Abstract
In Caenorhabditis elegans, germ granules called P granules are directly inherited from mother to daughter and segregate with the germ lineage as it separates from the soma during initial embryonic cell divisions. Here we define meg-1 and meg-2 (maternal-effect germ-cell defective), which are expressed in the maternal germline and encode proteins that localize exclusively to P granules during embryonic germline segregation. Localization of MEG-1 to P granules depends upon the membrane-bound protein MES-1. meg-1 mutants exhibit multiple germline defects: P-granule mis-segregation in embryos, underproliferation and aberrant P-granule morphology in larval germ cells, and ultimately, sterility as adults. The penetrance of meg-1 phenotypes increases when meg-2 is also absent. Loss of the P-granule component pgl-1 in meg-1 mutants increases germ-cell proliferation, while loss of glh-1 decreases proliferation. Because meg-1 is provided maternally but its action is required in the embryonic germ lineage during segregation from somatic lineages, it provides a critical link for ensuring the continuity of germline development from one generation to the next.
GERM cells are fundamentally distinct from all somatic cells, because they ultimately produce the gametes that give rise to new individuals. By contrast, somatic cells have a restricted developmental fate and persist only as long as the life of a single individual. A full understanding of the functional properties that distinguish germ cells from somatic cells is critical if current reproductive therapies are to be improved.
A distinguishing characteristic of the germ lineage in many species, including Caenorhabditis elegans, Drosophila, Xenopus, zebrafish, and male mammals, is the presence of a germ-cell-specific structure called germ plasm or germ granules (Seydoux and Braun 2006). Some protein components of germ granules are conserved, while others are specific to a particular species. The common presence of diverse RNA regulatory proteins and mRNAs indicates a role in controlling RNA stability, localization, and/or accessibility to the translational machinery. However, the exact molecular function of germ granules, and how they contribute to germline development, remains elusive.
Another conserved characteristic of the germline is its very early establishment and separation from somatic tissues during embryonic development. In C. elegans, the one-cell zygote, P0, undergoes a series of asymmetric divisions to establish early somatic founder cells (AB, E, MS, C, and D) and the embryonic germ lineage (P1–P4) (Figure 1A, reviewed by Strome 2005). Each division of the P cell gives rise to one daughter that will ultimately contribute to somatic tissue and one daughter that retains the ability to generate the germ lineage. Only during this brief window of time does the germline fate mix with somatic fates. The birth of P4 around the 28-cell stage signals the point of germline restriction, because P4 gives rise to all germ cells and only germ cells. P4 divides once to produce two equivalent primordial germ cells, Z2 and Z3, which remain mitotically inactive until after hatching.
Figure 1.—
meg-1 is expressed in the proximal germline and is required for fertility. (A) Diagram of early embryonic divisions highlighting P lineage. (B) In situ hybridization of meg-1 with antisense probe in wild-type gonad. No staining was observed with sense probe. Arrow marks onset of expression at mid-pachytene. Bar, 50 μm. (C) Schematic of meg-1 genomic locus showing vr10 and vr11 deletions. (D) Temperature-sensitive, maternal-effect sterility of meg-1(vr10) and meg-1(vr11) alleles. Homozygous mutants were shifted to the restrictive temperature as L4's and the progeny were assessed for sterility. Results of three experiments were averaged. Error bars indicate standard deviation.
The newly fertilized C. elegans zygote inherits germ granules, called P granules, via the oocyte. P granules are segregated specifically into the P lineage during the early embryonic cell divisions. P granules are initially dispersed in the cytoplasm of the oocyte, the zygote, and P1 of the two-cell embryo, but begin to associate with nuclear pores in P2 and all subsequent germ cells (Strome and Wood 1982; Pitt et al. 2000). The mechanisms governing embryonic P-granule segregation are poorly understood, although a protein with similarity to receptor tyrosine kinases, MES-1, controls the asymmetric partitioning of P granules during the divisions of P2 and P3 (Berkowitz and Strome 2000).
Several components of P granules have been identified. The PGL and GLH family members are localized specifically to P granules at all stages of germline development. The four GLH proteins encode RNA helicases related to Drosophila Vasa, a conserved component of germ plasm (Gruidl et al. 1996; Kuznicki et al. 2000). PGL-1 and PGL-3 encode RGG-box proteins with predicted RNA-binding capability (Kawasaki et al. 1998, 2004). These constitutive components likely play some role in assembly and function of P granules, although loss of any one of these factors does not disrupt P-granule formation. Both pgl-1 and glh-1 mutants display reduced fertility due to decreased proliferation and defects in gametogenesis, but do not lack germ cells entirely (Kawasaki et al. 1998; Kuznicki et al. 2000). Similar to other germ granules, P granules are likely to regulate mRNA stability and/or translation, perhaps to correctly localize germline determinants or suppress somatic fates. However, a more precise molecular mechanism for P-granule function, and how distinct P-granule protein components might interact, is currently unclear.
We have identified two genes whose protein products localize specifically to P granules only in the germline blastomeres of early embryos. These two genes, named meg-1 and meg-2 (maternal-effect germ-cell defective), are required for germ-cell function. Loss of meg-1 activity by mutation or RNA interference (RNAi) results in sterile animals that lack normal germ cells and sometimes have ectopic P granules in somatic embryonic blastomeres, a phenotype that is enhanced by loss of meg-2. The protein MES-1 is required for recruitment of MEG-1 to embryonic P granules, and meg-1 and meg-2 function synergistically with mes-1 to establish proper germ-cell fate. Moreover, we found that meg-1 interacts with other P-granule components: loss of pgl-1 ameliorates the meg-1 phenotype, while loss of glh-1 exacerbates it. These data provide evidence for a unique, specific role for embryonic germ granules in establishing and maintaining the larval and adult germline of C. elegans.
MATERIALS AND METHODS
Strains and maintenance:
Nematode strain maintenance was as described (Sulston and Hodgkin 1988). C. elegans strain N2 was used as the wild-type strain in addition to the following variants: LG I, glh-1(ok100), glh-1(ok439), bnIs1 [pie-1∷gfp∷pgl-1 unc-119(+)]; LG III, glp-1(e2141), ced-4(n1162), unc-119(ed3); LG IV, pgl-1(bn101); LG V, emb-4(hc60); LG X, mes-1(bn74), meg-1(vr10 and vr11). For temperature-sensitive mutants, 15° or 20° was the permissive temperature and 25° was the restrictive temperature.
Deletion mutant identification:
To isolate mutations in K02B9.1, a library of mutagenized worms was screened for deletion alleles by PCR. The deletion library was constructed and screened as described (Ahringer 2006). Deletion breakpoints were the following: vr10, AGATGCCACATACAAACGCT/CTGGCGGAAGACGATGCAAA, and vr11, CAGTTCCAAATGAATCAAAG/CTGGCGGAAGACGATGCAAA. After deletion mutations had been identified, frozen worms from corresponding wells were recovered and homozygous mutants were isolated. Prior to phenotypic and genetic analysis, vr10 and vr11 were backcrossed to N2 eight times to remove potential additional mutations. Double- and triple-mutant combinations were built using PCR to follow deletions in each strain, combined with analysis of sterility. A Fisher's exact test was used to determine statistical significance for differences in percentage of sterility or severity between genotypes (Table 3), and a Student's t-test was used to determine statistical significance for differences in germ-cell number between genotypes (Figure 6B).
TABLE 3.
Percentage of sterile progeny and germline development in P-granule mutants
| Germline development
|
||||
|---|---|---|---|---|
| Genotype | % sterile progeny (n) | % severely affected | % moderately affected | n |
| glh-1 | 3 (62) | 0 | 100 | 17 |
| pgl-1 | 85 (55) | 15 | 85 | 13 |
| meg-1 | 53 (193) | 70 | 30 | 10 |
| glh-1;meg-1 | 100 (38) | 91 | 9 | 11 |
| glh-1;pgl-1 | 100 (21) | 45 | 55 | 11 |
| pgl-1;meg-1 | 77 (156) | 26 | 74 | 19 |
| glh-1;pgl-1;meg-1 | 96 (28) | 90 | 10 | 10 |
L4 hermaphrodites were shifted from 20° to 25°, and progeny were raised at 25° and scored for sterility as described in materials and methods. Sterile adults were fixed and stained with DAPI to assess germline development. Underlining highlights meg-1 and pgl-1 comparison.
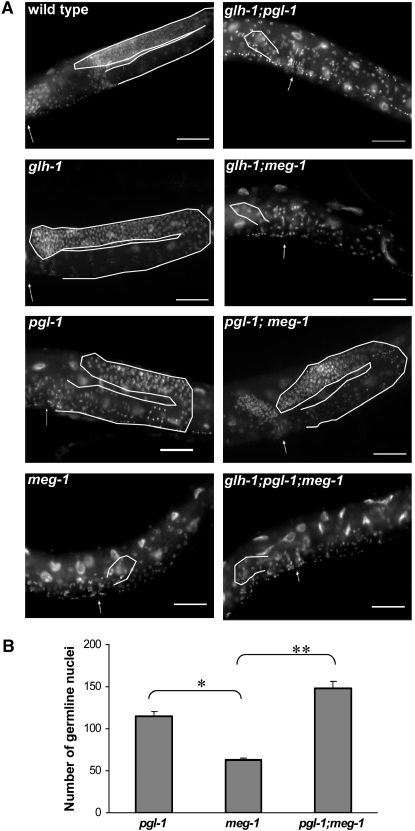
Figure 6.—
meg-1 exhibits diverse genetic interactions with glh-1 and pgl-1. (A) DAPI-stained animals showing the most common germline phenotype in wild-type, glh-1, pgl-1, meg-1, glh-1;pgl-1, glh-1;meg-1, pgl-1;meg-1, and glh-1;pgl-1;meg-1 adults. Arrow indicates position of vulva. Bar, 50 μm. (B) pgl-1;meg-1 sterile adults have more germ cells than meg-1 sterile adults. Average number of germ cells was counted in fixed sterile adults stained with DAPI to visualize nuclei. n ≥ 17 sterile animals for each genotype; three independent trials were averaged together and standard error was determined (*P < 0.05, **P < 0.005).
Sterile progeny assay:
Hermaphrodites raised at the permissive temperature to the L4 stage were placed at the indicated temperature of 15°, 20°, or 25° as the assay required. These hermaphrodites were permitted to lay eggs overnight before transfer to a new plate, to produce successive plates containing >20 progeny that were staged within 12–24 hr. The progeny were then held at the indicated temperature long enough to reach young adulthood (e.g., 3 days at 25°) and then scored for the presence or absence of eggs in the uterus under the dissecting microscope. The presence of eggs in the uterus indicates successful fertilization and serves as a reliable proxy method for monitoring the frequency of sterility in large numbers of animals.
In situ hybridization:
In situ hybridization was performed as described (Jones et al. 1996). Probes were prepared from cDNA cloned in the pCRII vector (Invitrogen, San Diego). The cDNA fragment used for the meg-1 probe corresponded to nucleotides 4–2585, where numbers indicate nucleotides from ATG to the predicted spliced coding region. The cDNA was amplified by PCR from ∼2 ng of plasmid using gene-specific primer pairs. To label either a sense or an antisense single-stranded probe, 800 ng of purified PCR product was used with a single-gene-specific primer for repeated primer extension with digoxigenin-11-dUTP. These probes were diluted 1:2 in hybridization buffer (5× SSC, 50% deionized formamide, 100 μg/ml salmon sperm DNA, 50 μg/ml heparin, 0.1% Tween20) and then applied to gonads in borosilicate tubes and hybridized at 48° for 24–30 hr. After hybridization, gonads were washed with hybridization buffer and PBST (PBS, 0.1% Tween20). The gonads were then incubated with alkaline-phosphatase-conjugated anti-Dig (Fab2 fragment) (Roche, Indianapolis) at 4° overnight. Gonads were stained with alkaline phosphatase substrate tablets (Roche), mounted, and viewed using a Zeiss Axioplan 2 imaging epifluorescence microscope.
RNAi:
RNAi was performed by injection as described (Ahringer 2006). dsRNA was prepared by in vitro transcription of PCR products amplified by primers with T7 sites. Primer sequences used to amplify a 750-bp region of meg-1 were 5′-taatacgactcactatagggtgacgctcccaagcatcagttg-3′ and 5′-taatacgactcactatagggtttgcttgtccgattcttgtgg-3′. Primers used to amplify a 529-bp region of meg-2 were 5′-taatacgactcactatagggagcctgggaactccgtgaagc-3′ and 5′-taatacgactcactatagggtctaccatccgtagatcagtgg-3′. The concentration of injected dsRNA was 250–1000 ng/μl. After injection, hermaphrodites were allowed to lay eggs for 12–18 hr. Only progeny produced after this period were scored for the presence or absence of eggs to determine the percentage of sterility.
Antibody production:
A K02B9.1 1908-bp cDNA was amplified by PCR using the following primers with NdeI sites: 5′-ggaattccatatggataatcgtggtcatttttcttc-3′ and 5′-ggaattccatatgtcattcattgcttggcaattccttg-3′. The resulting fragment was cloned into the NdeI site of the expression vector pET19b (Novagen). The 6× His-tagged fusion protein was expressed in Escherichia coli BL21 (DE3) cells after induction with isopropyl thiogalactosidase overnight at 25°. The protein was then purified over a Ni-NTA resin (QIAGEN, Valencia, CA) under denaturing conditions. The resulting purified protein (4 mg) was subjected to SDS–polyacrylamide gel electrophoresis. The fusion protein was cut out of the gel, homogenized using a glass Dounce homogenizer, and resuspended in a total volume of 8 ml PBS. Homogenized protein (2 ml) was injected with MPL + TDM adjuvant (Sigma, St. Louis) into rabbits (Pocono Rabbit Farm and Laboratory, Canadensis, PA) every 4 weeks. Antibodies against MEG-1 were purified from serum by affinity purification to the 6× His fusion MEG-1 on nitrocellulose membrane strips followed by elution with 0.1 m glycine–HCl (pH 2.5).
Immunofluorescence:
Embryos were fixed as previously described (Strome and Wood 1982). Antibodies and dilutions used for immunofluoresence were affinity-purified rabbit anti-PGL-1 (1:30,000), mouse anti-PGL-1 OICD14 (1:2), rabbit affinity-purified anti-GLH-1 (1:2000), rat anti-PGL-3 (1:2000) (gifts from S. Strome), affinity-purified rat anti-MES-1 (1:5) (a gift from L. Berkowitz), rabbit anti-NOS-2 (a gift from G. Seydoux), rabbit anti-H4Ac16 (Serotec, Raleigh, NC). After slides were stained with DAPI and washed with PBST (1× PBS, 0.1% Tween 20), they were incubated at room temperature for 2–3 hr with a fluorescent secondary antibody (1:500, Molecular Probes, Carlsbad, CA). Slides were then washed in PBST, mounted in mounting medium with DABCO (Sigma), and viewed using a Zeiss Axioplan 2 imaging epifluorescence microscope.
GFP fusion proteins:
The K02B9.1 or K02B9.2 coding sequence was PCR amplified and cloned in frame to GFP in vector pID3.01b, which contains the pie-1 promoter, GFP coding sequence, Gateway recombination sites, and the pie-1 3′-UTR, as well as an unc-119 rescuing genomic fragment (Pelletieri et al. 2003). The resulting plasmids were transformed into unc-119(ed3) animals by microparticle bombardment as described (Praitis et al. 2001) and lines bearing 100% non-Unc animals, presumed to be integrated, were examined for GFP expression.
RESULTS
meg-1 is a novel maternal-effect sterile gene:
Previous microarray studies identified a set of genes expressed in the proximal germline of C. elegans (Leacock and Reinke 2006). In the course of an RNAi screen to investigate the function of these genes, we defined a locus required for germline development. This locus consists of two related, adjacent X-linked genes, K02B9.1 and K02B9.2. In situ hybridization using a probe corresponding to K02B9.1, which probably also cross-hybridizes to K02B9.2, shows expression specifically in the proximal germline of the adult hermaphrodite (Figure 1B). The proteins encoded by K02B9.1 and K02B9.2 share three regions that are almost identical, interspersed with unique regions (supplemental Figure 1 at http://www.genetics.org/supplemental/). Both proteins do not have recognizable functional domains, but are enriched approximately twofold each for asparagine, glutamine, and serine, relative to the amino acid frequency in metazoan proteins. Because of the unique localization and function of the encoded proteins, described below, we have designated K02B9.1 as meg-1 and K02B9.2 as meg-2 (meg, maternal-effect germ-cell defective).
Our initial RNAi results did not distinguish whether one or both of these proteins were required for fertility (data not shown). To determine the relative contributions of meg-1 and meg-2 to the sterile phenotype, we isolated two deletion alleles of meg-1, vr10 and vr11 (Figure 1C). The vr10 deletion allele is predicted to produce the N-terminal 279 amino acids followed by 21 novel amino acids before a stop codon is introduced. The vr11 allele is an in-frame deletion that eliminates 121 amino acids from the central region of the protein. Several lines of evidence suggest that both alleles are very strong loss-of-function or null mutants: each mutant fails to stain with a polyclonal antibody raised against full-length MEG-1 (described below), and vr10/vr10, vr11/vr11, vr10/vr11, and meg-1(RNAi) animals all display very similar phenotypes (Tables 1 and 2).
TABLE 1.
Temperature-sensitive maternal-effect sterility of meg-1 mutants
| % sterile (n)
|
|||
|---|---|---|---|
| Genotype | 15° | 20° | 25° |
| Maternal-effect sterility of meg-1 mutants | |||
| P0meg-1(vr10)/+ at 20° | |||
| F1meg-1(vr10) | 0 (5) | 0 (5) | 0 (4) |
| F2meg-1(vr10)a | 13 (379) | 1 (925) | 75 (734) |
| F1meg-1(vr10)/+ | 0 (8) | 0 (9) | 0 (5) |
| F2 +/+, meg-1(vr10)/+, and meg-1(vr10)a | 0 (619) | 0 (1344) | 0 (736) |
| P0meg-1(vr11)/+ at 20° | |||
| F1meg-1(vr11) | 0 (3) | 0 (4) | 0 (3) |
| F2meg-1(vr11)a | 8 (239) | 0 (461) | 45 (447) |
| F1meg-1(vr11)/+ | 0 (9) | 0 (7) | 0 (8) |
| F2 +/+, meg-1(vr11)/+, and meg-1(vr11)a | 0 (855) | 0 (882) | 0 (1011) |
| Zygotic meg-1 does not rescue sterility | |||
| meg-1(vr10) | ND | ND | 49 (220) |
| meg-1(vr10) × N2 male | ND | ND | 49 (384) |
| meg-1(vr11) | ND | ND | 47 (187) |
| meg-1(vr11) × N2 male | ND | ND | 30 (279) |
Animals were considered sterile if they displayed an “empty uterus” phenotype when observed under the dissecting microscope. F1's and F2's were raised at the indicated temperature. ND, not determined.
These F2 progeny are derived from the F1 mothers from the previous row.
TABLE 2.
Genetic interactions and rescue by GFP fusion proteins
| % sterile progeny (n)
|
|||
|---|---|---|---|
| Genotype | 15° | 20° | 25° |
| meg-1(RNAi) | 19 (230) | ND | 83 (262) |
| meg-2(RNAi) | <1% (732) | ND | 0 (436) |
| meg-1(vr10) | 59 (1399) | 15 (2351) | 80 (1864) |
| meg-1(vr11) | 23 (1091) | 4 (1875) | 59 (2411) |
| meg-1(vr10)meg-2(RNAi) | ND | 100 (1922) | ND |
| meg-1(vr11)meg-2(RNAi) | ND | 93 (1423) | ND |
| mes-1(bn74) | 6 (1637) | ND | 57 (2506) |
| mes-1(bn74)meg-1(RNAi) | 88 (509) | ND | 84 (822) |
| mes-1(bn74)meg-2(RNAi) | 40 (722) | ND | 83 (180) |
| GFP∷MEG-1 | ND | ND | 0 (445) |
| meg-1(vr10); GFP∷MEG-1 | ND | ND | 0 (149) |
| meg-1(vr11); GFP∷MEG-1 | ND | ND | 0 (354) |
| GFP:MEG-2 | ND | ND | 0 (145) |
| meg-1(vr10); GFP∷MEG-2 | ND | ND | 22 (308) |
| meg-1(vr11); GFP∷MEG-2 | ND | ND | 9 (125) |
The incidence of the meg sterile phenotype is variable and is higher in this experiment than in Table 1. ND, not determined.
meg-1(vr10) and meg-1(vr11) hermaphrodites exhibit maternal-effect sterility (Table 1). Homozygous mutants born of a heterozygous mother are fertile, but progeny born of first-generation homozygous mutants are sterile. Zygotic meg-1 activity cannot rescue the sterility: meg-1/+ heterozygotes born from a cross between meg-1 homozygous hermaphrodites and wild-type males are still sterile. The penetrance of the sterile progeny phenotype is incomplete, variable, and temperature sensitive, despite the fact that both alleles are likely to retain essentially no function (Table 1, Figure 1D). A severe loss-of-function mutation that exhibits a temperature-sensitive phenotype implies that the gene product normally functions in a cellular process that is inherently temperature sensitive. When meg-1(vr10) and meg-1(vr11) homozygous mutants are raised at 20°, only 1–15% of the offspring are sterile, whereas at 25°, the incidence of sterility increases to 45–80%. We also unexpectedly saw that a smaller but consistent increase in sterility is evident at 15° compared to 20° (10–50%, depending on the experiment). Sterile animals at all temperatures had similarly profound germline defects, while fertile siblings appeared normal (Figure 2); thus expressivity was not highly variable. Following our initial characterization of the temperature-sensitive maternal-effect sterility, we maintained both alleles as homozygous mutants at 20°. To induce the meg-1 phenotype for the analyses described below, we shifted meg-1(vr10) or meg-1(vr11) parents from 20° to 25° as L4 larvae and then analyzed the offspring as embryos, larvae, or adults at 25°. We refer to these offspring as meg-1 mutants throughout this report, and any exceptions are noted.
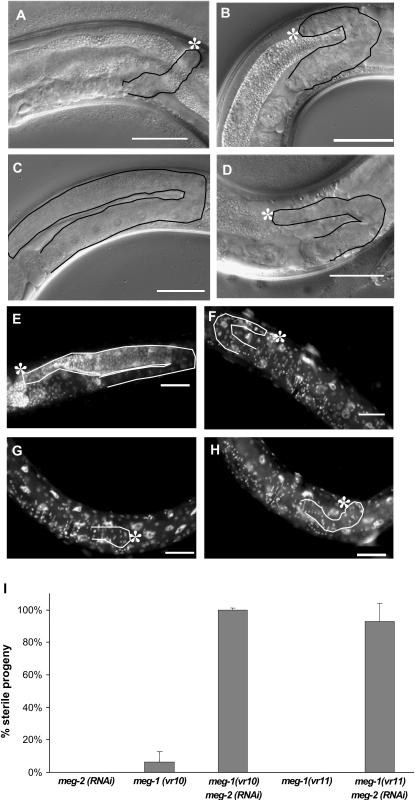
Figure 2.—
meg-1 mutants have a severely underproliferated germline phenotype that is enhanced by meg-2(RNAi). (A–D) DIC. (A and B) meg-1(vr10) sterile adult. (C) meg-1(vr10) fertile adult, which appears very similar to wild type. (D) meg-1(vr10)meg-2(RNAi) sterile adult. (E–H) DAPI. (E) Wild-type (N2) fertile adult. (F) meg-1(vr10) sterile adult with very small distal germline. (G) meg-1(vr10) sterile adult. (H) meg-1(vr11) sterile adult. (A–H) All animals were raised at 25°; asterisks indicate the distal end of the germline, which is left of the image border in C. Approximate location of gonads is outlined. Bar, 50 μm. (I) RNAi of meg-2 was performed in a meg-1(vr10) or a meg-1(vr11) background at 20° and progeny were scored for the presence or absence of eggs to determine the percentage of sterility. Error bars, standard deviation.
To determine the contribution of meg-2 to the sterile phenotype, we performed RNAi using dsRNA corresponding to a unique portion of meg-2. meg-2(RNAi) produced no phenotype when injected into wild-type animals at 20°, but dramatically increased the sterility of meg-1(vr10) progeny from 15 to 100% and meg-1(vr11) progeny from 4 to 93% (Figure 2I; Table 2). This result suggests that meg-2 may exhibit genetic redundancy with meg-1 and might compensate for the loss of meg-1 at 20°. Transgenic rescue experiments (see below) are consistent with this possibility.
meg-1 larvae exhibit defective germ-cell proliferation or survival:
The adult germlines of most sterile progeny of meg-1 mutant hermaphrodites are extremely underdeveloped, bearing only a few germ cells with aberrant, diffuse morphology and lacking features of either meiotic progression or gamete differentiation (Figure 2). meg-1 mutant males born from meg-1 mutant hermaphrodites raised at 25° can exhibit a mild germ-cell underproliferation, but it is not as severe as in their hermaphrodite siblings, and sperm are often present (16/24 animals).
To better understand when the onset of the defect in hermaphrodites occurs, we analyzed germ-cell development during larval stages. Using a strain in which P granules were marked by GFP∷PGL-1 to distinguish germ cells from somatic cells, we monitored the number of GFP+ germ cells in control and meg-1 larvae at 25°. The number of germ cells counted by GFP expression appeared comparable to the number of germ cells observed by DIC. We found that Z2 and Z3 are present in GFP∷PGL-1; meg-1(vr10) hatched larvae as in wild type. Early divisions of germ cells occurred in most larvae during the L1 and L2 stages (Figure 3, A–C). However, while the germ cells in GFP∷PGL-1 larvae proliferate extensively in the L3 and L4 stages, the GFP∷PGL-1; meg-1(vr10) germ cells exhibited much less proliferation (Figure 3, A, D, and E). In many GFP∷PGL-1; meg-1(vr10) germ cells, GFP∷PGL-1 became diffuse instead of granular (Figure 3E), suggesting that these cells have lost the ability to assemble P granules or maintain GFP∷PGL-1 on P granules.
Figure 3.—
meg-1 germ cells fail to proliferate extensively. (A) Number of GFP-positive germ cells in GFP∷PGL-1 and GFP∷PGL-1; meg-1(vr10) animals at 25° was scored throughout development. Larvae that would ultimately become sterile as adults could not be distinguished from those that would remain fertile, so all animals were included in the analysis. (B–E) Representative GFP images from GFP∷PGL-1 (left) and GFP∷PGL-1; meg-1 (right) at L1 (B), L2 (C), L3 (D), and L4 (E) stages. Brackets mark germ cells in the developing gonad or in one gonad arm (B and D). Boxes mark regions that are enlarged below to show germ-cell and P-granule morphology (C and E). Bar, 10 μm.
To determine if the reduction in germ-cell number in meg-1 mutant hermaphrodites was caused by aberrant apoptosis of germ cells, we combined the meg-1(vr10) allele with ced-4(n1162), a strain that cannot undergo apoptosis. ced-4; meg-1(vr10) progeny developed into sterile adults with high penetrance (75%, n = 60) and displayed germ-cell defects that were as extensive as those seen in the meg-1(vr10) mutant alone. Because blocking apoptosis does not restore fertility or germ-cell development to meg-1 mutants, the severe reduction in germ-cell number is likely due to decreased proliferation or some form of non-apoptotic cell death.
MEG-1 localizes to embryonic P granules:
To determine the subcellular localization of MEG-1, we raised a polyclonal antibody against the full-length MEG-1 protein. The antibody recognized a single band of the expected size of 70 kDa in Western analysis of wild-type worm lysate (data not shown). In immunofluorescence studies, we found that MEG-1 is detectable only in the embryonic P lineage and only in structures that costain with anti-PGL-1, indicating that they are P granules (Figure 4). MEG-1 first localizes to P granules at the 4- to 8-cell stage after P2 is born, remains on P granules through subsequent P cell divisions, and begins to fade when Z2 and Z3 are born at the 100-cell stage. MEG-1 was not detected in the adult germline or in any somatic tissues. Additionally, meg-1(vr10) and meg-1(vr11) embryos fail to stain with the MEG-1 antibody (supplemental Figure 2 at http://www.genetics.org/supplemental/).
Figure 4.—
MEG-1 localizes to embryonic P granules. (A–E) Wild-type embryos stained with DAPI (blue), PGL-1 antibody (red), and MEG-1 antibody (green). (A) Two-cell stage. (B) Four-cell stage. (C) Nine-cell stage. (D) Twenty-eight-cell stage. (E) Pre-comma stage. (F) meg-1(vr10) 8-cell embryo stained with DAPI (blue), PGL-1 (red), and PGL-3 (green), showing segregation of P granules to two cells.
To independently verify the P-granule association of MEG-1 and investigate the localization of MEG-2, we generated transgenic lines expressing GFP∷MEG-1 and GFP∷MEG-2 fusion proteins in the germline. Both GFP∷MEG-1 and GFP∷MEG-2 localize to P granules specifically in P2, P3, P4, and Z2/Z3, consistent with anti-MEG-1 antibody staining (supplemental Figure 3 at http://www.genetics.org/supplemental/). The localization of MEG-2 to P granules is consistent with the genetic data that meg-2 functions redundantly with meg-1. Each transgene can rescue the sterility of meg-1(vr10) or meg-1(vr11) mutants at 25°, indicating that they produce functional proteins (Table 2). The partial rescue of meg-1 mutants by GFP∷MEG-2 suggests that extra copies of MEG-2 can compensate for the absence of MEG-1, implying that the overall level of MEG-1 and MEG-2 is important for proper germline development.
We noted that GFP∷MEG-1 and GFP∷MEG-2 remain detectable in germ cells beyond early Z2/Z3. Possibly, the pie-1 regulatory elements driving transgene expression promote prolonged GFP∷MEG-1 and GFP∷MEG-2 expression in the germline, or the GFP could enhance the stability or detectability of the protein. Strikingly, although GFP remains detectable in Z2 and Z3 in larvae, it does not localize to P granules but is diffusely cytoplasmic (supplemental Figure 3). This observation suggests that some regulated activity, which is not present in Z2 and Z3, is required to promote MEG-1 localization to P granules in P2, P3, and P4. This activity may be mes-1 signaling (see below).
Independent localization of MEG-1 and other P-granule components:
To determine whether localization of MEG-1 to embryonic P granules depends on other P-granule proteins, we stained pgl-1 and glh-1 mutants with anti-MEG-1 antibodies (supplemental Figure 2). MEG-1 localized to P granules in both of these mutants as in wild type. We also asked whether constituent components of P granules in embryos are affected by the loss of meg-1. We stained meg-1 embryos with antibodies to three known P-granule proteins, PGL-1, PGL-3, and GLH-1 (Figure 4F and data not shown). All three correctly localize to perinuclear granular structures in embryonic germline blastomeres in the absence of meg-1 activity. We conclude that MEG-1 can associate with P granules independently of these P-granule components, and vice versa.
meg-1 mutants retain many characteristics of primordial germ cells:
The embryonic P-granule-specific expression of MEG-1 led us to examine several characteristics of primordial germ cells: translation of NOS-2, chromatin modifications in Z2 and Z3, and proliferation arrest in starved larvae. nos-2 mRNA is maternally deposited in the embryo, but its translation is delayed in the P lineage until late P4 or early Z2 and Z3 stage (Subramaniam and Seydoux 1999; D'Agostino et al. 2006). meg-1 embryos stained positively for NOS-2 in late P4 and Z2/Z3 at the same frequency seen in wild-type embryos (∼52 and ∼55%, respectively; n ≥ 19), although the staining is perhaps reduced in intensity in meg-1 embryos (supplemental Figure 4 at http://www.genetics.org/supplemental/).
After P4 divides to give rise to Z2 and Z3, these primordial germ cells lose two chromatin modifications that persist in somatic cells: methylated histone H3 (H3MeK4) and acetylated histone H4 (H4AcK8) (Schaner et al. 2003). We examined Z2 and Z3 in wild-type and meg-1 mutant embryos at the >100-cell stage for H3MeK4 status and found that H3MeK4 in Z2/Z3 was decreased in 66% of meg-1 embryos, which is similar to wild type (82%; supplemental Figure 5 at http://www.genetics.org/supplemental/). A subset of meg-1 mutant embryos may abnormally retain H3MeK4, but at a frequency much less than the incidence of sterility. Thus, failure to lose the H3MeK4 modification is not the primary cause of sterility in meg-1 mutants.
Z2 and Z3 remain mitotically quiescent throughout embryogenesis until the L1 larvae hatch and begin to feed. The intake of food is directly linked to proliferation, since Z2 and Z3 do not initiate mitotic division in starved larvae (Subramaniam and Seydoux 1999). We used the GFP∷PGL-1-expressing strain to examine divisions of Z2 and Z3 under starved and fed conditions. Control GFP∷PGL-1 larvae hatched in the absence of food did not exhibit Z2 and Z3 divisions, but after 8 hr of exposure to food, more than two GFP+ cells were observed in 67% of larvae (n = 6). Similarly, Z2 and Z3 did not divide in most GFP∷PGL-1; meg-1(vr10) starved L1's (92%), but proceeded to divide after feeding (86%; n = 7). Therefore, the primordial germ cells in meg-1 mutant larvae retain the ability to arrest in the absence of food. We conclude from this series of experiments that meg-1 mutants have only minor defects in several primordial germ-cell characteristics.
meg-1 interacts with mes-1 to control embryonic P-granule segregation:
Although many aspects of primordial germ-cell development appeared normal in meg-1(vr10) mutant embryos at 25°, we did observe that ∼13% of embryos showed mis-segregation of P granules to somatic blastomeres C and D (Figure 4F). Additionally, 17% of GFP∷PGL-1; meg-1(vr10) L1 larvae had additional GFP∷PGL-1-positive cells outside of the somatic gonad in locations where the descendants of the D blastomere are typically found (not shown). This percentage (∼13–17%) is significantly less than the percentage of sterile adults observed at this temperature (∼60%). Moreover, although the frequency of mis-segregation increases to 50% when both meg-1 and meg-2 are inactivated by RNAi, it is still less than the frequency of sterility (>90%). Because sterility develops in meg-1(vr10) mutants even when P granules segregate correctly, we conclude that P-granule mis-segregation is not the sole defect underlying sterility in meg-1 mutants. However, this mis-segregation phenotype indicates that the embryonic P lineage is defective, at least in some meg-1 mutants.
Mutations in the gene mes-1 cause a very similar embryonic P-granule mis-segregation phenotype and exhibit a temperature-sensitive, incompletely penetrant, maternal-effect sterility, similar to meg-1 (Strome et al. 1995). Unlike meg-1, however, the percentage of mes-1 mutant embryos with P-granule segregation defects does correlate with the percentage of sterility. Notably, the MES-1 protein, which shows similarity to receptor tyrosine kinases, localizes to the membrane of the P blastomere adjacent to the endodermal blastomere from the 4- to 24-cell stage (Berkowitz and Strome 2000) at the same time and in the same cells as MEG-1.
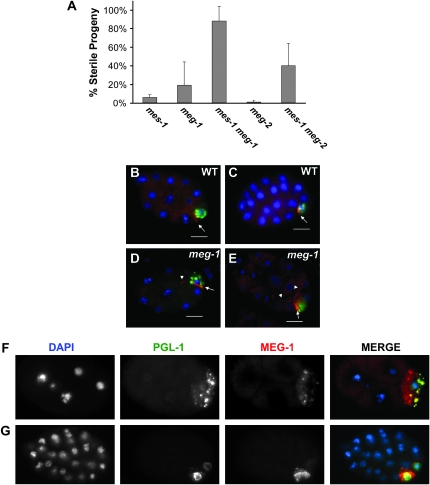
On the basis of the similar mutant phenotype and expression of mes-1 and meg-1, we investigated whether these genes might act within the same genetic pathway. We performed RNAi of meg-1 or meg-2 in mes-1 mutants at the permissive temperature (15°). Both mes-1(bn74) meg-1(RNAi) and mes-1(bn74) meg-2(RNAi) produced a significantly greater (88 and 40%, respectively) percentage of sterile progeny than mes-1 alone (6%), meg-1(RNAi) alone (19%), or meg-2(RNAi) alone (1%) (Figure 5A, Table 2). Thus, meg-1 and meg-2 act synergistically with mes-1.
Figure 5.—
mes-1 interacts synergistically with meg-1 and meg-2. (A) Percentage of sterile progeny produced by mes-1(bn74), meg-1(RNAi), mes-1(bn74)meg-1(RNAi), meg-2(RNAi), and mes-1(bn74)meg-2(RNAi) at 15°. Error bars, standard deviation. Number of animals scored is >180 over two or more independent trials per genotype. (B–E) Embryos at two different stages stained for DAPI (blue), anti-PGL-1 (green), and anti-MES-1 (red). (B and C) Wild type. (D and E) meg-1(vr11). Arrow indicates region of normal MES-1 localization. Arrowheads indicate ectopic MES-1 localization. Bar, 10 μm. (F and G) mes-1(bn74) 4-cell (F) and ∼24-cell (G) embryos at 25° stained with DAPI (blue), anti-PGL-1 (green), and anti-MEG-1 (red).
We next examined the localization of MES-1 in meg-1 mutants using an antibody to MES-1. In wild-type embryos at 25°, MES-1 is restricted to the membrane of the P cell bordering the adjacent mesendodermal blastomere (Berkowitz and Strome 2000; Figure 5, B and C). A small percentage (∼15%) of meg-1 mutant embryos had an expanded domain of MES-1 localization to both sides of the germline blastomere that bordered cells other than the endodermal blastomere and sometimes became diffuse (Figure 5, D and E). The frequency of aberrantly localized MES-1 correlates with the percentage of meg-1 mutant embryos and larvae with abnormal P-granule segregation (13–17%), suggesting that the P-granule mis-segregation in meg-1 embryos could be due to a failure to properly localize MES-1.
We then examined the localization of MEG-1 in mes-1 mutants at 25°. We found that in ∼60% of mes-1 embryos, MEG-1 and PGL-1 no longer tightly colocalize. While PGL-1 remains granular, a significant portion of MEG-1 becomes diffuse in the cytoplasm or can be found in granules lacking PGL-1 (Figure 5, F and G: compare to Figure 4, B–D). Because mes-1 mutants mis-segregate P granules and MEG-1 is no longer tightly associated with the PGL-1-containing P granules, MEG-1 and PGL-1 often become segregated to different cells as embryonic development progresses. Notably, at the permissive temperature (15°), P-granule segregation occurs properly in ∼90% of mes-1 embryos, and MEG-1 and PGL-1 remain colocalized in 84% of embryos. Thus, the incidence of sterility in mes-1 null mutants at low and high temperatures correlates with the ability of MEG-1 to associate with P granules.
Loss of pgl-1 activity can improve the meg-1 mutant phenotype:
The PGL and GLH protein families are exclusively found on P granules throughout all stages of germline development. Additionally, pgl-1 and glh-1 mutants also display temperature-sensitive, partially penetrant sterility. meg-1 and meg-2 share these properties, although their expression is restricted to P granules in early embryos. To investigate the relationships among pgl-1, glh-1, and meg-1, we constructed three double mutants: glh-1(ok439); pgl-1(bn101), glh-1(ok439); meg-1(vr10), and pgl-1(bn101); meg-1(vr10), as well as the triple mutant glh-1(ok439); pgl-1(bn101); meg-1(vr10). After shifting L4 hermaphrodites to the restrictive temperature of 25°, we scored their progeny for the presence or absence of eggs in the uterus, as a proxy measurement for sterility that permits us to count large numbers of animals. The pgl-1 and meg-1 single mutants produced a clearly visible empty uterus phenotype (85 and 53%, respectively) that correlates tightly with sterility; however, only ∼3% of glh-1(ok439) mutants exhibit an empty uterus phenotype, although ∼40% are sterile when individually tested. The triple mutant, as well as the glh-1; pgl-1 and glh-1; meg-1 double mutants, exhibited more highly penetrant sterility than any single mutant, at or close to 100% (Table 3). However, we noted that the pgl-1; meg-1 double mutant does not exhibit an increased incidence of sterile progeny relative to the pgl-1 single mutant (P < 0.065).
To more precisely examine the extent of germline development in these mutant combinations, we fixed and stained sterile adults with DAPI to visualize germline nuclei (Figure 6A). We classified these sterile adults into two phenotypic categories: a “severely affected” germline, which contains few identifiable germ cells, or a “moderately affected” germline, which contains a larger number of germ cells in the distal arm, but generally does not have gametes (Table 3). Few glh-1 and pgl-1 mutants are severely affected (0 and 15%, respectively), whereas the majority of meg-1 mutants are severely affected (70%). Strikingly, the incidence of severely affected germline development in meg-1 single mutants is significantly decreased in pgl-1; meg-1 mutants (from 70 to 26%; P < 0.03) (Table 3, Figure 6A).
To confirm this observation, we counted the number of germ cells in sterile adults stained with DAPI. The average number of germ cells in the pgl-1; meg-1 mutant was significantly higher than in the meg-1 mutants (P < 0.005), but not significantly different from the number of germ cells in pgl-1 mutants (P < 0.29; Figure 6B). Thus, two distinct aspects of the meg-1 phenotype are altered by the loss of pgl-1: relative to meg-1 single mutants, pgl-1; meg-1 double mutants have both an increased frequency of sterility and an increased germ-cell number. Both of these phenotypes closely resemble the pgl-1 single-mutant phenotype. Notably, even though germ-cell number is increased in pgl-1;meg-1 double mutants, gamete differentiation is still abnormal and the likely cause of sterility, as in pgl-1 mutants (Kawasaki et al. 1998). This result indicates that wild-type pgl-1 activity is required for full manifestation of the meg-1 phenotype. The P-granule components PGL-1, PGL-3, and GLH-1 all localize to P granules in meg-1 mutant embryos, and conversely, MEG-1 is correctly localized to P granules in pgl-1 or glh-1 mutants (Figure 2; supplemental Figure 2). Thus, interdependent P-granule localization cannot explain the genetic relationship between pgl-1 and meg-1.
DISCUSSION
Our examination of the function and localization of meg-1 and meg-2 has uncovered a role for these embryonic P-granule components in promoting germ-cell development during the time of germline restriction. MEG-1 and MEG-2 localize specifically to embryonic P granules and require signaling by MES-1 for this localization. Even though MEG expression is apparently limited to a very narrow window during germline specification, the absence of MEG activity has a profound effect on subsequent germline development. meg-1 mutants exhibit a temperature-sensitive sterile phenotype similar to that seen in mutants of other P-granule components, with defects in both germ-cell proliferation and differentiation. Notably, germ-cell proliferation in meg-1 mutants is greatly improved by mutation of pgl-1, which also encodes a P-granule-specific component. Because of their very specific localization and phenotype, MEG-1 and MEG-2 are uniquely situated as components that link germline development between generations.
How does MEG-1 promote germline development?
MEG-1 and MEG-2 do not share any sequence similarity to proteins in other organisms. The only obvious feature is that both proteins are enriched for the noncharged polar amino acids serine, glutamine, and asparagine, although their specific location within each protein is not conserved. Thus, the function of the MEG proteins could potentially rely on the overall abundance of these residues, not their organization into domains. Species-specific proteins are a common feature of germline development, possibly as a consequence of the diverse and rapid evolutionary pressure on the germline in different species. For instance, in Drosophila, pole plasm assembly is mediated by the novel protein Oskar (Santos and Lehmann 2004). Alternatively, MEG-1 and MEG-2 may serve a structural role in P granules that does not require high sequence conservation to be achieved in other species.
The sterility caused by loss of meg activity appears to have two components, a mild P-granule mis-segregation defect in embryos and a profound failure of germ cells to proliferate and survive in larvae. The simplest explanation for this variable phenotype is that MEG-1 and MEG-2 are required for the retention of some aspect of germ-cell character in embryonic germline blastomeres. The different phenotypes would then arise depending on whether meg activity falls below a threshold prior or subsequent to the point of germline restriction. If meg activity falls below the threshold before birth of P4, then P3 mis-segregates P granules into the somatic daughter cell. In this case, somatic factors are also likely mis-segregated into P4, as has been described for mes-1 mutants (Strome et al. 1995), and germline fate is disrupted. If meg activity falls below the threshold in P4, some aspect of germline character is also disrupted and the animal becomes sterile. However, in some animals, sufficient meg activity is maintained until Z2/Z3 are born and these animals develop a fertile germline.
Despite the occasional instance of mis-segregation, P granules appear largely intact in meg-1 mutant embryos. The absence of an obvious P-granule defect might be due to the fact that P granules do not need to extensively multiply in embryos. However, once germ-cell proliferation is initiated in larvae, every daughter cell must inherit sufficient P granules for proper germline function. In the absence of efficient propagation of functional P granules, germ cells might fail to divide or survive. On the basis of this idea, one possibility is that lack of meg-1 activity in the embryo also results in a defect in generation of new P granules that contributes to the failure of larval germ cells to populate the gonad.
How do MEG-1 and MEG-2 become localized to P granules?
The onset of MEG-1 and MEG-2 localization to P granules in P2 is concomitant with the association of P granules to the nuclear envelope. This association might represent a new phase of P-granule function or development that requires MEG activity. Conversely, MEG-1 and MEG-2 disappear from P granules shortly after the point of germline restriction in P4, suggesting that they are no longer needed. We noted in our experiments that even if their expression is forced beyond this window, MEG-1 and MEG-2 fail to localize to P granules and remain diffusely cytoplasmic. This narrow window of P-granule association coincides with the expression and function of another protein, MES-1 (Strome et al. 1995).
Our investigation of the relationship between meg-1 and mes-1 suggests that these proteins likely act in distinct pathways that converge upon a common property of P cells. mes-1 and meg-1 appear to have distinct roles in promoting fertility, as mutation of both causes a synergistic enhancement of sterility. Sterility in mes-1 mutants is due to P-granule mis-segregation while sterility in meg-1 mutants is primarily due to failure of larval germ-cell proliferation. Moreover, the localization of MES-1 and MEG-1 to their respective subcellular compartment is partially dependent on each other's activity.
MES-1 could be responsible for directly promoting localization of MEG-1 to P granules; alternatively, MES-1 could deliver a polarity cue to the P cell or a segregation cue to P granules, and MEG-1 dissociation from P granules in mes-1 mutants could be an indirect consequence of the absence of this cue. Conversely, MES-1 localization also depends on proper cell polarity (Berkowitz and Strome 2000), and abnormal MES-1 localization in a small percentage of meg-1 mutant embryos may indicate that the P blastomere is not properly polarized. We suggest that the aberrant regulation of P-granule function in the absence of mes-1 feeds back to disrupt the localization and function of meg-1, and vice versa, possibly by disturbing P-cell polarity or identity.
How does MEG-1 interact with existing P-granule components?
Genes encoding P-granule components such as MEG-1, PGL-1, and GLH-1 exhibit several commonalities in their null or strong loss-of-function phenotypes. First, the phenotypes of a single mutant are typically only partially penetrant. Both pgl-1 and glh-1 are members of multi-gene families that exhibit redundancy (Kuznicki et al. 2000; Kawasaki et al. 2004). The pgl-1 phenotype is enhanced by loss of pgl-3, and the glh-1 phenotype is enhanced by loss of glh-4. Similarly, we have made multiple observations consistent with meg-2 functioning redundantly with meg-1. MEG-1 and MEG-2 are highly similar, and they are expressed at the same time and colocalize to P granules in the embryonic blastomeres P2, P3, and P4. Additionally, extra copies of meg-2 can compensate for loss of meg-1, while loss of meg-2 enhances the penetrance of the defects seen in meg-1 mutants. Thus, for each family of P-granule components, one member seems to take on the primary role (meg-1, pgl-1, and glh-1), while other family members provide secondary roles. The prevalence of this functional distribution among related genes suggests that support systems are critical for robust P-granule activity.
Second, the mutant phenotypes of pgl-1, glh-1, and meg-1 are temperature sensitive; moreover, pgl-1 and meg-1 even share the highly unusual feature of dual cold and heat sensitivity (Kawasaki et al. 1998; this work). This common characteristic suggests that P-granule function is intrinsically sensitive to temperature. Possibly temperature affects the rate of a cellular process occurring at P granules, which have been implicated in many aspects of mRNA function, including localization, translation, and degradation. Altering the rate of any of these processes by temperature could influence the relative abundance of gene products and ultimately germline development.
Our investigation of the genetic interaction between the primary effectors of these P-granule family members revealed that loss of pgl-1 lessens the severity of the meg-1 phenotype. This result is in sharp contrast with our observation that mutation of glh-1 increases the severity of either the meg-1 or the pgl-1 mutant phenotype. The more extensive germline development in the pgl-1;meg-1 double mutant suggests that meg-1 might function to repress pgl-1 in the early germline blastomeres. The specific molecular function of pgl-1 has not been identified, although PGL-1 does have an RGG box that is a predicted RNA-binding domain (Kawasaki et al. 1998). Potentially, meg-1 could influence interaction of RNAs with PGL-1 or another unknown aspect of PGL-1 function.
MEG-1 is the only protein identified to date that localizes exclusively to P granules only in the embryo. All other proteins associated with P granules only in the embryo are also found in other subcellular compartments. MEG-1 is present in the P lineage from the time that P granules first associate with the nuclear envelope until the time that the primordial germ cells Z2 and Z3 are born. Possibly, MEG-1 might act in this critical window to limit P-granule function during the time when somatic and germ fates are present in the embryonic P lineage. In this scenario, removing PGL-1 activity would also cripple inappropriate P-granule activity, making MEG-1's role less critical for future germline development and thus ameliorating the phenotype of meg-1 mutants. Our results demonstrate that P-granule function is likely subject to complex regulation to promote germline development. Understanding the function of P granules, as well as their similarity to germ-plasm components in other systems, is crucial for a complete understanding of the biology of reproduction and cellular totipotency.
Acknowledgments
We thank Susan Strome, Geraldine Seydoux, Karen Bennett, and Laura Berkowitz for reagents and strains used in this study. We also thank Michelle Kudron and Marina Santiago for experimental assistance. Some strains were received from the Caenorhabditis Genetics Center. This work was supported by National Institutes of Health grant R01 GM65682 (V.R.).
References
- Ahringer, J. (Editor), 2006. Reverse genetics, in WormBook, edited by The C. elegans Research Community. WormBook, http://www.wormbook.org.
- Berkowitz, L. A., and S. Strome, 2000. MES-1, a protein required for unequal divisions of the germline in early C. elegans embryos, resembles receptor tyrosine kinases and is localized to the boundary between the germline and gut cells. Development 127: 4419–4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Agostino, I., C. Merritt, P. L. Chan, G. Seydoux and K. Subramaniam, 2006. Translational repression restricts expression of the C. elegans nanos homolog NOS-2 to the embryonic germline. Dev. Biol. 292: 244–252. [DOI] [PubMed] [Google Scholar]
- Gruidl, M. E., P. A. Smith, K. A. Kuznicki, J. S. McCrone, J. Kirchner et al., 1996. Multiple potential germ-line helicases are components of the germ-line-specific P granules of Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 93: 13837–13842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, A. R., R. Francis and T. Schedl, 1996. GLD-1, a cytoplasmic protein essential for oocyte differentiation, shows stage-and sex-specific expression during Caenorhabditis elegans germline development. Dev. Biol. 180: 165–183. [DOI] [PubMed] [Google Scholar]
- Kawasaki, I., Y. H. Shim, J. Kirchner, J. Kaminker, W. B. Wood et al., 1998. PGL-1, a predicted RNA-binding component of germ granules, is essential for fertility in C. elegans. Cell 94: 635–645. [DOI] [PubMed] [Google Scholar]
- Kawasaki, I., A. Amiri, Y. Fan, N. Meyer, S. Dunkelbarger et al., 2004. The PGL family proteins associate with germ granules and function redundantly in Caenorhabditis elegans germline development. Genetics 167: 645–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznicki, K. A., P. A. Smith, W. M. Leung-Chiu, A. O. Estevez, H. C. Scott et al., 2000. Combinatorial RNA interference indicates GLH-4 can compensate for GLH-1; these two P granule components are critical for fertility in C. elegans. Development 127: 2907–2916. [DOI] [PubMed] [Google Scholar]
- Leacock, S. W. and V. Reinke, 2006. Expression profiling of MAP kinase-mediated meiotic progression in Caenorhabditis elegans. PLoS Genet. 2: e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletieri, J., V. Reinke, S. K. Kim and G. Seydoux, 2003. Coordinate activation of maternal protein degradation in the egg-to-embryo transition in C. elegans. Dev. Cell 5: 451–462. [DOI] [PubMed] [Google Scholar]
- Pitt, J., J. A. Schisa and J. Priess, 2000. P granules in the germ cells of Caenorhabditis elegans adults are associated with clusters of nuclear pores and contain RNA. Dev. Biol. 219: 315–333. [DOI] [PubMed] [Google Scholar]
- Praitis, V., E. Casey, D. Collar and J. Austin, 2001. Creation of low-copy integrated transgenic lines in Caenorhabditis elegans. Genetics 157: 1217–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos, A. C., and R. Lehmann, 2004. Germ cell specification and migration in Drosophila and beyond. Curr. Biol. 14: R578–R589. [DOI] [PubMed] [Google Scholar]
- Schaner, C. E., G. Deshpande, P. D. Schedl and W. G. Kelly, 2003. A conserved chromatin architecture marks and maintains the restricted germ cell lineage in worms and flies. Dev. Cell 5: 747–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seydoux, G., and R. Braun, 2006. Pathway to totipotency: lessons from germ cells. Cell 127: 891–904. [DOI] [PubMed] [Google Scholar]
- Strome, S., 2005. Specification of the germ line, in WormBook, edited by The C. elegans Research Community. WormBook, http://www.wormbook.org. [DOI] [PMC free article] [PubMed]
- Strome, S., and W. B. Wood, 1982. Immunofluorescence visualization of germ-line-specific cytoplasmic granules in embryos, larvae, and adults of Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 79: 1558–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strome, S., P. Martin, E. Schierenberg and J. Paulsen, 1995. Transformation of the germ line into muscle in mes-1 mutant embryos of C. elegans. Development 121: 2961–2972. [DOI] [PubMed] [Google Scholar]
- Subramaniam, K., and G. Seydoux, 1999. nos-1 and nos-2, two genes related to Drosophila nanos, regulate primordial germ cell development and survival in Caenorhabditis elegans. Development 126: 4861–4871. [DOI] [PubMed] [Google Scholar]
- Sulston, J., and J. Hodgkin, 1988. Methods, pp. 587–606 in The Nematode Caenorhabditis elegans, edited by W. B. Wood. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.