Abstract
Although most living organisms reproduce sexually, some have developed a uniparental reproduction where the embryo usually derives from the female parent. A unique case of paternal apomixis in plants has been recently reported in Cupressus dupreziana, an endangered Mediterranean conifer. This species produces unreduced pollen that develop into all-paternal embryos within the seed tissues. We analyzed seedlings produced by open-pollinated C. dupreziana seed trees using morphological descriptors, ploidy levels assessed through flow cytometry, and AFLP genetic diversity. In situ C. dupreziana seed trees (from Algeria) produced only diploid C. dupreziana progeny. In contrast, only one-third of the progeny produced by ex situ C. dupreziana seed trees planted in French collections were similar to C. dupreziana seedlings; the other progeny were haploid or diploid C. sempervirens seedlings. These results demonstrate that C. dupreziana ovules allow for the development of all-paternal embryos from pollen produced by another species, C. sempervirens. Thus, the in planta androgenesis is achieved through the combination of the embryogenic behavior of pollen grains and the ability of seed tree ovules to act as a surrogate mother. This phenomenon offers a unique opportunity to produce, by natural means, highly valuable material for genetic studies and selection of sterile cultivars.
SEXUAL reproduction is the general rule in higher plants and animals. More than 99% of multicellular eukaryotes reproduce sexually (Bell 1982; Barton and Charlesworth 1998; West et al. 1999). The sexual cycle follows the alternation of haploid and diploid cells delimited by meiosis and syngamy, two events that modify the nuclear chromosome number (Vrijenhoek 1998). However, in some asexual species or lineages, the diploid embryo is of uniparental origin. In plants, at least 300 species in 40 angiosperm families (mainly Poaceae, Asteraceae, Rosaceae, and Rutaceae) exhibit patterns of apomixis where the embryo is derived solely from maternal tissue, either a somatic cell or a gamete with the somatic chromosome number (unreduced gamete) (Vielle Calzada et al. 1996). In rarer cases, the parthenogenetic development of a reduced gametophyte produces a haploid embryo (Foroughi-Wehr and Wenzel 1993). This last feature, also called nonrecurrent apomixis, occurs naturally in some female gametophytes. It is also intensively used in breeding programs through the in vitro culture of male gametophytes (androgenesis). Diploid homozygous genotypes can then be obtained through natural or artificial doubling of the chromosome number.
Until recently, apomixis had been reported only in Angiosperms. The highly endangered Mediterranean conifer, Cupressus dupreziana A. Camus (or Tassili cypress), is to our knowledge the first plant species where in planta “paternal apomixis” (i.e., embryogenic development of diploid pollen in seed tissues) was hypothesized (Pichot et al. 2000). This monoecious species is the most notable tree species of the mountainous region of the Tassili N'Ajjer plateau in the central Algerian Sahara desert (Balachowsky 1955). The natural population today is reduced to 233 trees spread over 1200 km2 on the southwestern border of the Tassili N'Ajjer plateau (≥1500 m above sea level) (Abdoun and Beddiaf 2002). Low accessibility due to high cliffs has contributed to protecting these trees from traditional harvesting for timber supply in Djanet and Ghat oases. In more accessible regions, overexploitation led to the extinction of the populations. Both extreme climatic conditions (the mean annual rainfall averages 30 mm) and a high human pressure may explain the very poor natural regeneration observed. The species produces unreduced diploid pollen whose embryogenic ability was demonstrated in interspecific pollinations; the controlled pollination of ovules of Cupressus sempervirens L. (or Mediterranean cypress) by pollen of C. dupreziana produces diploid embryos of C. dupreziana in C. sempervirens seeds (Pichot et al. 2001). Reciprocally, we demonstrate here that the C. dupreziana surrogate mother ability makes it possible to develop haploid embryos from reduced pollen produced by another cypress species. To our knowledge, this is the first report of natural in planta androgenesis in plants.
MATERIALS AND METHODS
Seedling production:
Seed lots were collected from 6 open-pollinated in situ C. dupreziana trees (Tassili N'Ajjer desert, Algeria) and from 26 open-pollinated C. dupreziana trees from four southeastern France, ex situ, cypress collections (Carpentras, 1 seed tree; Estérel, 18 seed trees; Montpellier, 5 seed trees; and Ruscas, 2 seed trees. Ex situ collections contained other cypress species that may have contributed to pollination. Seeds were sown in petri dishes and germinating seeds were transplanted in 400 cm3 containers and cultivated in nursery.
Morphological observations and ploidy level:
Number of cotyledons, length and shape of juvenile leaves, and foliage color were recorded on 4-month-old seedlings to discriminate C. dupreziana and C. sempervirens phenotypes. Seedling ploidy level was assessed by flow cytometry. Nuclei of fresh somatic tissues were stained with 4′,6-diamidino-2-phenylindole and analyzed using a PARTEC Ploidy Analyser PA following the protocol described by Pichot and El Maâtaoui (2000).
The effect of seed tree geographic origin on the proportion of the different phenotypes and ploidy levels of the progeny was tested using a generalized linear model with the logit link function (Collett 1991). Statistical analyses were performed with the R software (R Development Core Team 2004).
Genetic diversity:
To infer the genetic origin of the seedlings, we compared their genetic patterns revealed by amplified fragment length polymorphism (AFLP) (Vos et al. 1995) to those of 14 cypress species available from INRA (French National Institute for Agricultural Research) collections: C. sempervirens (7 samples), C. atlantica (5), C. dupreziana (43), C. arizonica (5), C. goveniana (5), C. forbesii (4), C. lusitanica (5), C. macnabiana (2), C. macrocarpa (5), C. benthamii (5), C. funebris (5), C. cashmeriana (5), C. chengiana (5), C. torulosa (5). AFLP analysis was conducted using the protocol (slightly modified) of Zabeau and Vos (1993). DNA was digested with EcoRI and MseI restriction enzymes. Amplifications were performed using a Gradient 96 Stratagene (La Jolla, CA) Robotcycler. Selective amplifications were performed using EcoRI + ACG and MseI + CAG primer pairs. Silver staining was conducted according to the procedure (slightly modified) described by Creste et al. (2001). The presence/absence of AFLP marker bands was scored for all individuals.
The individual AFLP profiles of the seedlings were plotted as supplemental points on the plane defined by the first two axes of the discriminant analysis performed on the genetic profiles of the cypresses used as the control (14 species). The discriminant multivariate analysis was performed using the Multidim package (Carlier and Croquette 2002) within the R software.
RESULTS
Characteristics of control cypress species used for the identification of the progeny are described first. Progeny features are then reported and summarized in Table 1.
TABLE 1.
Classification of cypress progeny produced by open-pollinated C. dupreziana seed trees on the basis of their morphological and genetic characteristics
| Geographic origin | No. of seed trees | No. of seedlings | Morphologya | Ploidyb | AFLP profilec | Figure |
|---|---|---|---|---|---|---|
| In situ Tassili N'Ajjer, Algeria | 6 | 38 | Cd | 2C | Cd | Figure 1A |
| Ex situ southeastern France | 26 | 54 | Cd | 2C | Cd | Figure 1A |
| 32 | Cs | 2C | Cs | Figure 1B | ||
| 74 | Cs | 1C | Cs | Figure 1C |
Cd, C. dupreziana-like with long and sharp juvenile leaves and light-green foliage; Cs, C. sempervirens-like with short juvenile leaves and dark-green foliage.
Relative DNA content revealed by flow cytometry as compared to diploid (2C) somatic tissues used as the control.
Based on a discriminant analysis of cypress species using 32 AFLP markers. Cd, C. dupreziana profile; Cs, C. sempervirens profile.
Morphological characteristics and ploidy level of the controls:
The embryos of Mediterranean and Asian species have two or, more rarely, three cotyledons while American species have three to five cotyledons. Juvenile leaves of C. dupreziana seedlings are longer and sharper than those of C. sempervirens. C. dupreziana foliage has a lighter green color. All cypress species used as the control were diploid and exhibited similar DNA contents in flow cytometry measurements.
AFLP pattern of the controls:
Genetic diversity was estimated with 32 polymorphic AFLP markers. The discriminant analysis of AFLP profiles for the control species (106 individuals) clearly discriminated their three main geographic origins—America, Asia, and the Mediterranean basin—and also led to a clear differentiation among Mediterranean species. Moreover, five AFLP bands were diagnostic markers for C. sempervirens (one band) and C. dupreziana (four bands). A nuclear biparental inheritance is usually reported for AFLP markers (Lerceteau and Szmidt 1999; Kakehi et al. 2005). A nuclear inheritance was also demonstrated for 6 of our 32 markers by analyzing segregation of AFLP bands in progeny produced by C. sempervirens-controlled crosses (data not shown). The 26 remaining markers were not analyzable due to the lack of polymorphism.
Characteristics of the progeny from in situ C. dupreziana seed trees (Table 1):
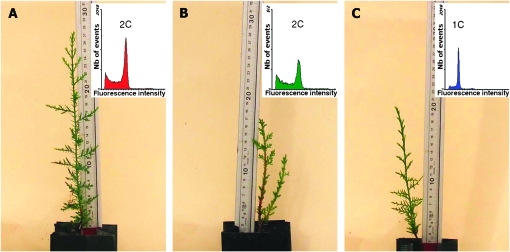
All 38 seedlings produced by the six in situ C. dupreziana seed trees had two cotyledons and exhibited a C. dupreziana phenotype (Figure 1A). Their relative fluorescence intensity peaks corresponded to those of somatic diploid tissues used as the control. AFLP patterns of these seedlings matched those of C. dupreziana controls.
Figure 1.—
Morphology and ploidy of the progeny produced by open-pollinated C. dupreziana seed trees. (A) Diploid C. dupreziana phenotype from in situ and ex situ seed trees. (B) Diploid C. sempervirens phenotype from ex situ seed trees. (C) Haploid C. sempervirens phenotype from ex situ seed trees.
Characteristics of the progeny from ex situ C. dupreziana seed trees (Table 1):
Within the 160 seedlings produced by the ex situ open-pollinated C. dupreziana seed trees, two contrasted phenotypes were observed. A typical C. dupreziana-like phenotype was observed for 54 seedlings (Figure 1A) and a C. sempervirens-like phenotype was observed for 106 seedlings. C. dupreziana-like seedlings had two cotyledons except for one with three cotyledons. They were diploid and had AFLP profiles like C. dupreziana (Figure 2).
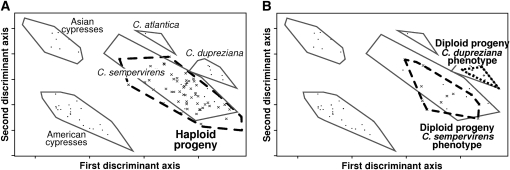
Figure 2.—
Investigation of the genetic origin of progeny using AFLP markers. Only progeny produced by C. dupreziana seed trees planted in ex situ collections were studied. The individual genetic profiles were plotted as supplemental points on the plane defined by the first two axes of a discriminant analysis of cypress species used as a control (the three Mediterranean species C. sempervirens, C. atlantica, and C. dupreziana; one American cypress group; and one Asian cypress group). All haploid progeny (A) were assigned to C. sempervirens. For diploid progeny (B), the genetic assignment either to C. dupreziana or to C. sempervirens was fully consistent with the classification based on the morphological type (Table 1, Figure 1).
Most of the C. sempervirens-like seedlings had reduced growth and sometimes exhibited an unusual bushy shape. About one-third (32 of 106) of these seedlings were diploid (Figure 1B). They had two cotyledons, except for one with three cotyledons. The remaining two-thirds produced peaks of fluorescence intensity corresponding to half the diploid DNA amount and were consequently considered as haploid (Figure 1C). All haploid seedlings had two cotyledons. Irrespective of their ploidy levels, all C. sempervirens-like seedlings exhibited AFLP profiles that matched those of the C. sempervirens control trees (Figure 2). Almost all ex situ C. dupreziana seed trees produced haploid seedlings. Only five seed trees, with a very low number of seedlings (four with one and one with three), produced only diploid progeny.
The proportion of C. sempervirens-like progeny vs. C. dupreziana-like progeny varied strongly from one site to the other, but the proportion of haploid seedlings within the C. sempervirens-like progeny was less variable (Table 2).
TABLE 2.
Variability of the proportion of the different phenotypes and ploidy levels according to the geographic origin of the seed trees
|
Ex situ C. dupreziana collection
|
|||||
|---|---|---|---|---|---|
| Carpentras | Estérel | Montpellier | Ruscas | P-valuea | |
| No. of seedlings | 17 | 84 | 41 | 18 | |
| % of C. sempervirens-like progeny | 41 | 90 | 34 | 50 | 4.6 × 10−11 |
| % of haploid cypresses within C. sempervirens-like progeny | 100 | 70 | 50 | 78 | 5.2 × 10−2 |
All seed trees were open-pollinated C. dupreziana cypresses planted in the ex situ collection in southeastern France.
Probability of the observed proportions assuming no site effect and computed from a logit generalized linear model.
DISCUSSION
All-paternal genetic origin of the seedlings:
The morphological and genetic characteristics of the C. sempervirens-like progeny produced by ex situ C. dupreziana seed trees indicate a strictly paternal origin of these seedlings. This result confirms the ability of cypress pollen to produce an embryo within the seed tissues of another cypress species but without genetic contribution of the seed tree, as reported when C. sempervirens seed trees were pollinated by C. dupreziana pollen (Pichot et al. 2001). The androgenetic process occurred from unreduced diploid pollen of C. dupreziana (Pichot and El Maâtaoui 2000; El Maâtaoui and Pichot 2001) and all the progeny from C. sempervirens seed trees were diploid. In contrast, C. sempervirens produces haploid pollen grains and both haploid and diploid C. sempervirens-like progeny were produced from ex situ, open-pollinated C. dupreziana seed trees. The haploid progeny would thus directly derive from the embryogenic development of the microgametophytes, initiated by haploid C. sempervirens pollen grains in a way similar to that of the diploid C. dupreziana microgametophytes, which produce diploid androgenic embryos. We demonstrate that the diploid progeny were pure C. sempervirens genotypes. However, an origin from unreduced gametes seems unlikely since no diploid nuclei were observed in C. sempervirens mature pollen (Pichot and El Maâtaoui 2000). They could derive either from the fusion of two male gametes or from the early natural diploidization of the haploid embryo. The reduced growth and the abnormalities observed in most of the diploid C. sempervirens-like seedlings support the diploidization hypothesis or the fusion of two genetically identical gametes (i.e., deriving from the same pollen grain). Indeed, it is well known that homozygosity produces inbreeding depression in allogamous species (Charlesworth and Charlesworth 1987). Complementary genetic analyses using codominant nuclear markers are planned to check for the putative homozygosity of these progeny.
Species compatibility:
Although C. dupreziana seed trees planted in French cypress collections were surrounded by many other cypress species, all the seedlings deriving from alien pollen were C. sempervirens trees. It must be stressed that no seedlings from American cypress species were observed although many C. dupreziana seed trees were close to C. arizonica cypresses and that their flowering periods clearly overlap (Pichot 2000). The large genetic distance between these species as compared to the proximity between the two Mediterranean species may explain this feature. According to recent studies, the genus Cupressus is paraphyletic (Little et al. 2004; Little 2006). Cypresses from the New World, now recombined into the genus Callitropsis, and cypresses from the Old Word (Mediterranean and Asian species), keeping the genus name Cupressus, do not share the same phylogenetic origin.
Significant variation in the proportion of C. sempervirens-like vs. C. dupreziana-like progeny observed among sites (Table 2) may be due to the relative contribution of the two species to the pollen cloud. Indeed, the highest rate of C. sempervirens-like progeny was observed in seeds collected from the Estérel plantation where C. dupreziana seed trees are the smallest and have a very low pollen production.
Conditions for natural androgenesis:
Although the production of all-maternal progeny by apomixis or parthenogenesis is a rather frequent phenomenon, the production of all-paternal progeny has been very rarely reported (McKone and Halpern 2003). In animals, it has been reported to occur naturally in freshwater clams (Corbicula genus) (Komaru et al. 1998) and in interspecific hybrids of the Sicilian stick insect Bacillus rossius-grandii (Tinti and Scali 1995). It was also observed in Drosophila melanogaster mutants (Komma and Endow 1995) and following fish egg irradiation (Araki et al. 1995). To our knowledge, C. dupreziana is the only plant in which progeny are produced by the apomictic development of pollen grains. The scarcity of androgenic cases reported compared to the amount of gynogenic case reports may be explained not only by the probable evolutionary dead end of this reproductive process but also by the difficulty in detecting it (McKone and Halpern 2003). The male component ability to produce an embryo without female gamete contribution may not be so rare. In fact, it is intensively used in Angiosperms to produce haploid genotypes from in vitro culture of immature anthers. Thus, favorable growth conditions combined with a lack of syngamy opportunity may often lead to androgenesis. Such conditions occur naturally in the C. dupreziana × C. sempervirens cross-pollination system due to the production of unreduced diploid male and female spores in C. dupreziana (Pichot et al. 1998; Pichot and El Maâtaoui 2000).
Consequences for C. dupreziana genetic preservation:
Natural production of C. sempervirens cypresses by C. dupreziana seed trees is an unusual type of genetic pollution although it is, according to our observations, restricted to seed admixture and does not include hybridization. The apomictic reproductive process protected the species from extinction for millennia and today prevents it from interspecific hybridization. This feature greatly facilitates the genetic conservation of the Tassili cypress and its safe propagation by seeds. However, only seeds collected from C. dupreziana seed trees that are very distant from other cypress species can be considered as pure C. dupreziana seeds. In other cases, seedling phenotype has to be checked to eliminate all non-C. dupreziana individuals, even if these alien seedlings probably would have been naturally counterselected due to the expression of deleterious genes. Unfortunately, this short-term advantage is also a long-term dead end as there is no more possibility for genetic evolution.
Production of haploid genotypes:
Our findings also demonstrate the opportunity to produce haploid genotypes in C. sempervirens species. This material is highly valuable for genetic studies and mapping, especially in C. sempervirens where the tetrasporic origin of the megagametophyte (El Maâtaoui et al. 1998) prevents analysis of marker segregation as usually performed in conifers (Wu et al. 1999). Finally, the highly probable sterility of the haploid genotypes offers a unique opportunity of producing cone-free cultivars. This may be very attractive in practical terms since the heavy production of female cones depreciates the aesthetic value of ornamental cypresses, while pollen produced by male cones causes severe pollinosis in Mediterranean regions (Charpin et al. 2005).
Acknowledgments
We thank F. Abdoun, G. Bettachini, P. Brahic, W. Brunetto, B. Jouaud, and J. Thévenet for their contribution to seed collection, seedling culture, and DNA marker experiments. We also thank two anonymous reviewers for their valuable comments.
References
- Abdoun, F., and M. Beddiaf, 2002. Cupressus dupreziana A. Camus: répartition, dépérissement et régénération au Tassili n'Ajjer, Sahara central. C. R. Acad. Sci. III 325: 617–627. [DOI] [PubMed] [Google Scholar]
- Araki, K., H. Shinma, H. Nagoya, I. Nakayama and H. Onozato, 1995. Androgenetic diploids of rainbow trout (Oncorhynchus mykiss) produced by fused sperm. Can. J. Fish. Aquat. Sci. 52: 892–896. [Google Scholar]
- Balachowsky, A. S., 1955. Une relique rarissime du Sahara central: Le cyprès de Duprez. La Nature 3237: 20–24. [Google Scholar]
- Barton, N. H., and B. Charlesworth, 1998. Why sex and recombination? Science 281: 1986–1990. [PubMed] [Google Scholar]
- Bell, G., 1982. The Masterpiece of Nature: The Evolution and Genetics of Sexuality. University of California Press, Berkeley, CA.
- Carlier, A., and A. Croquette, 2002. Multidim, nouvelles fonctions pour l'analyse des données multidimensionnelles sous Splus, Version 2.2. Université Paul Sabatier, Toulouse, France.
- Charlesworth, D., and B. Charlesworth, 1987. Inbreeding depression and its evolutionary consequences. Annu. Rev. Ecol. Syst. 18: 237–268. [Google Scholar]
- Charpin, D., M. Calleja, C. Lahoz, C. Pichot, and Y. Waisel, 2005. Allergy to cypress pollen. Allergy 60: 293–301. [DOI] [PubMed] [Google Scholar]
- Collett, D., 1991. Modelling Binary Data. Chapman & Hall, London.
- Creste, S., A. Tulmann-Neto and A. Figueira, 2001. Detection of single sequence repeat polymorphisms in denaturing polyacrylamide sequencing gels by silver staining. Plant Mol. Biol. Rep. 19: 299–306. [Google Scholar]
- El Maâtaoui, M., and C. Pichot, 2001. Microsporogenesis in the endangered species Cupressus dupreziana A. Camus: evidence for meiotic defects yielding unreduced and abortive pollen. Planta 213: 543–549. [DOI] [PubMed] [Google Scholar]
- El Maâtaoui, M., C. Pichot, H. Alzubi and N. Grimaud, 1998. Cytological basis for a tetraspory in Cupressus sempervirens L. megagametogenesis and its implications in genetic studies. Theor. Appl. Genet. 96: 776–779. [Google Scholar]
- Foroughi-Wehr, B., and G. Wenzel, 1993. Andro- and parthenogenesis, pp. 261–277 in Plant Breeding: Principles and Prospects, edited by M. D. Hayward, N. O. Bosemark, and I. Romagosa. Chapman & Hall, London.
- Kakehi, Y., K. Nakayama, K. Watanabe and M. Nishida, 2005. Inheritance of amplified fragment length polymorphism markers and their utility in population genetic analysis of Plecoglossus altivelis. J. Fish Biol. 66: 1529–1544. [Google Scholar]
- Komaru, A., T. Kawagishi and K. Konishi, 1998. Cytological evidence of spontaneous androgenesis in the freshwater clam Corbicula leana Prime. Dev. Genes Evol. 208: 46–50. [DOI] [PubMed] [Google Scholar]
- Komma, D. J., and S. A. Endow, 1995. Haploidy and androgenesis in Drosophila. Proc. Natl. Acad. Sci. USA 92: 11884–11888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerceteau, E., and A. E. Szmidt, 1999. Properties of AFLP markers in inheritance and genetic diversity studies of Pinus sylvestris L. Heredity 82: 252–260. [DOI] [PubMed] [Google Scholar]
- Little, D. P., 2006. Evolution and circumscription of the true cypresses (Cupressaceae: Cupressus). Syst. Bot. 31: 461–480. [Google Scholar]
- Little, D. P., A. E. Schwarzbach, R. P. Adams and C. F. Hsieh, 2004. The circumscription and phylogenetic relationships of Callitropsis and the newly described genus Xanthocyparis (Cupressaceae). Am. J. Bot. 91: 1872–1881. [DOI] [PubMed] [Google Scholar]
- McKone, M. J., and S. L. Halpern, 2003. The evolution of androgenesis. Am. Nat. 161: 641–656. [DOI] [PubMed] [Google Scholar]
- Pichot, C., 2000. Variabilité de la pollinisation et du pollen chez les cyprès. Allerg. Immunol. 32: 132–133. [PubMed] [Google Scholar]
- Pichot, C., and M. El Maâtaoui, 2000. Unreduced diploid nuclei in Cupressus dupreziana A. Camus pollen. Theor. Appl. Genet. 101: 574–579. [Google Scholar]
- Pichot, C., A. Borrut and M. El Maâtaoui, 1998. Unexpected DNA content in the endosperm of Cupressus dupreziana A. Camus seeds and its implications in the reproductive process. Sex. Plant Reprod. 11: 148–152. [Google Scholar]
- Pichot, C., B. Fady and I. Hochu, 2000. Lack of mother tree alleles in zymograms of Cupressus dupreziana A. Camus embryo. Ann. For. Sci. 57: 17–22. [Google Scholar]
- Pichot, C., M. El Maâtaoui, S. Raddi and P. Raddi, 2001. Surrogate mother for endangered Cupressus. Nature 412: 39. [DOI] [PubMed] [Google Scholar]
- R Development Core Team, 2004. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna.
- Tinti, F., and V. Scali, 1995. Allozymic and cytological evidence for hemiclonal, all-paternal, and mosaic offspring of the hybridogenetic stick insect Bacillus rossius-grandii grandii. J. Exp. Zool. 273: 149–159. [Google Scholar]
- Vielle Calzada, J. P., C. F. Crane and D. M. Stelly, 1996. Apomixis: the asexual revolution. Science 274: 1322–1323. [Google Scholar]
- Vos, P., R. Hogers, M. Bleeker, M. Reijans, T. V. Lee et al., 1995. AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res. 23: 4407–4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrijenhoek, R. C., 1998. Clonal organisms and the benefits of sex. Adv. Mol. Ecol. 306: 151–172. [Google Scholar]
- West, S. A., C. M. Lively and A. F. Read, 1999. A pluralistic approach to sex and recombination. J. Evol. Biol. 12: 1003–1012. [Google Scholar]
- Wu, R. L., D. M. O'Malley and S. E. McKeand, 1999. Understanding the genetic architecture of a quantitative trait in gymnosperms by genotyping haploid megagametophytes. Theor. Appl. Genet. 99: 1031–1038. [Google Scholar]
- Zabeau, M., and P. Vos, 1993. Selective restriction fragment amplification: a general method for DNA fingerprinting. Pub. 0534858 A1, Bulletin 93/13. European Patent Office, Munich.