Abstract
Rates of Mu transposon insertions and excisions are both high in late somatic cells of maize. In contrast, although high rates of insertions are observed in germinal cells, germinal excisions are recovered only rarely. Plants doubly homozygous for deletion alleles of rad51A1 and rad51A2 do not encode functional RAD51 protein (RAD51−). Approximately 1% of the gametes from RAD51+ plants that carry the MuDR-insertion allele a1-m5216 include at least partial deletions of MuDR and the a1 gene. The structures of these deletions suggest they arise via the repair of MuDR-induced double-strand breaks via nonhomologous end joining. In RAD51− plants these germinal deletions are recovered at rates that are at least 40-fold higher. These rates are not substantially affected by the presence or absence of an a1-containing homolog. Together, these findings indicate that in RAD51+ germinal cells MuDR-induced double-strand breaks (DSBs) are efficiently repaired via RAD51-directed homologous recombination with the sister chromatid. This suggests that RAD51− plants may offer an efficient means to generate deletion alleles for functional genomic studies. Additionally, the high proportion of Mu-active, RAD51− plants that exhibit severe developmental defects suggest that RAD51 plays a critical role in the repair of MuDR-induced DSBs early in vegetative development.
THE Mutator transposon family of maize (Zea mays L.), first identified by its high forward mutation rate (Robertson 1978), consists of an autonomous (MuDR) and several nonautonomous elements, all of which share ∼200-bp conserved terminal inverted repeats (TIRs). The MuDR element carries the mudrA, which is required for transacting transposase activity (reviewed by Chandler and Hardeman 1992; Bennetzen 1996; Lisch 2002; Walbot and Rudenko 2002). In germinal cells, Mu transposition frequencies can be as high as more than once per element per plant generation (Alleman and Freeling 1986; Walbot and Warren 1988). Germinal revertant events from Mu-insertion alleles are, however, recovered only rarely (Brown et al. 1989b; Levy et al. 1989; Schnable et al. 1989). In contrast, somatic excision events occur at high rates (Raizada et al. 2001; Walbot and Rudenko 2002). Double-strand breaks (DSBs), including those generated by the excision of transposons, can be repaired via two major pathways: homologous recombination (HR) and nonhomologous end joining (NHEJ) (Pastwa and Blasiak 2003; West et al. 2004).
Two models have been proposed to reconcile the differential behavior of Mu transposons in germinal and late somatic cells (Figure 1). In both models Mu transposes via a “cut-and-paste” mechanism in late somatic cells. According to model A, Mu transposes exclusively via the cut-and-paste mechanism, i.e., this mechanism is also utilized in germinal cells. To explain the low rate of germinal revertants, this model states that in germinal cells, but not in late somatic cells, Mu-induced DSBs are repaired via HR using the sister chromatid or homologous chromosome as a template (Donlin et al. 1995; Lisch et al. 1995; Hsia and Schnable 1996). The proposed role of HR in the repair of Mu-induced DSBs was based on the recovery of internal deletions of Mu elements thought to have arisen via a gap repair model (Donlin et al. 1995; Lisch et al. 1995; Hsia and Schnable 1996), which was originally described to explain the behavior of Drosophila P elements (Engels et al. 1990).
Figure 1.—
Two competing models for Mu transposition mechanism are presented. Triangles designate Mu transposons. The parentheses represent DSBs. The dashed line represents a deletion. In the right column, two single-strand nicks are illustrated in an enlarged rectangle, where each line represents a single-strand of DNA. Elsewhere each line represents double-strand DNA. Our data provide strong experimental support for the view that repair of Mu-induced DSBs in germinal cells and early somatic cells occurs via the mechanism outlined in model A.
According to the alternative model B, although Mu transposons utilize a cut-and-paste mechanism in late somatic cells, replicative transposition is used in germinal cells, thereby explaining the low rate of germinal revertants (Craig 1995; Raizada et al. 2001; Walbot and Rudenko 2002). This model is based on the finding that the bacterial transposon Tn7 is competent to make a switch between cut-and-paste and replicative transposition (May and Craig 1996) and the fact that the maize mudrA gene produces multiple transcripts that could at least potentially enable the switch between cut-and-paste and replicative transposition (Raizada et al. 2001; Walbot and Rudenko 2002).
RAD51, the RecA homolog in eukaryotes, plays a central role in the HR pathway (Baumann and West 1998; Thacker 1999), including that of maize (Franklin et al. 1999; Li et al. 2007). We have compared the behavior of a MuDR transposon in RAD51+ and RAD51− maize plants. Our data establish that RAD51-directed HR plays a major role in the repair of MuDR-induced DSBs in germinal cells. As such this study provides strong experimental support for the excision of Mu transposons in cell lineages that are inherited (germinal events), as well as in those that occur late in somatic cell development. This finding suggests that developmental differences in the regulation of endogenous DSBs repair pathways are responsible for the different behaviors of Mu transposons in germinal and late somatic cells.
MATERIALS AND METHODS
Genetic stocks:
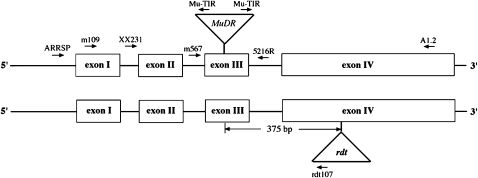
The a1-sh2 interval of chromosome 3 serves as a model for the study of meiotic recombination (Civardi et al. 1994; Xu et al. 1995; Yao et al. 2002; Yandeau-Nelson et al. 2005, 2006; Yao and Schnable 2005). The a1 gene encodes dihydroflavonol 4-reductase (O'Reilly et al. 1985) and is necessary for accumulation of anthocyanins in several plant tissues, including the aleurone (Wienand et al. 1990). The a1-m5216 allele contains a MuDR transposon insertion in exon III of the a1 gene and conditions a spotted kernel phenotype due to somatic excision of MuDR (Hsia and Schnable 1996; Figure 2). The a1∷rdt (Brown et al. 1989a; Figure 2; GenBank accession no. AF072704), a1-mrh (Shepherd et al. 1989) and a1-mr102b (Cuypers et al. 1988; GenBank accession no. AY687856) alleles all contain transposon insertions that disrupt a1 gene function and condition a nonspotted kernel phenotype in the absence of appropriate active transposases. The a1-dl allele has an 8-bp insertion in exon III that disrupts a1 gene function (Hsia and Schnable 1996; GenBank accession no. U46056). Kernels that lack a functional Sh2 allele are shrunken (Tsai and Nelson 1966). The a1 and sh2 genes are both physically deleted in the ax-1 allele (Yandeau-Nelson et al. 2006). The functional characterization of wx1 locus was described previously (Shure et al. 1983; Baran et al. 1992).
Figure 2.—
Structures of the a1-m5216 and a1∷rdt alleles. Triangles represent MuDR or rdt transposons. The a1-m5216 (top) and a1∷rdt (bottom) alleles contain MuDR and rdt transponson insertions, respectively. Primer positions are designated by arrows.
The maize genome contains only two rad51 genes (Franklin et al. 1999; Li et al. 2007). Deletion derivatives (rad51A1-54F11d1 and rad51A2-98E7d4) of both of these genes were isolated as described (Li et al. 2007). Plants that are homozygous for both of these mutant alleles are referred to as RAD51−; all other genotypes with functional RAD51 are referred to as RAD51+. A material transfer agreement governs the distribution of rad51 alleles; inquiries should be directed to Robert Meeley, Pioneer Hi-Bred, Johnston, Iowa.
Oligonucleotides:
The following oligonucleotides were used in this study: 5216R, 5′ TAA ATA AAA GGT GTC GTC AGC G 3′; A1.2, 5′ GAT TGT TGC TTA AGC GCC AAT CGT 3′; ARRSP, 5′ GAC TAG TTG CAG CGT GTG GTG TT 3′; IDPa1-dl, 5′ CGT CGG TCC AGC ACT CCA 3′; m109, 5′ AGC AGC AGC TAA AGA AGC AAG TC 3′; m567, 5′ CCT GAG GTA GAT CAG TCT TGG C 3′; Mu-TIR, 5′AGA GAA GCC AAC GCC A(AT)C GCC TC(CT) ATT TCG TC 3′; Mu1211, 5′ GTG GAA GGA GGA GGA CTA CT 3′; Mu1253, 5′ ATG AGC AAG GGT TTA GCG TGG AAT G 3′; Mu1805, 5′ AGG TAT TTC CGT ATG CTG AGA G 3′; Mu1936, 5′ ACA TTT CTG ACC TTG CTA AC 3′; Mu2332r, 5′ TGC CAT TCC TCA CAA GAA CAC TG 3′; Mu2400, 5′ CCT CTG CTA CGT CTG GCT GTA CTG G 3′; Mu2903, 5′ CCT CTG CTA CGT CTG GCT GTA CTG G 3′; Mu3102, 5′ CCA AGA AAA GAC TGA GGA TTA 3′; Mu3106r, 5′ GAG CAC TAA TCC TCA GTC TTT TC 3′; Mu3962, 5′ CGA CAA CCC TTC CGT AGA T 3′; Mu4536u, 5′ GAA CAC AGA ACA GCG GCT AGG 3′; Mu4700, 5′ ATC TTC CGT CGC CGA ATT GGA CTG C 3′; Mu534r, 5′ ATT AAA CTC ACC TCA CTG CCA CC 3′; MuDR2270, 5′ TGG CAG AGG TAC GAG ACA GC 3′; MUDR3960, 5′ TCA TCT ACG GAA GGG TTG TC 3′; rdt107, 5′ AGC GGT CAC CAA GCA ATA G 3′; wx2481, 5′ TAC CAG TCC CAC GGC ATC TAC A 3′; wx2659r, 5′ GGT AGG AGA TGT TGT GGA TGC AG 3′; and XX231, 5′ GCC AAA CTC TGA TTC GCT CCG TG 3′.
The approximate locations of some oligonucleotides are shown in Figure 2. All of the remaining oligonucleotides were designed on the basis of the MuDR sequence or wx1 gene.
Isolation of colored germinal revertants from a1-m5216:
Cross 1: RAD51+; a1-dl Sh2/a1-dl Sh2 Wx1/Wx1 × a1-m5216 Sh2/a1* Sh2; wx1/wx1
Cross 2: RAD51+; a1-m5216 Sh2/a1* Sh2 × a1-dl Sh2/a1-dl Sh2
Cross 3: RAD51+; a1-m5216 Sh2/ax-1 × a1-dl Sh2/a1-dl Sh2.
In all crosses female parents are listed first. The term a1* indicates either a1-mrh or a1-mr102b. To rule out possible pollen contamination, PCR was used to test for the presence of wx1 or a1 alleles contributed by the male parents. When amplified with primer pair wx2481 + wx2659r, the wx1 allele present in the male parent of cross 1 yields a distinctive (smaller) PCR product than produced by any tested Wx1 alleles. Because primer IDPa1-dl anneals to the 8-bp insertion in a1-dl, the primer pair ARRSP and IDPa1-dl amplifies the a1-dl allele contributed by the male parent of crosses 2–3, but not any other tested a1 alleles. Colored kernels from crosses 1–3 that carried the pollen markers were selected as putative germinal revertants from a1-m5216. These candidate kernels were germinated and self-pollinated to make homozygous stocks. These newly arisen A1 alleles were PCR-amplified from DNA extracted from homozygous seedlings using primers ARRSP and A1.2 (Figure 2), purified using QIAGEN (Valencia, CA) PCR purification kits (cat. no. 28106) and directly sequenced.
Isolation of nonspotted and pale germinal deletions from a1-m5216:
Cross 4: RAD51−; a1-m5216 Sh2/a1∷rdt sh2 × a1-dl sh2/a1-dl sh2
Cross 5: RAD51+; a1-m5216 Sh2/a1∷rdt sh2 × a1-dl sh2/a1-dl sh2
Cross 6: RAD51−; a1-m5216 Sh2/ax-1 × a1∷rdt sh2/a1∷rdt sh2
Cross 7: RAD51+; a1-m5216 Sh2/ax-1 × a1∷rdt sh2/a1∷rdt sh2.
Two strategies were used to isolate germinal deletions from a1-m5216. In strategy I, the a1-m5216 was heterozygous with a1∷rdt (crosses 4–5); in strategy II, it was made hemizygous using ax-1 (crosses 6–7). Because the genetic distance between a1 and sh2 is only 0.1 cM (Civardi et al. 1994), almost all round kernels from crosses 4–7 are derived from the a1-m5216 Sh2 haplotype. If no genomic or epigenetic changes occur at a1, all round kernels from progeny (crosses 4–7) will be spotted due to the somatic excision of MuDR from a1-m5216. Nonspotted round kernels from crosses 4–7 were selected as candidate germinal deletions of a1-m5216. The female parents of crosses 4 and 5 are siblings, as are the female parents of crosses 6 and 7. As such, crosses 5 and 7 are appropriate positive controls for crosses 4 and 6, respectively. Crosses 4 and 6 were conducted using approximately half of the RAD51− plants with active Mu that do not exhibit severe developmental abnormalities.
Nonspotted round kernels from crosses 4–7 were germinated and genomic DNA was isolated from 1-week-old seedlings using a modified high-throughput CTAB method (Dietrich et al. 2002). To rule out pollen contamination as a source of the nonspotted round kernel phenotype, PCR was used to test the presence of the expected a1 alleles from male parents. In progeny from crosses 4–5 (which should carry a1-dl) this was accomplished as described above; in progeny from crosses 6–7 (which should carry a1∷rdt) that was accomplished using the primer pair XX231 and rdt107 (Figure 2). To rule out rare recombination events as the origin of nonspotted round kernel phenotype, progeny from crosses 4–5 were also tested for the presence of a1∷rdt.
Plants that carried pollen markers and that did not arise via recombination between a1 and sh2 were further analyzed via PCR using two pairs of primers (Mu-TIR and XX231, Mu-TIR and A1.2) that anneal to MuDR and flanking a1 sequences (Figure 2). For those alleles that failed to amplify with both pairs of primers, two a1-flanking primers (m109 and A1.2) were used to directly amplify deletion products (Figure 2). It was possible to specifically amplify (and subsequently sequence) the a1-m5216 deletions from heterozygous progeny (crosses 4–7) because the primer m109 anneals to a1-m5216 but not to either a1∷rdt or a1-dl. For those alleles that were amplified with only one of the two primer pairs (Mu-TIR and XX231, Mu-TIR and A1.2), additional PCR was conducted using internal MuDR primers plus the appropriate a1-flanking primer. The resulting PCR products were subsequently sequenced to determine the deletion endpoints associated with each allele. Those alleles producing apparently normal PCR products using both primer pairs (Mu-TIR and XX231, Mu-TIR and A1.2) were subjected to temperature gradient capillary electrophoresis (TGCE) assays.
TGCE:
Because the PCR assay described above cannot detect very small deletions, nonspotted round kernels with apparently normal PCR products using two primer pairs (Mu-TIR and XX231, Mu-TIR and A1.2) were analyzed via TGCE, which is capable of detecting deletions as small as a single base (Hsia et al. 2005). Due to the large size (4.9 kb) of MuDR, two rounds of PCR were performed to prepare templates for TGCE analysis. Initially all haplotypes were analyzed with two pairs of primers (XX231 and Mu2332r, 5216R and MuDR2270). The resulting PCR products were purified using the QIAGEN PCR purification kit (cat. no. 28106) and then diluted 1000× with distilled water for a second round of PCR. If the first reaction was conducted using the primer pair XX231 and Mu2332r, four additional pairs of primers (m567 and Mu534r, Mu473 and Mu1253, Mu1211 and Mu1936, Mu1805 and Mu2332r) were used for the second round of PCR. If the first reaction was conducted with the primer pair 5216R and MuDR2270, five additional pairs of primers (MuDR2270 and Mu2903, Mu2400 and Mu3106R, Mu3102 and Mu3962, MuDR3960 and Mu4700, Mu4536u and 5216R) were used for the second round of PCR. PCR products from the second round of PCR reactions were subjected to TGCE analysis vs. the intact a1-m5216 control. TGCE was conducted using the Reveal System, model RVL 9612, rev. 2.0 (SpectruMedix, State College, PA). Sample preparation and TGCE conditions were as described previously (Hsia et al. 2005).
Crossing strategies for the observation of developmental defects associated with RAD51− in a Mu active genetic background:
Cross 8: rad51A1-54F11d1/Rad51A1; rad51A2-98E7d4/Rad51A2, A1 sh2/a1-dl sh2 X rad51A1-54F11d1/Rad51A1; rad51A2-98E7d4/Rad51A2, a1-m5216 Sh2/a1-dl sh2
Cross 9: rad51A1-54F11d1/Rad51A1; rad51A2-98E7d4/Rad51A2, A1 sh2/a1-dl sh2 X rad51A1-54F11d1/Rad51A1; rad51A2-98E7d4/Rad51A2, a1-m5216* Sh2/a1-dl sh2.
The male parent of cross 8 carries an active MuDR transposon, as demonstrated by the observation that >90% of its Sh2 progeny (i.e., those that carry a1-m5216) are spotted due to somatic excisions from a1 (Hsia and Schnable 1996; Figure 2). In contrast, the male parent of cross 9 exhibits little or no Mu activity because progeny carrying a1-m5216 are nonspotted. In this cross, the asterisk designates an inactive form of a1-m5216.
RESULTS
Colored germinal revertants from a1-m5216:
Kernels homozygous for stable mutant a1 alleles are colorless. The a1-m5216 allele conditions a spotted kernel phenotype due to somatic excisions of MuDR from a1 (Hsia and Schnable 1996). Sixteen independent confirmed, germinal colored revertants were isolated from normal RAD51+ plants carrying a1-m5216 (crosses 1–3, materials and methods) and sequenced (Figure 3A). Consistent with previous reports about rates of germinal revertants from Mu-insertion alleles (Brown et al. 1989b; Levy et al. 1989; Schnable et al. 1989), germinal revertants arose only rarely (2.60 × 10−5) from a1-m5216 (Table 1). All but four events (types 1–4, Figure 3A) were perfect germinal excisions (type 5, Figure 3A).
Figure 3.—
Sequence analysis of germinal revertants and class I germinal derivatives from a1-m5216. Open and solid boxes represent exons of the a1 gene and the MuDR insertion in a1-m5216, respectively. Retained a1 sequences flanking deletions are shown for reference in regular type. Sequences derived from the 9-bp target site duplication (TSD) of a1 are underlined. When nonzero, the number of base pairs of a1 sequences adjacent to MuDR that were deleted is presented in braces. Dashed lines between two double slashes designate deleted sequences. In most cases, the entire MuDR element was deleted. Retained portions of MuDR are shown in boldface type. Short filler sequences of the type described by Wessler et al. (1990) in spontaneous mutations are shown in lowercase letters, and the sizes of large fillers are indicated within brackets. Numbers of kernels in ear sectors are listed in parentheses. (A) Five types of germinal revertants from Table 1. One case of each of footprint types 1–4 was recovered. Twelve cases of the footprint type 5 (perfect excision) were recovered. (B) Class I events from RAD51−. Cross: a1-m5216 Sh2/a1∷rdt sh2 X a1-s sh2/a1-s sh2 (Figure 4). (C) Class I events from RAD51−. Cross: a1-m5216 Sh2/ax-1 X a1∷rdt sh2/a1∷rdt sh2 (Figure 4).
TABLE 1.
Rates and classes of germinal revertants isolated from a1-m5216 in RAD51+ plants
| Crossa | No. perfect excisions | No. imperfect excisions | Total no. revertants | Population size | Rate of germinal revertants |
|---|---|---|---|---|---|
| Cross 1 | 7b | 3c | 10 | 347,300 | 2.88 × 10−5 |
| Cross 2 | 3d | 0 | 3 | 110,000 | 2.72 × 10−5 |
| Cross 3 | 2e | 1f | 3 | 157,000 | 1.91 × 10−5 |
| Totalg | 12h | 4 | 16 | 614,300 | 2.60 × 10−5 |
Refer to materials and methods.
Type 5 in Figure 3A (plant identification nos. 03B-17-3, 03B-38-6, 03B-47-4, 03B-49-6, 03B-93-5, 03B-66-7, 02B-1132-L).
All three types are shown in Figure 3A [plant identification nos. 03B-16-2 (type 1), 03B-45-1 (type 2), 03B-68-3 (type 3)].
Type 5 in Figure 3A (plant identification nos. 03B-56-1, 03B-60-4, 03B-61-4).
Type 5 in Figure 3A (plant identification nos. 02B-1185 and 02B-1188).
Type 4 in Figure 3A (plant identification nos. 02B-1181).
No significant differences were detected among these three populations based on the chi-square homogeneity test. Therefore, data from the three crosses were pooled.
The high proportion of perfect excision events is probably due to selection, in that only colored kernels from crosses 1–3 were analyzed.
The effect of RAD51-directed HR repair on the rate of germinal deletions from a1-m5216:
Germinal revertants of Mu-insertion alleles arise via the excision of Mu transposons, which can subsequently insert (i.e., transpose) elsewhere in the genome. In contrast to the low rate of germinal reversions, germinal insertions of Mu transposons occur frequently (Chandler and Hardeman 1992; Bennetzen 1996; Lisch 2002; Walbot and Rudenko 2002). Model A explains this apparent discrepancy in germinal excision and insertion rates by invoking highly efficient HR-mediated repair of the MuDR-induced DSBs associated with the excision of Mu transposons (Figure 1). Specifically, following Mu excision, the sister chromatid or homologous chromosome is used as a template to replace the excised transposon via HR. This model predicts that in the absence of HR, Mu-insertion alleles will give rise to high rates of germinal deletions caused by alternative, error-prone DSBs repair pathways. To test this model, the rates at which germinal deletions arose from a1-m5216 in stocks with or without RAD51 (Li et al. 2007), which is central to HR, were compared via two crossing strategies (crosses 4–7, materials and methods).
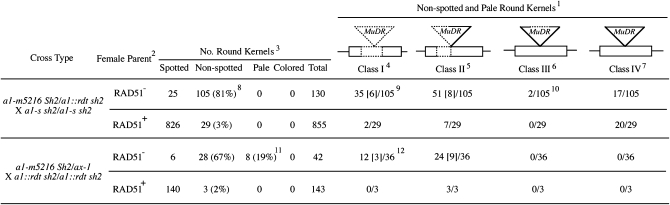
In strategy I, plants heterozygous for a1-m5216 and a recessive a1 allele were crossed by plants homozygous for another, distinguishable, recessive a1 allele (crosses 4—5). In strategy II, plants hemizygous for a1-m5216 were crossed by plants homozygous for a recessive a1 allele (crosses 6–7). From all crosses, progeny kernels that carried a1-m5216 were spotted due to somatic excisions of the MuDR transposon at a1. Partial or complete deletions of the MuDR insertion and/or a1 sequences resulted in a nonspotted kernel phenotype. Such germinal deletions were distinguished from nonspotted sibling kernels with the genotypes (a1∷rdt sh2/a1-dl sh2 or ax-1/a1∷rdt sh2) via the a1-m5216-coupled Sh2 marker that conditions round kernels (and subsequent PCR genotyping). As expected, only a small percentage of round kernels from RAD51+ plants (3% from cross 5 and 2% from cross 7) were nonspotted. In contrast, most round kernels from RAD51− plants (81% from cross 4 and 67% from cross 6) were nonspotted (Figure 4).
Figure 4.—
Rates and structures of repair products isolated from RAD51+ and RAD51− plants. (1) Fraction of nonspotted and pale round kernels that carry the indicated type of deletion of a1-m5216 is listed. (2) For a given cross type, the female parents are siblings that differ as indicated in their rad51 genotype. (3) Numbers of kernels within a family of this type are derived from multiple ears. (4) Class I events: loss of MuDR and part of a1 flanking sequences. Structures of deletion alleles were determined via PCR and sequencing of selected PCR products. (5) Class II events: deletions of terminal portions of MuDR and loss of flanking a1 sequences. Only deletions from one side are illustrated but deletions can occur on either side of MuDR. (6) Class III events: internal deletions of MuDR. (7) Class IV events: based on TGCE results these kernels carry apparently intact MuDR elements and therefore are nonspotted probably due to epigenetic inactivation. (8) Non-spotted (non-spotted round/total round). (9) Numbers in brackets indicate the total numbers of independent events (after correcting for ear sectors). Relevant plant identification numbers and their associated footprint types (Figure 3B) are: 04B-276-1, -2, -6, -14, -16, -18, -23 (type 6); 04B-276-21 (type 7); 04B-283-2, -4, -6 (type 8); 04B-283-8 (type 9); 05B-120-4, -7, -14, -15 (type 10); 05B-123-1–19 (type 11). (10) Relevant plant identification numbers are: 04B-277-6 (54-bp deletion) and 04B-279-2 (38-bp deletion). (11) All are class II. (12) Relevant plant identification numbers and their associated footprint types (Figure 3C) are 04B-279-1 (type 12), 04B-279-5 (type 13), 04B-128-1–10 (type 14).
All of the 165 nonspotted kernels from crosses 4–7 were subjected to PCR and sequencing analyses using two pairs of primers that anneal to MuDR and flanking a1 sequences (materials and methods; Figure 2). These analyses established that 134 of the nonspotted kernels carried alleles that had undergone deletions of MuDR and/or a1 sequences (classes I and II, Figure 4). The remaining 39 nonspotted kernels without apparent genomic rearrangements were subjected to TGCE, which is capable of detecting polymorphisms as small as a single base (Hsia et al. 2005). Using this assay and subsequent sequencing, two additional nonspotted round kernels were identified as carrying internal deletions of 54 bp (04B-277-6; GenBank accession no. 825704) and 38 bp (04B-279-2; GenBank accession no. 817897) of MuDR sequences as compared to a1-m5216 (class III, Figure 4). These two deletions likely arose via interrupted gap repair (Hsia and Schnable 1996).
Hence, in strategy I (a1-m5216 is heterozygous), class I, II, and III germinal deletions were recovered from RAD51− plants 64-fold more frequently than from RAD51+ controls {[(35 + 51 + 2)/130/(2 + 7)/855] = 64}. Similarly, in strategy II (a1-m5216 is hemizygous), germinal deletions are recovered from RAD51− plants 41-fold more frequently than from RAD51+ controls [(12 + 24)/42/(3/143) = 41]. When considering progeny from both strategies, all but two of the deletions responsible for the nonspotted kernel phenotypes included both MuDR and a1 sequences flanking the MuDR insertion site (Figure 3, B and C; class I and II, Figure 4). These results strongly suggest that in RAD51+ germinal cells, MuDR-induced DSBs occur frequently, but are usually successfully repaired via RAD51-directed HR. Because low rates of germinal deletions were also observed in control crosses 5 and 7, MuDR-induced DSBs are repaired via NHEJ at low rates even in the presence of RAD51. The loss of somatic excision activity in the 37 kernels that did not contain deletions (class IV, Figure 4) may be due to epigenetic changes at a1-m5216 (Chandler et al. 1988; Chomet et al. 1991).
Ear sectors of germinal deletions from RAD51− plants:
Ear sectors of germinal deletions could be generated if MuDR excises prior to meiosis. Two or more kernels from an ear were considered to be part of a sector if they had the same deletion endpoints. Based on this criterion, nine ear sectors were identified from RAD51− plants. These sectors contained 2, 3, 4, 7, 7, 8, 10, 19, and 39 kernels. All kernels from the sectors that contained 2–19 kernels were sequenced. For the sector of 39 kernels, 17 kernels were sequenced; DNA from the remaining 22 kernels yielded the same-sized PCR product as from the sequenced kernels. These excision ear sectors recovered from RAD51− plants are much larger than the Mu-insertion sectors described previously (Robertson 1980, 1981). The rate of recovering ear sectors is at least 52-fold higher in RAD51− plants as compared to RAD51+ controls (9/172 vs. 0/998). This indicates that RAD51-directed HR is required for repairing MuDR-induced DSBs in premeiotic cells.
RAD51 plays a critical role early in vegetative development of Mu active plants:
Maize RAD51− plants develop well under normal conditions, but are highly sensitive to radiation treatment, presumably due to the absence of RAD51-mediated repair of radiation-induced DSBs (Li et al. 2007). To test the role of RAD51 in the repair of MuDR-induced DSBs in early vegetative development, an active MuDR was introduced into RAD51− plants (cross 8, materials and methods). Approximately half of the Mu-active, RAD51− plants exhibit severe developmental defects (Table 2 and Figure 5). These defects were not observed among sibling plants that have functional RAD51 or related plants that lack active MuDR (Table 2). This finding strongly suggests that RAD51-directed HR is also involved in the repair of MuDR-induced DSBs during early vegetative development.
TABLE 2.
RAD51 is required for repair of MuDR-induced DSBs in vegetative tissues
| Genotype | Mu activity | No. plants with severe developmental defectsa | No. normal plants |
|---|---|---|---|
| RAD51−b | Active | 10c | 13 |
| RAD51+b | Active | 0 | 30 |
| RAD51−d | Inactive | 0 | 15 |
The severe developmental defects are described in Figure 5.
Progeny of cross 8 (materials and methods). Because plants from these two categories are siblings, they can be directly compared.
One defective plant died 2 weeks after germination.
Progeny of cross 9. With the exception of rad51 genotypes and Mu activity, this category of plants possesses almost the same genetic background as the other two categories.
Figure 5.—
Developmental defects associated with RAD51− plants and Mu activity. Many RAD51− plants with Mu activity are short (less than one-third the height of nonmutant siblings), have tiny tassels or no tassels, and do not produce ears (Table 2). These developmental defects were observed in multiple families and planting seasons including our 2005 greenhouse, 2006 (S. Liu and P. S. Schnable, unpublished data), and 2007 summer nurseries. (A) Two Mu active siblings from the 2007 summer nursery (plant identification nos. 07-3616-1 and 07-3616-2). Left and right plants are RAD51+ and RAD51−, respectively. The ruler is 12-inches long. (B–D) RAD51− plants with Mu activity from 2005 greenhouse season (plant identification nos. 05B-1594-25, 05B-1594-66, 05B-1597-36; Table 2). The white stake is 6-inches long. (E–G) RAD51− plants with Mu activity from the 2007 summer nursery (plant identification nos. 07-1238-5, 07-3616-2, 07-3617-2). The ruler is 12 in. long.
DISCUSSION
The different behaviors of Mu transposons are a consequence of differential developmental regulations of DSB repair pathways:
High rates of Mu excisions and insertions are often observed only in somatic tissues formed late in development. In contrast, one of the long-standing puzzles associated with Mu biology has been the low rate of germinal reversions, even though germinal insertions are frequent. Two models have been proposed to explain this apparent discrepancy (Figure 1). Model A proposes that different DSB repair pathways are utilized in germinal and late somatic cells (Donlin et al. 1995; Lisch et al. 1995; Hsia and Schnable 1996). In contrast, model B proposes that different Mu transposition mechanisms are utilized in germinal and late somatic cells (Raizada et al. 2001; Walbot and Rudenko 2002). In yeast, Rad51p-mediated recombination requires ∼70–100 bp homology on each side of the DSB (Ira and Haber 2002). Because RAD51 is not involved in the search for microhomologies, model B predicts that the absence of RAD51 would not have an effect on the outcome of Mu transposition in germinal or early somatic cells.
In the absence of RAD51, the a1-m5216 allele frequently gives rise to germinal deletions surrounding the MuDR insertion site (Figure 3, B and C, and Figure 4). Our findings strongly suggest that MuDR-induced DSBs occur frequently, but that in RAD51+ germinal cells these DSBs are usually repaired via RAD51-directed HR (model A, Figure 1). Most of germinal derivatives recovered from the RAD51− plants are nonspotted (Figure 4) as a consequence of deletions of a1 sequences that range from 5 to 352 bp (Figure 3, B and C). This finding indicates that the MuDR-induced DSBs are extensively resected, at least in the RAD51− background. The association of severe developmental defects with Mu activity in RAD51− plants (Table 2, Figure 5) indicates that RAD51 plays a critical role in the repair of MuDR-induced DSBs not only in germinal cells but also early in vegetative development (model A, Figure 1).
Hence, this study strongly suggests that the bulk of Mu transposition in both somatic and germinal cells occurs via a cut-and-paste mechanism, and the difference in outcomes of Mu transpositions is a consequence of the differing availabilities of DSB repair pathways during development (model A, Figure 1). Specifically, in late somatic cells, MuDR-induced DSBs are primarily repaired via the error-prone NHEJ pathway (resulting in somatic sectors of “empty sites” which may restore gene function and in the case of a1-m5216 result in colored spots on a colorless aleurone); in germinal cells and early somatic cells, usually (but not always) repaired via the highly accurate RAD51-directed HR pathway (resulting in the restoration of the Mu transposon; model A, Figure 1).
Sister chromatids serve as the major template for the repair of MuDR-induced DSBs:
Substantially higher rates of germinal deletions were obtained from RAD51− than from RAD51+ plants. The majority of these deletions removed portions of both MuDR sequences and a1 sequences that flank the MuDR-insertion site (Figure 3, B and C; classes I and II, Figure 4). This observation and the central role of RAD51 in HR strongly suggest that these germinal deletions in RAD51− plants arise via the error-prone NHEJ pathway.
In yeast, Rad51p is required for equal sister chromatid exchange (Gonzalez-Barrera et al. 2003), but not for HR between homologous chromosomes (Shinohara et al. 1997, 2000). If these specialized functions are conserved between yeast and maize, rates of recombination between sister chromatids, but not between homologous chromosomes, would be expected to be significantly reduced in RAD51− maize plants. Evidence for at least partial conservation of this functional specialization was obtained from our observation that rates of meiotic crossovers in surviving female gametes produced by RAD51− maize plants were not significantly lower than those in RAD51+ controls (Li et al. 2007).
In strategy I, a1-m5216 was heterozygous with the a1∷rdt allele (crosses 4–5, materials and methods). Hence, the template for HR-mediated repair of the MuDR-induced DSBs could have been either the sister chromatid or the homologous chromosome. All round progeny from cross 4 carry either a1-m5216 or imperfect footprints derived from a1-m5216 (Figure 4), suggesting that the homologous chromosome carrying a1∷rdt was not used as a template to repair MuDR-induced DSBs. In contrast, in strategy II only the sister chromatid could have been used as the repair template because a1-m5216 was hemizygous (crosses 6–7). Similarly increased rates of germinal deletions were observed in both strategies from RAD51− plants (Figure 4). We, therefore, conclude that sister chromatids are at least the major template for the repair of MuDR-induced DSBs via RAD51-directed HR.
On the other hand, our results do not demonstrate that the homologous chromosome is never used as a template for the repair of MuDR-induced DSBs. Indeed, the fact that the presence of an active Mutator increases rates of intragenic crossovers in a heterozygote involving a Mu-insertion allele, suggest that the homologous chromosome can at least sometimes be used as a repair template during meiosis (Yandeau-Nelson et al. 2005). Our finding that germinal revertants are recovered only rarely from RAD51+ plants that are heterozygous for Mu-insertion alleles (e.g., 2.60 × 10−5 for a1-m5216, Table 1) indicates that either the homologous chromosome is not often used as the repair template (perhaps because these events arise in premeiotic cells) or that the transposon insertions in the a1 allele (crosses 1–2 and similar to strategy I) inhibit recombination between homologous chromosomes.
The two termini of Mu transposons are not cut and/or repaired simultaneously:
For Mu transposons to excise, DSBs must be introduced adjacent to both termini. Over 60% (82/136) of the germinal deletions recovered in this study have lost only one terminus of the MuDR transposon (class II, Figure 4). Similar observations have been observed in somatic events (Raizada et al. 2001). Because these “one-sided” deletions would not be expected in RAD51− plants (where RAD51-directed HR is blocked) if both MuDR termini are cut or repaired simultaneously, we hypothesize that the two termini of Mu transposons are not cut and/or repaired simultaneously during Mu transposition. Consistent with this hypothesis, class I events (Figure 3, B and C, and Figure 4) show polarity of deletion sizes from the two sides.
Germinal deletions of Mu-insertion alleles can be recovered at a higher rate in RAD51− plants:
In RAD51+ germinal cells, a DSB on one side of a Mu transposon has at least three possible fates. First, following the introduction of a second DSB, the Mu transposon could excise and the empty site will be mainly repaired via HR or rarely via NHEJ. Second, it is possible that the first DSB could be repaired directly via HR without the introduction of a second DSB. Third, the one-sided DSB could be repaired directly by the error-prone NHEJ. If this repair occurs after nucleotide resection, a partial deletion of the host gene could result. Germinal adjacent deletions are recovered only at low rates from RAD51+ plants, and it has not been possible to identify adjacent deletions of some loci even after extensive screening (Table 3). In contrast, germinal deletions of the host gene are readily recovered from RAD51− plants (classes I and II, Figure 4). These results suggest that RAD51 is required to repair MuDR-induced DSBs and prevent deletion of flanking DNA.
TABLE 3.
Summary of germinal adjacent deletions derived from Mu-insertion alleles
| Locus | Allele | Mu insertion | No. adjacent deletions | Genic deletion (bp) | Rate | Source |
|---|---|---|---|---|---|---|
| adh1 | Adh1-S3034 | Mu1 | 1 | 74 | NDa | Taylor and Walbot (1985) |
| bz2 | bz2∷mu1 | Mu1 | 3 | ∼75–77b | ∼2.5% (3/118) | Levy and Walbot (1991) |
| dmc1a | dmc1a-93F11 | Mu1 | 0 | 0 | 0/1,250 | S. Liu and P. S. Schnable (unpublished data) |
| hcf106 | hcf106-mum1 | Mu1 | 1 | 244 | ∼0.3% (1/350)c | Das and Martienssen (1995) |
| pdc1 | pdc1-mu4365 | dMuDR | 1 | 202 | ∼0.2% (1/550) | Y. Fu and P. S. Schnable (unpublished data) |
| rad51A1 | rad51A1-54F11 | dMuDR | 1 | 363 | ND | Li et al. (2007) |
| rad51A2 | rad51A2-98E7 | dMuDR | 4 | ∼69–179 | ∼0.8% (4/500) | |
| rf2a | rf2a-m8122d | Mu1 | 0 | NAe | 0/1,200 | D. Skibbe and P. S. Schnable (unpublished data) |
| rf2c | rf2c-m1307 | Mu1 | 0 | NA | 0/1,500 | |
| rf2d | rf2d-m1310 | Mu8 | 0 | NA | 0/1,500 | |
| rth1 | rth1-2 | Mu7 | 1 | 383 | ∼0.02% (1/5,400) | Wen et al. (2005) |
No data.
Includes a partial deletion of Mu1 (16–22 bp). All other adjacent deletion alleles listed in this table have intact Mu transposons.
Two recovered Mu-insertion alleles are not included.
This allele was described by Cui et al. (2003).
Not applicable.
Mu transposons are widely used for reverse genetics (Bensen et al. 1995; Lunde et al. 2003; May et al. 2003; Settles et al. 2004; McCarty et al. 2005). Even so, Mu-insertions in promoters, introns, and 3′ ends may not disrupt gene function. Coupled with the preference of Mu transposons to insert in the 5′ ends of at least some genes (Dietrich et al. 2002; May et al. 2003), many Mu-insertion alleles isolated via reverse genetics are at least partially functional and not ideal substrates for functional genomic studies. One approach for dealing with this challenge is to isolate derivative alleles that have lost genic sequences adjacent to the Mu insertion (Table 3). The high rate at which partial deletions of the a1 gene were recovered in this study suggests that RAD51− stocks may offer an attractive means to generate knock-out mutants from Mu-insertion alleles.
Acknowledgments
We thank An-Ping Hsia for valuable discussions and technical assistance, graduate students Yan Fu, Sanzhen Liu, and David Skibbe for sharing unpublished data, undergraduate student Kenny Tsang for assistance in caring for plants, Debbie Chen for assistance with TGCE, and graduate student Sanzhen Liu for assistance with figures. This research was supported by grants from Pioneer Hi-Bred International and from the National Research Initiative of the USDA Cooperative State Research, Education, and Extension Service, grant numbers 03-00940 and 05-00962. Additional support was provided by Hatch Act and State of Iowa funds.
References
- Alleman, M., and M. Freeling, 1986. The Mu transposable elements of maize: evidence for transposition and copy number regulation during development. Genetics 112: 107–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran, G., C. Echt, T. Bureau and S. Wessler, 1992. Molecular analysis of the maize wx-B3 allele indicates that precise excision of the transposable Ac element is rare. Genetics 130: 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann, P., and S. C. West, 1998. Role of the human RAD51 protein in homologous recombination and double-stranded-break repair. Trends Biochem. Sci. 23: 247–251. [DOI] [PubMed] [Google Scholar]
- Bennetzen, J. L., 1996. The Mutator transposable element system of maize. Curr. Top. Microbiol. Immunol. 204: 195–229. [DOI] [PubMed] [Google Scholar]
- Bensen, R. J., G. S. Johal, V. C. Crane, J. T. Tossberg, P. S. Schnable et al., 1995. Cloning and characterization of the maize An1 gene. Plant Cell 7: 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, J. J., M. G. Mattes, C. O'Reilly and N. S. Shepherd, 1989. a Molecular characterization of rDt, a maize transposon of the “Dotted” controlling element system. Mol. Gen. Genet. 215: 239–244. [DOI] [PubMed] [Google Scholar]
- Brown, W. E., D. S. Robertson and J. L. Bennetzen, 1989. b Molecular analysis of multiple Mutator-derived alleles of the bronze locus of maize. Genetics 122: 439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler, V. L., and K. J. Hardeman, 1992. The Mu elements of Zea mays. Adv. Genet. 30: 77–122. [DOI] [PubMed] [Google Scholar]
- Chandler, V. L., L. E. Talbert and F. Raymond, 1988. Sequence, genomic distribution, and DNA modification of a Mu1 element from nonmutator maize stocks. Genetics 119: 951–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomet, P., D. Lisch, K. J. Hardeman, V. L. Chandler and M. Freeling, 1991. Identification of a regulatory transposon that controls the Mutator transposable element system in maize. Genetics 129: 261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civardi, L., Y. Xia, K. J. Edwards, P. S. Schnable and B. J. Nikolau, 1994. The relationship between genetic and physical distances in the cloned a1-sh2 interval of the Zea mays L. genome. Proc. Natl. Acad. Sci. USA 91: 8268–8272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig, N. L., 1995. Unity in transposition reactions. Science 270: 253–254. [DOI] [PubMed] [Google Scholar]
- Cui, X., A. P. Hsia, F. Liu, D. A. Ashlock, R. P. Wise et al., 2003. Alternative transcription initiation sites and polyadenylation sites are recruited during Mu suppression at the rf2a locus of maize. Genetics 163: 685–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuypers, H., S. Dash, P. A. Peterson, H. Saedler and A. Gierl, 1988. The defective en-I102 element encodes a product reducing the mutability of the En/Spm transposable element system of Zea mays. EMBO J. 7: 2953–2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das, L., and R. Martienssen, 1995. Site-selected transposon mutagenesis at the hcf106 locus in maize. Plant Cell 7: 287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich, C. R., F. Cui, M. L. Packila, J. Li, D. A. Ashlock et al., 2002. Maize Mu transposons are targeted to the 5′-untranslated region of the gl8 gene and sequences flanking Mu target-site duplications exhibit nonrandom nucleotide composition throughout the genome. Genetics 160: 697–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donlin, M. J., D. Lisch and M. Freeling, 1995. Tissue-specific accumulation of MURB, a protein encoded by MuDR, the autonomous regulator of the Mutator transposable element family. Plant Cell 7: 1989–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels, W. R., D. M. Johnson-Schlitz, W. B. Eggleston and J. Sved, 1990. High-frequency P element loss in Drosophila is homolog dependent. Cell 62: 515–525. [DOI] [PubMed] [Google Scholar]
- Franklin, A. E., J. McElver, I. Sunjevaric, R. Rothstein, B. Bowen et al., 1999. Three-dimensional microscopy of the Rad51 recombination protein during meiotic prophase. Plant Cell 11: 809–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Barrera, S., F. Cortes-Ledesma, R. E. Wellinger and A. Aguilera, 2003. Equal sister chromatid exchange is a major mechanism of double-strand break repair in yeast. Mol Cell 11: 1661–1671. [DOI] [PubMed] [Google Scholar]
- Hsia, A. P., and P. S. Schnable, 1996. DNA sequence analyses support the role of interrupted gap repair in the origin of internal deletions of the maize transposon, MuDR. Genetics 142: 603–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia, A. P., T. J. Wen, H. D. Chen, Z. Liu, M. D. Yandeau-Nelson et al., 2005. Temperature gradient capillary electrophoresis (TGCE)–a tool for the high-throughput discovery and mapping of SNPs and IDPs. Theor. Appl. Genet. 111: 218–225. [DOI] [PubMed] [Google Scholar]
- Ira, G., and J. E. Haber, 2002. Characterization of RAD51-independent break-induced replication that acts preferentially with short homologous sequences. Mol. Cell. Biol. 22: 6384–6392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy, A. A., and V. Walbot, 1991. Molecular analysis of the loss of somatic instability in the bz2:mu1 allele of maize. Mol. Gen. Genet. 229: 147–151. [DOI] [PubMed] [Google Scholar]
- Levy, A. A., A. B. Britt, K. R. Luehrsen, V. L. Chandler, C. Warren et al., 1989. Developmental and genetic aspects of Mutator excision in maize. Dev. Genet. 10: 520–531. [DOI] [PubMed] [Google Scholar]
- Li, J., C. L. Harper, I. Golubovskaya, C. R. Wang, D. Weber et al., 2007. Functional analysis of maize RAD51 in meiosis and DSBs repair. Genetics 176: 1469–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisch, D., 2002. Mutator transposons. Trends Plant Sci. 7: 498–504. [DOI] [PubMed] [Google Scholar]
- Lisch, D., P. Chomet and M. Freeling, 1995. Genetic characterization of the Mutator system in maize: behavior and regulation of Mu transposons in a minimal line. Genetics 139: 1777–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunde, C. F., D. J. Morrow, L. M. Roy and V. Walbot, 2003. Progress in maize gene discovery: a project update. Funct. Integr. Genomics 3: 25–32. [DOI] [PubMed] [Google Scholar]
- May, B. P., H. Liu, E. Vollbrecht, L. Senior, P. D. Rabinowicz et al., 2003. Maize-targeted mutagenesis: a knockout resource for maize. Proc. Natl. Acad. Sci. USA 100: 11541–11546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May, E. W., and N. L. Craig, 1996. Switching from cut-and-paste to replicative Tn7 transposition. Science 272: 401–404. [DOI] [PubMed] [Google Scholar]
- McCarty, D. R., A. Mark Settles, M. Suzuki, B. C. Tan, S. Latshaw et al., 2005. Steady-state transposon mutagenesis in inbred maize. Plant J. 44: 52–61. [DOI] [PubMed] [Google Scholar]
- O'Reilly, C., N. S. Shepherd, A. Pereira, Z. Schwarz-Sommer, I. Bertram et al., 1985. Molecular cloning of the a1 locus of Zea mays using the transposable elements En and Mu1. EMBO J. 4: 877–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastwa, E., and J. Blasiak, 2003. Non-homologous DNA end joining. Acta Biochim. Pol. 50: 891–908. [PubMed] [Google Scholar]
- Raizada, M. N., G. L. Nan and V. Walbot, 2001. Somatic and germinal mobility of the RescueMu transposon in transgenic maize. Plant Cell 13: 1587–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson, D. S., 1978. Characterization of a mutator system in maize. Mutat. Res. 51: 21–28. [Google Scholar]
- Robertson, D. S., 1980. The timing of Mu activity in maize. Genetics 94: 969–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson, D. S., 1981. Mutator activity in maize: timing of its activation in ontogeny. Science 213: 1515–1517. [DOI] [PubMed] [Google Scholar]
- Schnable, P. S., P. A. Peterson and H. Saedler, 1989. The bz-rcy allele of the Cy transposable element system of Zea mays contains a Mu-like element insertion. Mol. Gen. Genet. 217: 459–463. [DOI] [PubMed] [Google Scholar]
- Settles, A. M., S. Latshaw and D. R. McCarty, 2004. Molecular analysis of high-copy insertion sites in maize. Nucleic Acids Res. 32: e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd, N. S., M. M. Rhoades and E. Dempsey, 1989. Genetic and molecular characterization of a-mrh-Mrh, a new mutable system of Zea mays. Dev. Genet. 10: 507–519. [DOI] [PubMed] [Google Scholar]
- Shinohara, A., S. Gasior, T. Ogawa, N. Kleckner and D. K. Bishop, 1997. Saccharomyces cerevisiae recA homologues RAD51 and DMC1 have both distinct and overlapping roles in meiotic recombination. Genes Cells 2: 615–629. [DOI] [PubMed] [Google Scholar]
- Shinohara, M., S. L. Gasior, D. K. Bishop and A. Shinohara, 2000. Tid1/Rdh54 promotes colocalization of Rad51 and Dmc1 during meiotic recombination. Proc. Natl. Acad. Sci. USA 97: 10814–10819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shure, M., S. Wessler and N. Fedoroff, 1983. Molecular identification and isolation of the waxy locus in maize. Cell 35: 225–233. [DOI] [PubMed] [Google Scholar]
- Taylor, L. P., and V. Walbot, 1985. A deletion adjacent to the maize transposable element Mu-1 accompanies loss of Adh1 expression. EMBO J. 4: 869–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacker, J., 1999. A surfeit of RAD51-like genes? Trends Genet. 15: 166–168. [DOI] [PubMed] [Google Scholar]
- Tsai, C. Y., and O. E. Nelson, 1966. Starch-deficient maize mutant lacking adenosine dephosphate glucose pyrophosphorylase activity. Science 151: 341–343. [DOI] [PubMed] [Google Scholar]
- Walbot, V., and G. N. Rudenko, 2002. MuDR/Mu transposable elements of maize, pp. 533–564 in Mobile DNA II, edited by N. L. Craig, R. Craigie, M. Gellert and A. Lambowitz. American Society for Microbiology, Washington, DC.
- Walbot, V., and C. A. Warren, 1988. Regulation of Mu element copy number in maize lines with an active or inactive Mutator transposable element system. Mol. Gen. Genet. 211: 27–34. [DOI] [PubMed] [Google Scholar]
- Wen, T. J., F. Hochholdinger, M. Sauer, W. Bruce and P. S. Schnable, 2005. The roothairless1 gene of maize encodes a homolog of sec3, which is involved in polar exocytosis. Plant Physiol. 138: 1637–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessler, S., A. Tarpley, M. Purugganan, M. Spell and R. Okagaki, 1990. Filler DNA is associated with spontaneous deletions in maize. Proc. Natl. Acad. Sci. USA 87: 8731–8735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West, C. E., W. M. Waterworth, P. A. Sunderland and C. M. Bray, 2004. Arabidopsis DNA double-strand break repair pathways. Biochem. Soc. Trans. 32: 964–966. [DOI] [PubMed] [Google Scholar]
- Wienand, U., J. Paz-Arez, B. Scheffler and H. Saedler, 1990. Molecular analysis of gene regulation in the anthocyanin pathway of Zea mays, pp 111–124 in Plant Gene Transfer, edited by C. J. Lamb and R. N. Beachy. Wiley-Liss, New York.
- Xu, X., A. P. Hsia, L. Zhang, B. J. Nikolau and P. S. Schnable, 1995. Meiotic recombination break points resolve at high rates at the 5′ end of a maize coding sequence. Plant Cell 7: 2151–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yandeau-Nelson, M. D., Q. Zhou, H. Yao, X. Xu, B. J. Nikolau et al., 2005. MuDR transposase increases the frequency of meiotic crossovers in the vicinity of a Mu insertion in the maize a1 gene. Genetics 169: 917–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yandeau-Nelson, M. D., Y. Xia, J. Li, M. G. Neuffer and P. S. Schnable, 2006. Unequal sister chromatid and homolog recombination at a tandem duplication of the a1 locus in maize. Genetics 173: 2211–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, H., and P. S. Schnable, 2005. Cis-effects on meiotic recombination across distinct a1-sh2 intervals in a common Zea genetic background. Genetics 170: 1929–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, H., Q. Zhou, J. Li, H. Smith, M. Yandeau et al., 2002. Molecular characterization of meiotic recombination across the 140-kb multigenic a1-sh2 interval of maize. Proc. Natl. Acad. Sci. USA 99: 6157–6162. [DOI] [PMC free article] [PubMed] [Google Scholar]