFLP analysis and by the return-to-growth protocol (Game et al. 1980; Borts et al. 1986; Soustelle et al. 2002). In mitotic growth, the MAT heterozygous rad57 diploid is 10- to 100-fold more sensitive to 800 Gy IR than the corresponding RAD57 diploid even though repair of a single DSB appears to be normal. We suggest the kinetics of DSBR are considerably slower in the absence of Rad55-Rad57. Slower or inefficient processing may suffice for repair of a single DSB (Aylon et al. 2003), but not for multiple lesions. This decrease in the efficiency of repair of multiple lesions could account for the IR sensitivity and the sporulation defect of rad57 MAT heterozygous diploids. Although failure to repair some DSBs might be tolerated in diploids, the haploid products of meiosis would not be viable if half of the 150 or so meiotic DSBs were unrepaired.
Acknowledgments
The authors thank R. Rothstein (Columbia University) and members of the Rothstein lab for assistance with microscopy and helpful discussions. We thank J. Haber, R. Rothstein, and S. Marcand for yeast strains and plasmids and W. K. Holloman for comments on the manuscript. This research was supported by Public Health Service grants GM054099 (L.S.S.), T32 GM08224 (C.W.F.), and T32 AI07161 (C.W.F.) from the National Institutes of Health and by a National Science Foundation F31 predoctoral fellowship to A.M.M.
References
- Akamatsu, Y., Y. Tsutsui, T. Morishita, M. S. Siddique, Y. Kurokawa et al., 2007. Fission yeast Swi5/Sfr1 and Rhp55/Rhp57 differentially regulate Rhp51-dependent recombination outcomes. EMBO J. 26: 1352–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylon, Y., B. Liefshitz, G. Bitan-Banin and M. Kupiec, 2003. Molecular dissection of mitotic recombination in the yeast Saccharomyces cerevisiae. Mol. Cell. Biol. 23: 1403–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai, Y., and L. S. Symington, 1996. A Rad52 homolog is required for RAD51-independent mitotic recombination in Saccharomyces cerevisiae. Genes Dev. 10: 2025–2037. [DOI] [PubMed] [Google Scholar]
- Bartsch, S., L. E. Kang and L. S. Symington, 2000. RAD51 is required for the repair of plasmid double-stranded DNA gaps from either plasmid or chromosomal templates. Mol. Cell. Biol. 20: 1194–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeke, J. D., J. Trueheart, G. Natsoulis and G. R. Fink, 1987. 5-Fluoroorotic acid as a selective agent in yeast molecular genetics. Methods Enzymol. 154: 164–175. [DOI] [PubMed] [Google Scholar]
- Borts, R. H., M. Lichten and J. E. Haber, 1986. Analysis of meiosis-defective mutations in yeast by physical monitoring of recombination. Genetics 113: 551–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenneman, M. A., B. M. Wagener, C. A. Miller, C. Allen and J. A. Nickoloff, 2002. XRCC3 controls the fidelity of homologous recombination: roles for XRCC3 in late stages of recombination. Mol. Cell 10: 387–395. [DOI] [PubMed] [Google Scholar]
- Courcelle, J., C. Carswell-Crumpton and P. C. Hanawalt, 1997. recF and recR are required for the resumption of replication at DNA replication forks in Escherichia coli. Proc. Natl. Acad. Sci. USA 94: 3714–3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, Z., and M. Fasullo, 2003. Multiple recombination pathways for sister chromatid exchange in Saccharomyces cerevisiae: role of RAD1 and the RAD52 epistasis group genes. Nucleic Acids Res. 31: 2576–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firmenich, A. A., M. Elias-Arnanz and P. Berg, 1995. A novel allele of Saccharomyces cerevisiae RFA1 that is deficient in recombination and repair and suppressible by RAD52. Mol. Cell. Biol. 15: 1620–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin, G. S., and L. S. Symington, 2002. Mutations in yeast Rad51 that partially bypass the requirement for Rad55 and Rad57 in DNA repair by increasing the stability of Rad51-DNA complexes. EMBO J. 21: 3160–3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank-Vaillant, M., and S. Marcand, 2001. NHEJ regulation by mating type is exercised through a novel protein, Lif2p, essential to the Ligase IV pathway. Genes Dev. 15: 3005–3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman, J. A., and S. Jinks-Robertson, 2002. Genetic requirements for spontaneous and transcription-stimulated mitotic recombination in Saccharomyces cerevisiae. Genetics 162: 15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis, J., and H. Roman, 1968. The effect of the mating-type alleles on intragenic recombination in yeast. Genetics 59: 33–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung, C. W., G. S. Fortin, S. E. Peterson and L. S. Symington, 2006. The rad51–K191R ATPase-defective mutant is impaired for presynaptic filament formation. Mol. Cell. Biol. 26: 9544–9554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Game, J. C., T. J. Zamb, R. J. Braun, M. Resnick and R. M. Roth, 1980. The role of radiation (rad) genes in meiotic recombination in yeast. Genetics 94: 51–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasior, S. L., A. K. Wong, Y. Kora, A. Shinohara and D. K. Bishop, 1998. Rad52 associates with RPA and functions with rad55 and rad57 to assemble meiotic recombination complexes. Genes Dev. 12: 2208–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasior, S. L., H. Olivares, U. Ear, D. M. Hari, R. Weichselbaum et al., 2001. Assembly of RecA-like recombinases: distinct roles for mediator proteins in mitosis and meiosis. Proc. Natl. Acad. Sci. USA 98: 8411–8418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays, S. L., A. A. Firmenich and P. Berg, 1995. Complex formation in yeast double-strand break repair: participation of Rad51, Rad52, Rad55, and Rad57 proteins. Proc. Natl. Acad. Sci. USA 92: 6925–6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry-Mowatt, J., D. Jackson, J. Y. Masson, P. A. Johnson, P. M. Clements et al., 2003. XRCC3 and Rad51 modulate replication fork progression on damaged vertebrate chromosomes. Mol. Cell 11: 1109–1117. [DOI] [PubMed] [Google Scholar]
- Hope, J. C., L. D. Cruzata, A. Duvshani, J. Mitsumoto, M. Maftahi et al., 2007. Mus81-Eme1-dependent and -independent crossovers form in mitotic cells during double-strand break repair in Schizosaccharomyces pombe. Mol. Cell. Biol. 27: 3828–3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiang, Y. H., M. G. Lihou and L. F. Liu, 1989. Arrest of replication forks by drug-stabilized topoisomerase I-DNA cleavable complexes as a mechanism of cell killing by camptothecin. Cancer Res. 49: 5077–5082. [PubMed] [Google Scholar]
- Huang, K. N., and L. S. Symington, 1994. Mutation of the gene encoding protein kinase C 1 stimulates mitotic recombination in Saccharomyces cerevisiae. Mol. Cell. Biol. 14: 6039–6045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito, H., Y. Fukuda, K. Murata and A. Kimura, 1983. Transformation of intact yeast cells treated with alkali cations. J. Bacteriol. 153: 163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov, E. L., N. Sugawara, J. Fishman-Lobell and J. E. Haber, 1996. Genetic requirements for the single-strand annealing pathway of double-strand break repair in Saccharomyces cerevisiae. Genetics 142: 693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, R. D., and L. S. Symington, 1995. Functional differences and interactions among the putative RecA homologs Rad51, Rad55, and Rad57. Mol. Cell. Biol. 15: 4843–4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadyk, L. C., and L. H. Hartwell, 1992. Sister chromatids are preferred over homologs as substrates for recombinational repair in Saccharomyces cerevisiae. Genetics 132: 387–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kans, J. A., and R. K. Mortimer, 1991. Nucleotide sequence of the RAD57 gene of Saccharomyces cerevisiae. Gene 105: 139–140. [DOI] [PubMed] [Google Scholar]
- Kowalczykowski, S. C., D. A. Dixon, A. K. Eggleston, S. D. Lauder and W. M. Rehrauer, 1994. Biochemistry of homologous recombination in Escherichia coli. Microbiol. Rev. 58: 401–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lea, D. E., and C. A. Coulson, 1948. The distribution of the numbers of mutants in bacterial populations. J. Genet. 49: 264–284. [DOI] [PubMed] [Google Scholar]
- Lemoine, F. J., N. P. Degtyareva, K. Lobachev and T. D. Petes, 2005. Chromosomal translocations in yeast induced by low levels of DNA polymerase a model for chromosome fragile sites. Cell 120: 587–598. [DOI] [PubMed] [Google Scholar]
- Lettier, G., Q. Feng, A. A. de Mayolo, N. Erdeniz, R. J. Reid et al., 2006. The role of DNA double-strand breaks in spontaneous homologous recombination in S. cerevisiae. PLoS Genet. 2: e194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liefshitz, B., A. Parket, R. Maya and M. Kupiec, 1995. The role of DNA repair genes in recombination between repeated sequences in yeast. Genetics 140: 1199–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisby, M., J. H. Barlow, R. C. Burgess and R. Rothstein, 2004. Choreography of the DNA damage response: spatiotemporal relationships among checkpoint and repair proteins. Cell 118: 699–713. [DOI] [PubMed] [Google Scholar]
- Liu, Y., J. Y. Masson, R. Shah, P. O'Regan and S. C. West, 2004. RAD51C is required for Holliday junction processing in mammalian cells. Science 303: 243–246. [DOI] [PubMed] [Google Scholar]
- Liu, Y., M. Tarsounas, P. O'Regan and S. C. West, 2007. Role of RAD51C and XRCC3 in genetic recombination and DNA repair. J. Biol. Chem. 282: 1973–1979. [DOI] [PubMed] [Google Scholar]
- Lobachev, K. S., D. A. Gordenin and M. A. Resnick, 2002. The Mre11 complex is required for repair of hairpin-capped double-strand breaks and prevention of chromosome rearrangements. Cell 108: 183–193. [DOI] [PubMed] [Google Scholar]
- Longtine, M. S., A. McKenzie, 3rd, D. J. Demarini, N. G. Shah, A. Wach et al., 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14: 953–961. [DOI] [PubMed] [Google Scholar]
- Lopes, M., M. Foiani and J. M. Sogo, 2006. Multiple mechanisms control chromosome integrity after replication fork uncoupling and restart at irreparable UV lesions. Mol. Cell 21: 15–27. [DOI] [PubMed] [Google Scholar]
- Lovett, S. T., 1994. Sequence of the RAD55 gene of Saccharomyces cerevisiae: similarity of RAD55 to prokaryotic RecA and other RecA-like proteins. Gene 142: 103–106. [DOI] [PubMed] [Google Scholar]
- Lovett, S. T., and R. K. Mortimer, 1987. Characterization of null mutants of the RAD55 gene of Saccharomyces cerevisiae: effects of temperature, osmotic strength and mating type. Genetics 116: 547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald, J. P., and R. Rothstein, 1994. Unrepaired heteroduplex DNA in Saccharomyces cerevisiae is decreased in RAD1 RAD52-independent recombination. Genetics 137: 393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimatsu, K., and S. C. Kowalczykowski, 2003. RecFOR proteins load RecA protein onto gapped DNA to accelerate DNA strand exchange: a universal step of recombinational repair. Mol. Cell 11: 1337–1347. [DOI] [PubMed] [Google Scholar]
- Nagaraju, G., S. Odate, A. Xie and R. Scully, 2006. Differential regulation of short- and long-tract gene conversion between sister chromatids by Rad51C. Mol. Cell. Biol. 26: 8075–8086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa, T., X. Yu, A. Shinohara and E. H. Egelman, 1993. Similarity of the yeast RAD51 filament to the bacterial RecA filament. Science 259: 1896–1899. [DOI] [PubMed] [Google Scholar]
- Rattray, A. J., and L. S. Symington, 1994. Use of a chromosomal inverted repeat to demonstrate that the RAD51 and RAD52 genes of Saccharomyces cerevisiae have different roles in mitotic recombination. Genetics 138: 587–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattray, A. J., and L. S. Symington, 1995. Multiple pathways for homologous recombination in Saccharomyces cerevisiae. Genetics 139: 45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattray, A. J., B. K. Shafer, C. B. McGill and J. N. Strathern, 2002. The roles of REV3 and RAD57 in double-strand-break-repair-induced mutagenesis of Saccharomyces cerevisiae. Genetics 162: 1063–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rine, J., and I. Herskowitz, 1987. Four genes responsible for a position effect on expression from HML and HMR in Saccharomyces cerevisiae. Genetics 116: 9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheraga, H. A., G. Nemethy and I. Z. Steinberg, 1962. The contribution of hydrophobic bonds to the thermal stability of protein conformations. J. Biol. Chem. 237: 2506–2508. [PubMed] [Google Scholar]
- Schild, D., 1995. Suppression of a new allele of the yeast RAD52 gene by overexpression of RAD51, mutations in srs2 and ccr4, or mating-type heterozygosity. Genetics 140: 115–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman, F., G. Fink and J. Hicks, 1986. Methods in Yeast Genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Signon, L., A. Malkova, M. L. Naylor, H. Klein and J. E. Haber, 2001. Genetic requirements for RAD51- and RAD54-independent break-induced replication repair of a chromosomal double-strand break. Mol. Cell. Biol. 21: 2048–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, J., and R. Rothstein, 1999. An allele of RFA1 suppresses RAD52-dependent double-strand break repair in Saccharomyces cerevisiae. Genetics 151: 447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soustelle, C., M. Vedel, R. Kolodner and A. Nicolas, 2002. Replication protein A is required for meiotic recombination in Saccharomyces cerevisiae. Genetics 161: 535–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara, N., E. L. Ivanov, J. Fishman-Lobell, B. L. Ray, X. Wu et al., 1995. DNA structure-dependent requirements for yeast RAD genes in gene conversion. Nature 373: 84–86. [DOI] [PubMed] [Google Scholar]
- Sugawara, N., X. Wang and J. E. Haber, 2003. In vivo roles of Rad52, Rad54, and Rad55 proteins in Rad51-mediated recombination. Mol. Cell 12: 209–219. [DOI] [PubMed] [Google Scholar]
- Sugiyama, T., E. M. Zaitseva and S. C. Kowalczykowski, 1997. A single-stranded DNA-binding protein is needed for efficient presynaptic complex formation by the Saccharomyces cerevisiae Rad51 protein. J. Biol. Chem. 272: 7940–7945. [DOI] [PubMed] [Google Scholar]
- Sung, P., 1997. Yeast Rad55 and Rad57 proteins form a heterodimer that functions with replication protein A to promote DNA strand exchange by Rad51 recombinase. Genes Dev. 11: 1111–1121. [DOI] [PubMed] [Google Scholar]
- Sung, P., and D. L. Robberson, 1995. DNA strand exchange mediated by a RAD51-ssDNA nucleoprotein filament with polarity opposite to that of RecA. Cell 82: 453–461. [DOI] [PubMed] [Google Scholar]
- Symington, L. S., 2002. Role of RAD52 epistasis group genes in homologous recombination and double-strand break repair. Microbiol. Mol. Biol. Rev. 66: 630–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata, M., M. S. Sasaki, S. Tachiiri, T. Fukushima, E. Sonoda et al., 2001. Chromosome instability and defective recombinational repair in knockout mutants of the five Rad51 paralogs. Mol. Cell. Biol. 21: 2858–2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, L. H., and D. Schild, 2001. Homologous recombinational repair of DNA ensures mammalian chromosome stability. Mutat. Res. 477: 131–153. [DOI] [PubMed] [Google Scholar]
- Toyn, J. H., P. L. Gunyuzlu, W. H. White, L. A. Thompson and G. F. Hollis, 2000. A counterselection for the tryptophan pathway in yeast: 5-fluoroanthranilic acid resistance. Yeast 16: 553–560. [DOI] [PubMed] [Google Scholar]
- Valencia, M., M. Bentele, M. B. Vaze, G. Herrmann, E. Kraus et al., 2001. NEJ1 controls non-homologous end joining in Saccharomyces cerevisiae. Nature 414: 666–669. [DOI] [PubMed] [Google Scholar]
- van Veelen, L. R., J. Essers, M. W. van de Rakt, H. Odijk, A. Pastink et al., 2005. Ionizing radiation-induced foci formation of mammalian Rad51 and Rad54 depends on the Rad51 paralogs, but not on Rad52. Mutat. Res. 574: 34–49. [DOI] [PubMed] [Google Scholar]
- Wang, T. C., H. Y. Chang and J. L. Hung, 1993. Cosuppression of recF, recR and recO mutations by mutant recA alleles in Escherichia coli cells. Mutat. Res. 294: 157–166. [DOI] [PubMed] [Google Scholar]
- Winzeler, E. A., D. D. Shoemaker, A. Astromoff, H. Liang, K. Anderson et al., 1999. Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science 285: 901–906. [DOI] [PubMed] [Google Scholar]
- Yokoyama, H., N. Sarai, W. Kagawa, R. Enomoto, T. Shibata et al., 2004. Preferential binding to branched DNA strands and strand-annealing activity of the human Rad51B, Rad51C, Rad51D and Xrcc2 protein complex. Nucleic Acids Res. 32: 2556–2565. [DOI] [PMC free article] [PubMed] [Google Scholar]