Abstract
The back-to-back geometry of sister kinetochores is essential in preventing loss or damage of chromosomes during mitosis. Kinetochore orientation is generated in part by a process of resolving kinetochores at the centromere (centromere resolution) prior to spindle interactions. Because few of the genes required for centromere resolution are known, we used Caenorhabditis elegans to screen for conditional mutants defective in orienting sister kinetochores during mitosis. C. elegans is ideal for such screens because its chromosomes are holocentric. Here we identified an essential gene, cin-4, required for centromere resolution and for removal of cohesin from sites near sister kinetochores during mitosis. Given that compromised cohesin function restores centromere resolution in the absence of cin-4, CIN-4 likely acts to remove cohesin from the CENP-A chromatin enabling centromere resolution. CIN-4 has a high amino acid identity to the catalytic domain of topoisomerase II, suggesting a partial gene duplication of the C. elegans topoisomerase II gene, top-2. Similar to CIN-4, TOP-2 is also required for centromere resolution; however, the loss of TOP-2 is phenotypically distinct from the loss of CIN-4, suggesting that CIN-4 and TOP-2 are topoisomerase II isoforms that perform separate essential functions in centromere structure and function.
A failure to accurately segregate mitotic chromosomes results in genome instability, severe consequences during development, and cancer progression (Lengauer et al. 1998). Centromere structure is particularly important for the accurate segregation of chromosomes. At the centromere, sister kinetochores assemble such that they face in opposite directions (a back-to-back orientation). This back-to-back geometry aids proper chromosome segregation by restricting attachment of one kinetochore to one centrosome, thereby preventing incorrect attachments (Rieder and Salmon 1998; Cimini et al. 2001). The back-to-back geometry of sister kinetochores is thought to be involved with the correction of inappropriate spindle–kinetochore interactions and with anaphase progression (Zhang and Walczak 2006; Baumann et al. 2007). The establishment of sister kinetochores back-to-back begins in G2/early prophase when centromere proteins are initially detected as single “dots” and later resolve into paired dots (Brenner et al. 1981). The centromere protein, CENP-A, is a histone H3 variant that forms the innermost layer of the kinetochore and is required for recruiting many kinetochore proteins (Howman et al. 2000; Moore and Roth 2001; Oegema et al. 2001; Blower et al. 2002). Thus, the separation or resolution of CENP-A chromatin (also referred to as centromere resolution) is an early step in positioning sister kinetochores back to back.
The mechanism of centromere resolution is not known, but it is independent of kinetochore microtubule interactions (Brenner et al. 1981; He and Brinkley 1996; Moore and Roth 2001). Both centromere resolution and kinetochore assembly depend on the centromere protein CENP-C, yet the mechanisms for such are not well understood (Moore and Roth 2001). However, it is known that the requirement for CENP-C in centromere resolution is bypassed by genetic disruption of cohesin function (Moore et al. 2005). Cohesin is a complex of four proteins that mediate cohesion between sister chromatids (Nasmyth 2001). The cohesin complex consists of two structural maintenance of chromosomes (SMC) proteins, SMC1 and SMC3, and two non-SMC proteins, SCC3 and SCC1/Mcd1/Rad21 (Losada et al. 1998; Toth et al. 1999). Cohesin forms a ring structure that is capable of encircling sister chromatids, which may form the basis of chromosome cohesion (Gruber et al. 2003; Huang and Moazed 2006). Cohesin is abundantly present in the nucleus during interphase and is sequentially removed from chromatin during mitosis. In vertebrates, the bulk of cohesin is removed during prophase (Losada et al. 2002; Sumara et al. 2002). This prophase removal pathway requires the wings apart-like (WAPL) protein and leads to the separation of chromosome arms (Gandhi et al. 2006; Kueng et al. 2006). Centromere resolution occurs during G2/early prophase, when cohesin is abundantly present, suggesting that a novel mechanism either removes or excludes cohesin from CENP-A chromatin. However, loss of CENP-C does not lead to retention of cohesin, suggesting that other factors are involved in the resolution of sister kinetochores (Moore et al. 2005).
To identify other potential factors required for centromere resolution, we performed a genetic screen using the nematode Caenorhabditis elegans. C. elegans is ideal for identifying mutants with centromere organization defects, because the chromosomes are holocentric and in the early embryo easily studied via immunofluorescence (Albertson and Thomson 1982; Moore et al. 1999). Here we have identified a gene, cin-4 (chromosome instability) that is 89% identical to the central catalytic domain of topoisomerase II. DNA topoisomerases catalyze the removal of topological complexities by breaking, manipulating, and rejoining DNA strands. Topological complexities, such as supercoils and catenanes, arise during DNA replication and, if they are not removed, chromosome segregation is inhibited. All topoisomerases are classified on the basis of the number of DNA strands that they cleave. Odd-numbered topoisomerases, such as topoisomerase I and III, cleave a single strand of duplex DNA, generating a gap through which another DNA strand may be passed. In contrast, the even-numbered topoisomerases, such as topoisomerase II, cleave both strands of a DNA duplex, generating a double-strand break; DNA topology is altered when a second DNA duplex is passed through the double-strand break. Thus, topoisomerase II is largely responsible for decatenating and separating entangled DNAs. Topoisomerase II is abundantly present at the centromere during mitosis, suggesting a role in centromere function (Rattner et al. 1996). At anaphase, topoisomerase II may act to cleave DNA connections between sister kinetochores in a tension-sensitive manner (Baumann et al. 2007). These DNA connections are accentuated by loss of cohesins, suggesting a functional interaction between cohesin and topoisomerase II in centromere structure and cell cycle progression. In addition to decatenation, topoisomerase II function includes regulating gene expression and centromere cohesion (Bachant et al. 2002; Ju et al. 2006; Lyu et al. 2006).
In this report, we demonstrate that cin-4 and the C. elegans topoisomerase II gene, top-2, are required for centromere resolution. The cin-4 gene appears to be a C. elegans unique partial gene duplication of top-2. In the cin-4 mutant, but not top-2(RNAi) animals, cohesin is present on mitotic chromosomes, suggesting that these two genes represent topoisomerase II isoforms with distinct roles in centromere structure and function.
MATERIALS AND METHODS
C. elegans strains and culture conditions:
The wild-type C. elegans Bristol strain (N2) was used. Strains were cultured on NGM plates with Escherichia coli OP50 as described by Brenner (1974). The following marker mutations and chromosomal rearrangements, listed by linkage group, were used for genetic mapping: LGI—dpy-5(e61); LGII—rol-6(e187), unc-4(e120), mnDf99, mnDf88, mnDf105, mnDf30, mnC1, lin-26(mc15), unc-104(e1625); LGIII—unc-32(e189); LGIV—unc-5(e53); LGV—dpy-11(e224); and LGX—lon-2(e678). All temperature-sensitive stocks were maintained at a permissive temperature of 15° and shifted to a restrictive temperature of 25° for 6 hr prior to analysis. The smc-1(e879) mutant was previously characterized (Chan et al. 2003). The double mutants cin-4(mr127);smc-1(e879) and cin-4(mr127);unc-119(ed3) were produced using standard genetic cross techniques.
Screen for chromosome instability mutants:
The collection of 254 temperature-sensitive embryonic lethal mutants previously described (Stear and Roth 2002) was screened for chromosome instability (cin) phenotypes using 4′,6-diamidino-2-phenylindole (DAPI) to stain DNA. We identified 22 mutants that at the nonpermissive temperature showed an aberrant distribution of DNA in the embryo. This aberrant DNA distribution is characteristic of defects in chromosome segregation (Moore et al. 1999), suggesting that these 22 mutants were defective in some aspect of chromosome segregation following the shift from the permissive to the nonpermissive temperature (15° vs. 25°). Because we were interested in genes required for mitotic chromosome structure and function, those mutants with embryos displaying defects only in meiosis or cytokinesis were not considered further. We also stained embryos from each mutant following a shift to the nonpermissive temperature with an antibody against tubulin. Mutants with defects in spindle organization were also not considered further. We linkage mapped and out-crossed three times the remaining nine mutants before reexamining them. Mutants found to be on the same linkage group were tested for noncomplementation. During the out-crossing, some mutants no longer demonstrated embryonic lethality or upon reexamination no longer showed chromosome instability. From this screen, we identified five chromosome instability genes, which we named chromosome instability (cin-1–cin-5).
Genetic mapping of cin-4(mr127):
A combination of three-point mapping and deletion mapping was used to map the cin-4(mr127) mutation. Briefly, heterozygous cin-4(mr127/+) males were crossed to hermaphrodites of MT3751 [dpy-5(e61)I, rol-6(e187)II, unc-32(e189)III] and MT464 [unc-5(e53)V, dpy-11(e224)V, lon-2(e678)X]. F2 progeny homozygous for each morphological class were scored for temperature-sensitive embryonic lethality. Seven of 36 dpy-5(e61), 0 of 35 rol-6(e187), 5 of 35 unc-32(e189), 4 of 34 unc-5(e53), 5 of 26 dpy-11(e224), and 6 of 34 lon-2(e678) were also cin-4(mr127), indicating that the cin-4 gene is on linkage group II. Three-point mapping was used to further map cin-4(mr127); for each heterozygote, the number of the total recombinant progeny selected that had a crossover event in a given interval is in parentheses between the markers flanking the interval—lin-26; unc-4/cin-4: cin-4 (0/10) lin-26 (10/10) unc-4 and unc-104; rol-6/cin-4: unc-104 (4/21) cin-4 (17/21) rol-6.
To further define the interval containing cin-4, we determined whether known deletions in this interval could complement cin-4(mr127) at the nonpermissive temperature. We crossed male cin-4(mr127) with hermaphrodites containing deletions in the target interval and then scored the heterozygous progeny for complementation. The cin-4(mr127) cross with mnDf1 or mnDf88 produced 10/10 and 14/14 viable F1 progeny, respectively, indicating that both deletions complemented cin-4(mr127). The cin-4(mr127) and mnDf30 cross did not complement as 14 of 15 F1 progeny were either sterile or embryonic lethal.
Transgenic rescue of cin-4(mr127):
To perform transgenic rescue experiments, we first cloned a 4.8-kb Bam HI/Xba I fragment from the cosmid ZK1127 into the vector pJN254, which contains the wild-type unc-119 gene. The plasmid pGS4.8ZK1127 contained the entire ZK1127.7 gene and the ∼1 kb upstream and downstream sequences necessary for gene expression and regulation. The plasmid pGS4.8ZK1127 was then bombarded into a cin-4(mr127)II;unc-119(ed3)III strain as previously explained in Praitis et al. (2001). Transgenic lines were identified by rescue of the unc-119 phenotype and shifted to the nonpermissive temperature to test for rescue of the cin-4(mr127) embryonic lethality phenotype.
DNA sequencing:
All sequencing was performed using ABI Prism 3730xl DNA sequencing (SeqWright). While sequencing the N2 wild-type cin-4 locus, we identified a difference between our DNA sequence and the DNA sequence reported for the C. elegans genome (Wormbase release WS182). We found an insertion of a G after nucleotide 7040775 on chromosome II, giving the sequence 5-ctcgcgagaaagctgagtctaaggcgaac G cgacgcgatggtatgcgatgatatgtcag. The G insertion changes the predicted reading frame of CIN-4 to include additional amino acids at the C terminus. To identify the mr127 lesion, we sequenced the cin-4 locus in cin-4(mr127) animals. Because of the DNA identity between cin-4 and top-2, we used PCR to amplify the cin-4 locus independently of the top-2 locus or any of the topoisomerase II pseudogenes in C. elegans. We performed PCR with oligos 5-CGTGCATGCGTATTCCAGGG and 5-CGAAATGAGCTGTGACGTCATAG to obtain a 3.3-kb fragment corresponding to the cin-4 locus. The G-to-A transition mutation changes glutamate 304 in the new predicted amino acid sequence to glycine.
RACE of cin-4 mRNA:
Messenger RNA was isolated from animals using RNeasy and the Oligotex mRNA isolation kit (QIAGEN, Valencia, CA). Reverse transcription using AccuScript (Stratagene, La Jolla, CA) and random hexamers was used to generate cDNA. For 5′-RACE, the oligos SL1 5-GGTTTAATTACCCAAG, and 5-CAGAATCAATGTACAC were used for PCR from the cDNA. For 3′-RACE, the oligos dT 5-TTTTTTTTTTTTTTTTTT and 5-ATGTCTGAATTGACAAGCAGGA were used. Longer cin-4 cDNA product was obtained using oligos 5-GAAGATGCGAACGACGCTG and 5-AAGCTTTCGCATACCATCGCGT for PCR from cDNA. The PCR products were sequenced and compared to the genomic sequence to determine intron–exon structure. The cin-4 sequence can be found on GenBank (EU191083).
Bioinformatics:
The C. elegans genome was searched using the translated basic local alignment search tool (TBLAST) tool (NCBI) and the amino acid sequence of human topoisomerase II α. The following loci were identified as having significant identity to human topoisomerase II α: K12D12.1, ZK1127.7, Y46H3C.4, F31E8.6, F32A11.5, K08E5.1, and R05D3.1. Predicted amino acid sequences were aligned using the Clustal W package of the LaserGene sequence analysis software package (DNASTAR). F31E8.6, F32A11.5, and K08E5.1 amino acid sequences contained frequent nonsense codons and are likely pseudogenes. R05D3.1 amino acid sequence was 43% identical to human topoisomerase II α and 64% identical to C. elegans top-2/K12D12.1 catalytic domains, suggesting that it is a topoisomerase II-related gene. The human WAPL amino acid sequence was used to search the C. elegans genome. Three predicted alternative products of the R08C7.10 gene (e-values of 3e–56) were identified as the likely C. elegans WAPL gene. Clustal W alignment of R08C7.10a with human WAPL showed 24% identity between the two. This identity was mostly present in the C-terminal portion of each protein.
RNA interference assay:
RNA-mediated interference (RNAi) was performed by established feeding or soaking procedures (Kamath et al. 2001). Bacterial strains for RNAi feeding were obtained from a whole-genome RNAi library (Kamath and Ahringer 2003). For RNAi soaking, double-strand RNA were generated in vitro using templates derived from PCR or from plasmids isolated from the genome RNAi library. The T7 promoter sequence was added to oligos for each gene to generate templates for in vitro synthesis as previously described (Moore et al. 1999). Oligonucleotides used for PCR were 5′-GGACGCGACAAATATGGAGT and 5′-TGTTCGTGGACCATCCAGTA for ZK1127.7/cin-4; 5′-GCGTGAAGTCAAAGTAGCTCAAT and 5′-CATCTTGATCAAAACATCGACAA for K12D12.1/top2; and 5′-GGCGCCAAATATGTGAAAGT and 5′-TGGGTATGGATCTCGTGTCA for R08C7.10/wapl-1.
Indirect immunofluorescence microscopy:
The cin-4(mr127) mutant exhibited a decrease in brood size with extended incubation at the nonpermissive temperature culminating in sterility (data not shown). To optimize embryo production for microscopy, we found that incubation of adult cin-4(mr127) hermaphrodites between 6 to 16 hr at the nonpermissive temperature produced sufficient numbers of embryos displaying the Cin phenotype for analysis. Embryos for immunofluorescence were fixed and stained using an N, N-dimethylformamide protocol previously described (Moore et al. 1999). Primary antibodies used in this work were anti-HCP-3/CENP-A (Buchwitz et al. 1999), anti-HCP-4/CENP-C (Moore and Roth 2001), and anti-SCC1/COH-2 (Pasierbek et al. 2001); a monoclonal antibody, mAb414 (Davis and Blobel 1986), directed against nuclear pore proteins; and an anti-β-tubulin antibody, YL 1/2 (Amersham Life Science, Little Chalfont, Buckinghamshire, UK). Staining was detected using AlexaFluor 594- and 488-conjugated secondary antibodies (Molecular Probes, Eugene, OR). The monoclonal antibody mAb414 was used to determine cell-cycle stage as previously described (Moore and Roth 2001). Slides were visualized either on a Zeiss AxioPlan 2E microscope equipped with a Sensys CCD camera (Photometric) or by three-dimensional multiple wavelength fluorescence microscopy using a Deltavision microscope (Applied Precision). Deltavision images were obtained at specified wavelengths using 0.2-μm optical sections. These sections were then deconvolved using Softworks software (Applied Precision) and examined as either single sections or projections of an entire stack of optical sections.
Real-time quantitative PCR assays:
Real-time quantitative PCR assay (RT–qPCR) was used to assay RNAi efficiency as well as potential secondary targets of RNAi. Messenger RNA was isolated from animals using RNeasy and the Oligotex mRNA isolation kit (QIAGEN). RNA quantity was determined using a Spectrophotometer (NanoDrop). RT–qPCR was performed using the Brilliant II SYBR Green kit (Stratagene) on a MX-4000 real-time PCR machine (Stratagene). Reactions were normalized against a nonvariable control gene, F22B2.13 (Link et al. 2003). Oligos for RT–qPCR were the following: for cin-4, 5-cgtggagttcgatgaagg and 5-aactaaccgctttaatcc; for top-2, 5-ccatcaataagtaaggag and 5-aagatgttagaagagttcc; for R05D3.1, 5-gcgctcgctccaacaactc and 5-ggttccacaaccattccc; and for F22B2.13, 5-catttgtggagaatgcc and 5-tggaatagcgatttgacg. The ΔΔCT with a reference gene method was used to calculate fold decrease in transcript (Real-Time PCR Applications Guide, Stratagene). Results were obtained from at least three independent experiments and each experiment was performed in triplicate.
RESULTS
Chromosomal instability mutant cin-4(mr127) is required for centromere resolution:
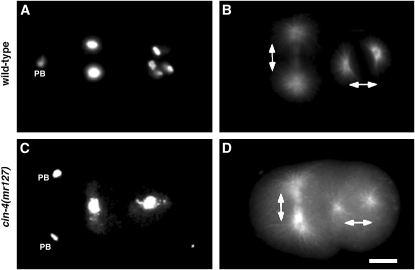
To better understand the mechanisms involved with maintaining chromosome stability, we screened 254 temperature-sensitive embryonic lethal mutants and identified five chromosome instability mutants (cin-1–cin-5), which demonstrated defects in mitotic chromosome structure and function. One of the mutants, cin-4(mr127), failed to properly segregate chromosomes during anaphase, yet the mitotic spindles were oriented bipolarly (Figure 1, C and D). Together, these results suggested that mitotic chromosome structure and function were defective in the cin-4(mr127) mutant.
Figure 1.—
Chromosome segregation is defective in the chromosome instability (cin) mutant, cin-4(mr127). Two-cell embryos from (A and B) wild-type or (C and D) cin-4(mr127) hermaphrodites incubated at the nonpermissive temperature for 6 hr. (A and C) DNA was visualized by DAPI staining. Cell division in early C. elegans embryos is well described and cell-cycle position is inferred by examination of the formation and orientation of the spindle (Strome 1993). (B and D) Microtubules visualized by staining with antitubulin antibodies indicated that mitotic spindles were bipolar and allowed inference of cell-cycle position. Mitotic spindle orientation is indicated by double-ended arrows. In wild-type embryos, chromosomes segregate equally to opposite poles whereas in cin-4(mr127) embryos chromosomes are observed as a single mass, indicating a defect in chromosome segregation. Polar bodies (PB) resulting from prior meiotic divisions are indicated. The second PB in the wild-type embryo is out of the plane of focus. Bar, 5 μm.
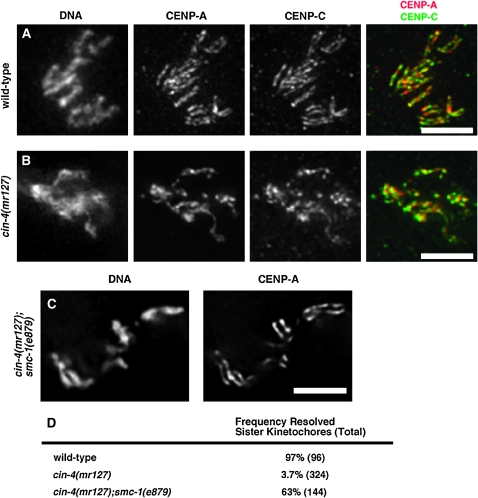
To further investigate the structure of mitotic chromosomes in cin-4(mr127) embryos at the nonpermissive temperature, embryos were stained with the cell-cycle marker antibodies anti-CENP-A/HCP-3 and mAb414. The mitotic chromosomes in these cin-4(mr127) embryos were partially condensed when compared with wild-type chromosomes (Figure 2, A and B, DNA). In wild-type embryos, mitotic chromosomes are holocentric and, as such, the centromeric histone CENP-A/HCP-3 is present along the length of each sister chromatid, which is visualized as a set of parallel lines (Figure 2A, CENP-A) (Buchwitz et al. 1999). Unlike in wild-type embryos, the mitotic chromosomes in cin-4(mr127) embryos, at the nonpermissive temperature, showed only a single line of CENP-A/HCP-3 staining (Figure 2B, CENP-A). To determine if the abnormal CENP-A/HCP-3 localization pattern, observed in cin-4(mr127) embryos, affects kinetochore structure, we examined proteins involved with kinetochore assembly. Similar to wild-type embryos, the kinetochore protein CENP-C/HCP-4 overlapped with CENP-A/HCP-3 in cin-4(mr127) embryos, indicating that CENP-C/HCP-4 localization with CENP-A/HCP-3 is not defective (Moore and Roth 2001) (Figure 2B). Other kinetochore proteins were also found localized with CENP-A/HCP-3 (data not shown). These results in addition to the observation that mitotic chromosomes still segregate (Figure 1) suggest that kinetochore assembly is not affected in cin-4(mr127) embryos.
Figure 2.—
cin-4 is necessary for centromere resolution. Nuclei in prometaphase from one- or two- cell embryos of (A) wild type, (B) cin-4(mr127), and (C) cin-4(mr127);smc-1(e879). Cell-cycle position was inferred by staining with the mAb414 antibody (not shown) to mark nuclear envelope breakdown. Embryos were obtained from cin-4(mr127) hermaphrodites after a 6-hr shift to the nonpermissive temperature and stained for DNA, CENP-A/HCP-3 (red in merged image), and CENP-C/HCP-4 (green in merged image). Colocalization between CENP-A/HCP-3 and CENP-C/HCP-4 was observed by yellow in the merged image. (D) Quantitative analysis of the incidence of resolved sister kinetochores. The number of chromosomes examined is in parentheses. Bar, 5 μm.
The failure of cin-4(mr127) mitotic blastomeres at the nonpermissive temperature to form parallel lines of CENP-A/HCP-3 could be due to the failure to resolve sister kinetochores (Moore and Roth 2001). Previously, we determined that loss of cohesin, via RNAi or genetic mutation, could suppress failed centromere resolution in hcp-4(RNAi) embryos (Moore et al. 2005). The smc-1(e879) mutation is a viable allele of the cohesin component, SMC1, and is able to reestablish centromere resolution in the absence of CENP-C/HCP-4 (Moore et al. 2005). To determine if sister kinetochores are present, but unresolved in cin-4(mr127) embryos, we constructed the double mutant cin-4(mr127);smc-1(e879). We found that in the cin-4(mr127);smc-1(e879) double mutant at the nonpermissive temperature the mitotic chromosomes were condensed and CENP-A/HCP-3 localization on opposite sides of mitotic chromosomes was more frequently observed (Figure 2, C and D). This result suggested that two duplicated, but unresolved, sister kinetochores are present in cin-4(mr127) embryos at the nonpermissive temperature.
Cohesin is associated with mitotic chromosomes in cin-4(mr127) embryos:
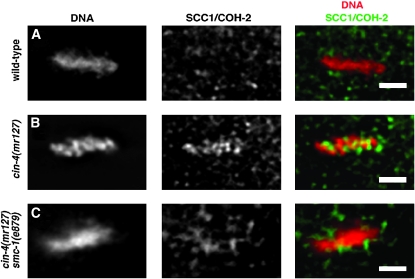
The observation that loss of cohesin function due to the smc-1(e879) allele restored parallel lines of CENP-A/HCP-3 in the cin-4(mr127) embryos suggested that cohesin might be inhibiting centromere resolution. To examine this possibility, we determined whether cohesin was present on mitotic chromosomes in cin-4(mr127) embryos at the nonpermissive temperature. In wild-type embryos, cohesin is not detected on mitotic chromosomes following nuclear envelope breakdown (Chan et al. 2003; Mito et al. 2003; Moore et al. 2005). We stained cin-4(mr127) embryos, shifted to the nonpermissive temperature, with antibodies to a cohesin protein, SCC1/COH-2, and determined that, unlike wild-type embryos, SCC1/COH-2 was present on mitotic chromosomes in cin-4(mr127) embryos (Figure 3, A and B). Interestingly, the SCC1/COH-2 staining did not completely colocalize with the mitotic chromosomes, suggesting that cohesin in cin-4(mr127) embryos is localized at specific sites. To determine if the localization of SCC1/COH-2 in cin-4(mr127) mitotic blastomeres required other cohesins, we compromised cohesin complex formation by producing a cin-4(mr127);smc-1(e879) double mutant. We reasoned that, if the observed staining was due to cohesin complex localization, then compromising SMC-1 should abrogate SCC1/COH-2 staining at mitotic chromosomes. Unlike the cin-4(mr127) embryos shifted to the nonpermissive temperature, in the cin-4(mr127);smc-1(e879) embryos SCC1/COH-2 localization on mitotic chromosomes was not observed (Figure 3C). Detection of other cohesin components using immunofluorescence showed similar localization patterns as SCC1/COH-2 in the cin-4(mr127) and cin-4(mr127);smc-1(e879) embryos (data not shown). These results support the idea that the cohesin complex is retained on mitotic chromosomes in cin-4(mr127) embryos at the nonpermissive temperature and that cin-4 is needed to prevent this cohesin localization.
Figure 3.—
Cohesin is localized on mitotic chromosomes in cin-4(mr127). Metaphase nuclei from two-cell embryos obtained from (A) wild-type, (B) cin-4(mr127), and (C) cin-4(mr127);smc-1(e879) hermaphrodites shifted to the nonpermissive temperature for 6 hr. Embryos were stained for DNA (red in merged image) and SCC1/COH-2 (green in merged image). Bar, 2 μm.
Mitotic cohesin staining is adjacent to unresolved sister kinetochores:
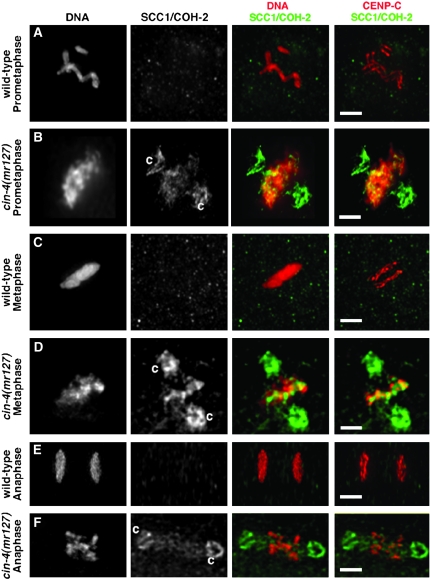
Our results suggested that mitotic cohesin was enriched at specific sites on the mitotic chromosomes of cin-4(mr127) embryos. To characterize these sites further, we examined the cohesin present on mitotic chromosomes in cin-4(mr127) embryos. Wild-type embryos and cin-4(mr127) embryos raised at the permissive temperature had similar CENP-C/HCP-4 and SCC1/COH-2 localization patterns (data not shown); CENP-C/HCP-4 formed parallel lines along the chromosomes and cohesin was absent from the mitotic chromosomes. In the cin-4(mr127) embryos, at the nonpermissive temperature, SCC1/COH-2 localized to the prometaphase chromosomes (Figure 4, A and B). Curiously, cohesin was observed on centrosomes in cin-4(mr127) embryos at the nonpermissive temperature (“c” in Figure 4B). The cohesin localized to mitotic chromosomes and centrosomes was still present in metaphase blastomeres (Figure 4, C and D). Further examination of the SCC1/COH-2 localization pattern in cin-4(mr127) mitotic chromosomes indicated that cohesin partially overlapped with DNA (Figure 4, B and D, DNA SCC1/COH-2) and in some instances, when SCC1/COH-2 localization did not overlap with DNA, it overlapped with CENP-C/HCP-4 localization (Figure 4,B and D, CENP-C SCC1/COH-2). When SCC1/COH-2 and CENP-C/HCP-4 localization did not overlap, the SCC1/COH-2 was adjacent to the CENP-C/HCP-4. In cin-4(mr127) embryos at the nonpermissive temperature, the SCC1/COH-2 localization to mitotic chromosomes, but not to centrosomes, was absent in anaphase blastomeres (Figure 4, E and F). These results suggested that cohesin was present near the sister kinetochores in cin-4(mr127) embryos and was removed from segregating chromosomes in anaphase.
Figure 4.—
Mitotic cohesin on chromosomes in cin-4(mr127) embryos localized near sites of unresolved sister kinetochores. Nuclei at indicated cell-cycle positions from embryos obtained from (A, C, and E) wild-type or (B, D, and F) cin-4(mr127) hermaphrodites shifted to the nonpermissive temperature for 6 hr. Prometaphase and metaphase nuclei were from two-cell embryos while anaphase nuclei were from four-cell embryos. Embryos were stained for DNA (red in first merged image), the kinetochore protein CENP-C (red in second merged image), and SCC1/COH-2 (green in both merged images). Centrosome (“c”) staining by the SCC1/COH-2 antibody (B, D, and F) was highly reproducible and dependent on the nonpermissive temperature. Bar, 2 μm.
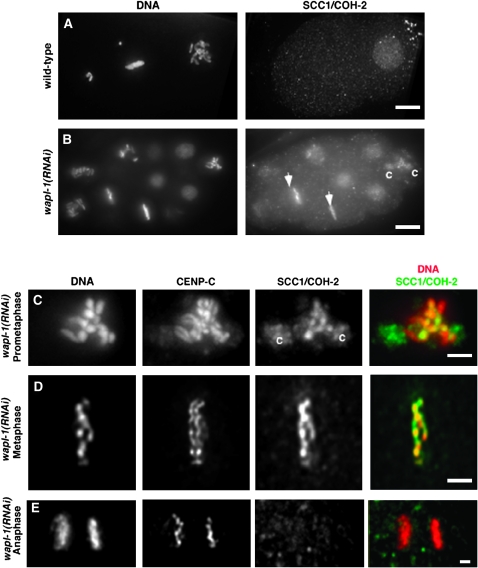
Cohesin removal, but not centromere resolution, is inhibited in wapl-1(RNAi) embryos:
The presence of cohesin on mitotic chromosomes in cin-4(mr127) embryos suggested that CIN-4 may function to remove cohesin upon entry into mitosis. In vertebrates, cohesin is removed from chromatid arms during prophase and requires the Aurora kinase B, Polo kinase, and WAPL (Losada et al. 2002; Sumara et al. 2002; Gandhi et al. 2006; Kueng et al. 2006). Previously it was shown that embryos with reduced levels of the Aurora kinase B homolog (AIR-2) did not retain cohesin on mitotic chromosomes in C. elegans (Moore et al. 2005). To further address whether a related pathway for cohesin removal exists in C. elegans, we used RNAi to reduce expression of a C. elegans gene that has similarity to the vertebrate gene WAPL. Others have suggested that the C. elegans gene R08C7.10 has similarity to WAPL (Gandhi et al. 2006). We found that there is a 24% amino acid identity between human WAPL and C. elegans R08C7.10 gene product. We determined that in the R08C7.10(RNAi) embryos cohesin is present on mitotic chromosomes (Figure 5, A and B). Similar to cin-4(mr127) embryos, the R08C7.10(RNAi) embryos contained SCC1/COH-2 localized to metaphase chromosomes (Figure 5B, arrows). Unlike cin-4(mr127) embryos, SCC1/COH-2 localization to centrosomes was infrequently observed (“c” in Figure 5B). These results suggest that R08C7.10 is the C. elegans ortholog of WAPL and will thus be referred to as wapl-1.
Figure 5.—
The cohesin dissociation pathway is conserved in C. elegans. (A and B) Embryos from (A) wild type and (B) wapl-1(RNAi) stained for DNA and SCC1/COH-2. Arrows in B indicate metaphase chromosomes with SCC1/COH-2 staining. Bars, 5 μm. (C–E) Closer view of wapl-1(RNA) nuclei at different cell-cycle stages stained for DNA (red in merged image), CENP-C, and SCC1/COH-2 (green in merged image). Overlap between DNA and SCC1/COH-2 staining is shown in merged image as yellow. Cohesin staining on centrosomes was rare, but observable in wapl-1(RNAi) embryos. Bar, 2 μm.
The cohesin localization on metaphase chromosomes in wapl-1(RNAi) suggests that cohesin may be retained until anaphase. Cohesin as detected by SCC1/COH-2 staining was present on mitotic chromosomes during prometaphase and metaphase (Figure 5, C and D) and completely overlapped with DNA in prometaphase and metaphase blastomeres (merged images in Figure 5, C and D). Cohesin was absent from anaphase chromosomes (Figure 5E). Thus, we concluded that the mechanism to remove cohesin from mitotic chromosomes prior to metaphase was conserved between C. elegans and humans.
The retention of cohesin on vertebrate chromosomes is known to inhibit sister-chromatid resolution; thus in C. elegans it is possible that the retention of cohesin on mitotic chromosomes inhibits centromere resolution (Losada et al. 2002). To address whether WAPL-1 was required for centromere resolution, we examined kinetochores and cohesin in wapl-1(RNAi) embryos. In wapl-1(RNAi) embryos, SCC1/COH-2 was present on prometaphase chromosomes, yet the sister kinetochores, as detected by CENP-C staining, were resolved (Figure 5C). This result suggested that WAPL-1 was not necessary for centromere resolution and that cohesin association with mitotic chromosomes is not sufficient to block centromere resolution.
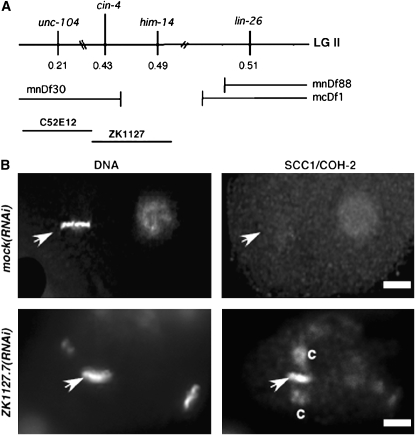
ZK1127.7 is the cin-4 gene:
Genetic mapping placed cin-4 between unc-104 and lin-26 genes on chromosome II (Figure 6). We further defined the interval containing mr127, using deletion mapping, and found that the deletion mnDf30 did not complement cin-4(mr127) at the nonpermissive temperature. Because the breakpoint for mnDf30 has been determined to be in the cosmid ZK1127 to the left of him-14 (Zalevsky et al. 1999), we examined candidate genes between unc-104 and him-14 via RNAi. RNAi of ZK1127.7 produced embryos with uneven distributions of DNA and anaphase bridging (data not shown). In ZK1127.7(RNAi) embryos, cohesin, as detected by SCC1/COH-2 staining, was present on mitotic chromatin (Figure 6B). To test whether ZK1127.7 is cin-4, we determined if the cin-4(mr127) phenotype could be rescued with the wild-type ZK1127.7 gene. A 4.8-kb stretch of DNA containing the ZK1127.7 gene with upstream and downstream regulatory elements was excised from the cosmid ZK1127. This 4.8-kb fragment was introduced into cin-4(mr127)II;unc119(ed3)III animals via a bombardment protocol (Praitis et al. 2001). We obtained three independent lines that stably integrated the wild-type ZK1127.7 gene into the genome. All three of these transgenic lines partially rescued the cin-4(mr127) phenotypes (data not shown). Together, our results indicate that cin-4 is ZK1127.7 and will be referred to as such.
Figure 6.—
The ZK1127.7 open reading frame encodes the cin-4 gene. (A) Summary of deletion mapping. (B) Two-cell embryos from mock(RNAi) or from ZK1127.7(RNAi) hermaphrodite stained for DNA and SCC1/COH-2. Arrowheads indicate mitotic DNA at metaphase. Centrosomes are labeled “c.” (B) Bar, 5 μm.
cin-4 has homology to the catalytic domain of TOP-2:
We determined the gene structure of cin-4 by comparing the sequences obtained from RT–PCR products with the genomic sequence of cin-4 in wild-type animals. Sequence analysis of the cin-4 locus indicates that a single G nucleotide not reported in the genome database (Wormbase release WS182) was present in the wild-type cin-4 genomic sequence (at nucleotide 7040775). This single nucleotide results in additional amino acids present at the C terminus of the predicted CIN-4 protein. The cin-4 mRNA was found to be SL1 trans-spliced and containing two open reading frames (ORFs) (Figure 7A). The longer ORF is predicted to encode a protein of 842 amino acids and further references to the CIN-4 amino acid sequence concern this ORF. Sequencing the genomic locus of cin-4 in the mr127 mutant strain identified a single A-to-G transition that results in glutamate 304 being changed to glycine (E304G) of the predicted CIN-4 amino acid sequence (Figure 7D). Comparing the predicted CIN-4 protein against both human and C. elegans genomes via BLAST analysis showed that CIN-4 has 89.1 and 54% amino acid identity to the central portion of the ORF K12D12.1 and to human topoisomerase II, respectively. K12D12.1 encodes a protein that has 52% identity over its entire length to topoisomerase II in humans. Given that K12D12.1 is the likely homolog of topoisomerase II in C. elegans, we refer to K12D12.1 as top-2. These findings suggest that cin-4 encodes a protein resembling the catalytic domain of topoisomerase II. Comparing the genomic DNA sequences for both cin-4 and top-2 showed that both share similar intron–exon structure with the major difference being a large deletion in cin-4 covering exon 2, exon 3, and all but the last part of exon 4 of top-2 (Figure 7B). Aligning the intron–exon boundaries of cin-4 and in top-2 revealed a high degree of identity (Figure 7C). These results together suggest that cin-4 arose by a duplication of the top-2 gene.
Figure 7.—
cin-4 is a duplication of the top-2 gene. (A) The cin-4 mRNA sequence following the SL1 spliced leader sequence. A potential upstream open reading frame is shown in the longer boxed region and the predicted cin-4 start codon is shown as boxed ATG. (B) Comparison of the structure of top-2 and cin-4 on the basis of DNA sequence alignment. A cartoon depicts the results from the alignment of the top-2 and cin-4 loci aligned using the DNA Strider Blocks alignment tool. A representation of the topoisomerase II functional domains is above the DNA alignment graphic. (C) DNA sequence alignment of intron–exon boundary sequences between top-2 and cin-4. The three intron DNA sequences (lowercase) of cin-4 are aligned with introns 1, 4, and 5 of top-2. Non-intronic DNA is shown in uppercase. (D) Amino acid sequence alignment of TOP-2 and CIN-4. Amino acid sequences were aligned using the Clustal W package of the Lasergene sequence analysis software package (DNASTAR). Amino acid identity is against a solid background. The box indicates the catalytic tyrosine present in HsTOP-2α and TOP-2 and the serine residue present in CIN-4. An asterisk indicates the glutamate 304-to-glycine change identified in mr127.
A TBLAST search of the C. elegans genome with the TOP-2 protein sequence identified six other loci in addition to the top-2 and cin-4 genes. Five of these six loci also appear to be gene duplications. The remaining ORF R05D3.1, which is annotated as a mitochondrial topoisomerase II, does not show a high degree of conservation of the DNA sequence with top-2 and cin-4, suggesting that it is a closely related, but distinct, topoisomerase II gene. The C. briggsae genome contains two predicted genes, CBG02746 and CBG18156, which share 53.9 and 35% with human topoisomerase II, respectively. CBG02746 has 79.5% amino acid identity with C. elegans TOP-2 and is likely the C. briggsae topoisomerase II gene whereas CBG18156 has 75.1% amino acid identity with R05D3.1. These results suggest that C. briggsae lacks a cin-4 gene.
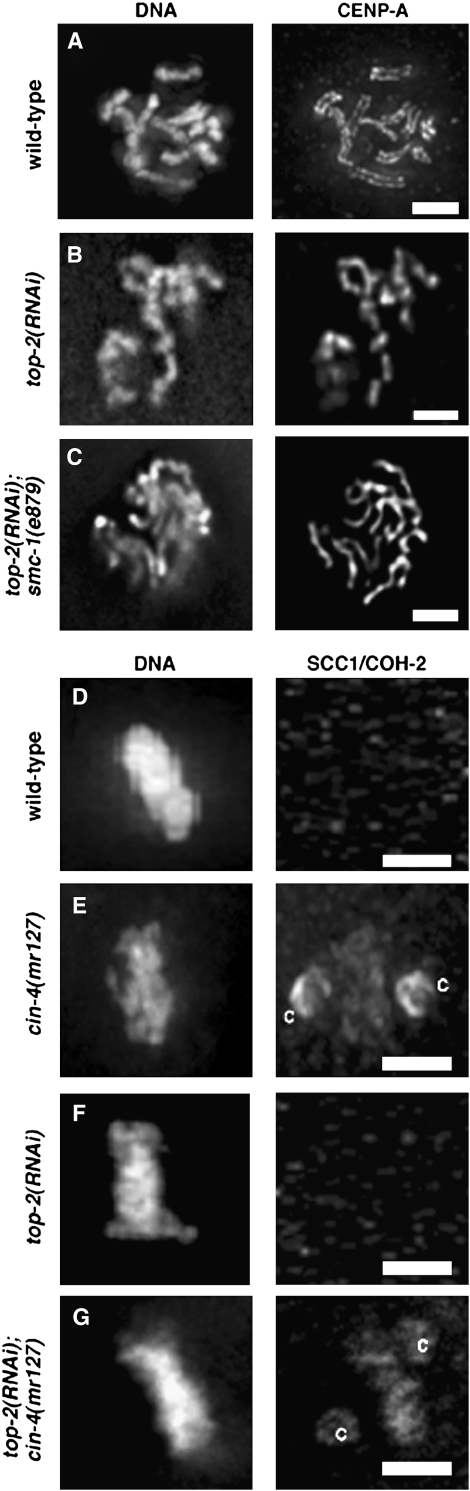
TOP-2 is required for centromere resolution:
CIN-4 and TOP-2 proteins closely resemble each other; thus, we determined if loss of top-2 via RNAi affected centromere resolution or cohesion localization. RNAi of top-2 resulted in embryonic lethality, indicating that top-2 is an essential gene (Kamath and Ahringer 2003). Because cin-4 and top-2 genes also share a high degree of DNA sequence identity, we used qRT–PCR to examine the reduction of each respective mRNA in various RNAi experiments. In our RNAi experiments, we reduced cin-4 or top-2 gene expression 9.5- to 11.5-fold, respectively (Table 1). In cin-4(RNAi) experiments, top-2 mRNA levels were also reduced 4-fold, but top-2(RNAi) did not reduce cin-4 mRNA levels (Table 1). As expected, neither cin-4(RNAi) nor top-2(RNAi) affected R05D3.1 mRNA levels, which were reduced 8-fold in R05D3.1(RNAi) experiments. As also observed by others (Kamath and Ahringer 2003), we found no detectable phenotype associated with R05D3.1(RNAi) embryos so we did not further investigate the R05D3.1 gene. As in cin-4(mr127) embryos, top-2(RNAi) embryos contained a single line of CENP-A/HCP-3 staining (Figure 8, A and B) and only 3 of 212 (1.4%) of the mitotic chromosomes examined showed any indication of resolved sister kinetochores. Similar to cin-4(mr127) embryos, CENP-C/HCP-4 was adjacent to CENP-A/HCP-3, suggesting that kinetochore assembly was not compromised in top-2(RNAi) embryos (data not shown). To determine if compromised cohesin function would restore paired sister kinetochores in top-2(RNAi) embryos, as it does in cin-4(mr127) embryos, we examined centromere resolution in smc-1(e879);top-2(RNAi) embryos. In the smc-1(e879);top-2(RNAi) embryos, only 2 of 302 (0.7%) mitotic chromosomes showed paired sister kinetochores, suggesting that loss of cohesin function did not result in centromere resolution in top-2(RNAi) embryos (Figure 8C). We examined cohesin localization in top-2(RNAi) embryos and found that, unlike cin-4(mr127) embryos at the nonpermissive temperature, cohesin was not detected on the mitotic chromosomes (Figure 8, D–F). Furthermore, we observed cohesin localized to the mitotic chromosomes in cin-4(mr127);top-2(RNAi) embryos, suggesting that loss of TOP-2 function did not suppress loss of cin-4 function (Figure 8G). These combined results suggest that cin-4 and top-2 have unique and separate essential functions.
TABLE 1.
Efficiency of RNAi experiments determined by RT–qPCR
| Fold decrease in mRNA levels ±SE (P-value)
|
|||
|---|---|---|---|
| Primary target locus | cin-4 | top-2 | R05D3.1 |
| cin-4(RNAi) | 9.5 ± 1.2 (0.01) | 4.0 ± 1.1 (0.03) | 1.2 ± 0.1 (0.14) |
| top-2(RNAi) | 1.0 ± 0.1 (0.5) | 10.9 ± 0.8 (0.01) | 1.1 ± 0.1 (0.18) |
| R05D3.1(RNAi) | 1.0 ± 0.1 (0.14) | 1.0 ± 0.3 (0.08) | 8.0 ± 1.0 (0.02) |
CT values for target cDNAs were normalized to the control cDNA, F22B2.13, to correct for minor differences in cDNA concentrations. The fold decrease in expression of target cDNAs relative to a mock(RNAi) was calculated using the ΔΔCT method. P-values were calculated using the Student's t-test. SE, standard error.
Figure 8.—
TOP-2 is necessary for centromere resolution. Prometaphase nuclei from two-cell embryos of (A and D) wild type, (B and F) top-2(RNAi), (C) top-2(RNAi);smc-1(e879), (E) cin-4(mr127), and (G) top-2(RNAi);cin-4(mr127). Cell-cycle position was inferred by staining with the mAb414 antibody (not shown), which identifies the nuclear envelope. Embryos from cin-4(mr127) hermaphrodites were obtained after a 6-hr shift to the nonpermissive temperature. Mitotic chromosomes were stained (A–C) for DNA and CENP-A/HCP-3 or (D–G) for DNA and SCC1/COH-2. SCC1/COH-2 centrosome staining is indicated by “c.” Bar, 2 μm.
DISCUSSION
The holocentric nature of C. elegans chromosomes is useful for identifying genes controlling chromosome structure and function (Moore and Roth 2001). Properly formed chromosomes will show a distinct two-line staining pattern of centromere and kinetochore proteins. We identified a temperature-sensitive mutant, cin-4(mr127), which failed to generate a two-line kinetochore structure at the nonpermissive temperature. The CIN-4 protein shares 89% identity with the central domain of the C. elegans TOP-2 protein. Our conclusion that cin-4 is a functional gene is based on several criteria. First, the cin-4(mr127) mutation was mapped to a position on chromosome II, which contained the ORF ZK1127.7. Second, RNAi of ZK1127.7 phenocopied the cin-4(mr127) mutation. Third, cin-4(mr127) was rescued by a 4.8-kb fragment containing the ZK1127.7 ORF. Finally, we identified a G-to-A transition that is predicted to change glutamate 304 of the predicted ZK1127.7 protein sequence into glycine. This change is in a highly conserved residue present in all topoisomerase II molecules examined.
cin-4 is a partial gene duplication of top-2:
The observations that CIN-4 protein has a high sequence similarity to the catalytic region of TOP-2 and that the genomic loci for cin-4 and top-2 are highly conserved suggest that cin-4 resulted from a gene-duplication event. Others have suggested that partial gene duplications are frequent in C. elegans (Katju and Lynch 2003). The C. briggsae genome contains orthologs of top-2 and R05D3.1 but does not contain a cin-4 ortholog, suggesting that the gene duplication giving rise to cin-4 arose after the divergence of C. elegans and C. briggsae. Interestingly, cin-4 is an essential gene, indicating either that cin-4 has acquired a new essential function (neofunctionalization) or that top-2 has lost an essential function that is now provided by cin-4 (subfunctionalization).
cin-4 is required for cohesin dynamics and centromere resolution:
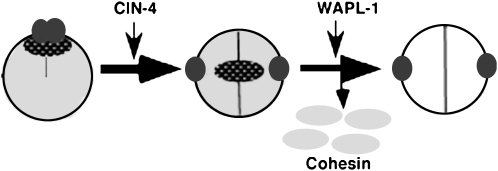
In cin-4(mr127) embryos at the nonpermissive temperature, cohesin was present on mitotic chromosomes and centromere resolution failed, suggesting a link between these two processes. Cohesin associates with chromosomes prior to DNA replication, but cohesion between sister chromatids is not established until DNA replication (Ciosk et al. 2000). The majority of cohesin is removed prior to metaphase. One protein implicated in cohesin dissociation is WAPL. In the absence of WAPL, cohesin is retained on mitotic chromosomes in vertebrates (Gandhi et al. 2006; Kueng et al. 2006). Likewise, cohesin was retained on mitotic chromosomes in C. elegans when WAPL-1 was removed via RNAi, indicating that this cohesin dissociation pathway is conserved between C. elegans and vertebrates. Given that this dissociation pathway is present in C. elegans, there were several differences between WAPL-1 and CIN-4 function, suggesting that they function in different aspects of cohesin dynamics. In wapl-1(RNAi), embryos resolved sister kinetochores, suggesting that WAPL-1 was not necessary for centromere resolution. Although it is possible that the efficiency of RNAi was not sufficient to block centromere resolution, we did observe cohesin on mitotic chromosomes, indicating that the presence of cohesin on mitotic chromosomes is not sufficient to inhibit centromere resolution. This result contrasted with results obtained with cin-4(RNAi) or cin-4(mr127) animals. In organisms with monocentric chromosomes, there are two populations of cohesin: one that is dissociated during prophase and a second population that is protected by Shugoshin (Salic et al. 2004; McGuinness et al. 2005). Whereas cohesin completely overlapped with mitotic chromosomes in wapl-1(RNAi) embryos, only a subset of each chromosome had cohesin staining in cin-4(mr127) embryos. These results suggested that the majority of cohesin is removed by WAPL-1 (Figure 9). Interestingly, the cohesin population that is present when cin-4 function is compromised is not removed solely by WAPL-1. As in the Shugoshin-protected centromeric cohesin, the cohesin on mitotic chromosomes in cin-4(mr127) embryos was localized on or near sister kinetochores. Unlike the cohesin population removed by WAPL-1, the cohesin near the sister kinetochores may interfere with centromere resolution. Consistent with this idea, the loss of cohesin function via a genetic mutant bypassed the need for CIN-4 in centromere resolution. The role of CIN-4 in centromere resolution may be to remove a centromere-associated cohesin population to enable centromere resolution. Alternatively, cohesin could be excluded from CENP-A chromatin in a CIN-4-dependent manner. Thus, in the absence of functional CIN-4, cohesin is retained near sister kinetochores and protected from WAPL-1-mediated dissociation.
Figure 9.—
Model for CIN-4 role in centromere resolution and cohesin dynamics. A cartoon of a cross section of a mitotic chromosome (large circle). The majority of cohesin (shading) is removed by WAPL-1; however, a second population of cohesin near sister kinetochores is not removed by WAPL-1 (stippled oval). CIN-4 removes or reorganizes this second cohesin population to enable centromere resolution. Sister kinetochores are depicted as small solid circles.
In Saccharomyces cerevisiae, evidence exists that topoisomerase II and cohesin interact to regulate centromere structure and function. The region near centromeres is enriched in cohesin proteins, yet is able to separate and rejoin repeatedly prior to anaphase (Blat and Kleckner 1999; Goshima and Yanagida 2000; He et al. 2000; Laloraya et al. 2000; Tanaka et al. 2000). Furthermore, centromeric cohesion is negatively regulated by SUMO-1 modification of TOP-2 (Bachant et al. 2002). As yet there is no evidence for a SUMO-1 modification of TOP-2 acting in centromere resolution in C. elegans (L. Moore, unpublished results). Thus it is possible that CIN-4 in C. elegans has co-opted this essential function of topoisomerase II. It will be interesting to examine TOP-2 from related organisms such as C. briggsae, which lack a cin-4 gene.
TOP-2 and CIN-4 function:
Both cin-4 and top-2 genes share significant identity with topoisomerase II and are essential, indicating that they each perform necessary topoisomerase II functions. RNAi of TOP-2 inhibited centromere resolution, as did the use of topoisomerase II inhibitor drugs (L. Moore, unpublished data). However, CIN-4 and TOP-2 function differently in centromere resolution in that genetic disruption of cohesin function did not restore centromere resolution in top-2(RNAi) embryos. Topoisomerase II function is necessary to remove catenations from the DNA. Two observations suggest that CIN-4 does not function in decatenation of DNA. First, the CIN-4 protein resembles the 92-kDa fragment of yeast TOP-2, which is not able to decatenate DNA (Lindsley and Wang 1991; Roca 2004). Second, examination of the aligned topoisomerase II protein sequences indicates that CIN-4 has a serine residue instead of the tyrosine residue contained in TOP-2 and topoisomerase II. Others have shown that this tyrosine residue forms a covalent bond between topoisomerase II and DNA during the catalytic cycle and is essential for decatenation (Worland and Wang 1989; Jensen et al. 1996). Thus, the essential decatenation function is likely to be performed by TOP-2 in C. elegans. Given that unresolved catenation of DNA acts as a form of cohesion, unresolved catenations present in top-2(RNAi) embryos might explain why inhibiting cohesin function does not restore centromere resolution when TOP-2 is absent.
As CIN-4 is not likely to alter DNA topology, CIN-4 may regulate cohesin function by its association with DNA. Topoisomerase II is known to associate with a variety of DNA structures involving a four-way junction (Roca et al. 1993; West and Austin 1999; Trigueros et al. 2004). In some models of cohesin function, such DNA structures could potentially block the translocation of cohesin along DNA, resulting in aberrant cohesin localization and thereby preventing the resolution of sister DNAs. Recruitment of topoisomerase II or CIN-4 in C. elegans may then be necessary to resolve these DNA structures and restore resolution. In this model, the presence of a tyrosine, or a serine in CIN-4, at the catalytic site is necessary to form one of the DNA-binding sites (Roca et al. 1996). Alternatively, CIN-4 may recruit factors needed for cohesin removal. An interesting observation is that cohesin was readily detected at centrosomes in cin-4(mr127) embryos. In humans, cohesin can be detected at centrosomes and may be required for centrosome function (Gregson et al. 2001). In wild-type C. elegans embryos, cohesin is not detected at centrosomes or at mitotic chromosomes (Chan et al. 2003; Mito et al. 2003; Moore et al. 2005). Given that in the absence of cin-4 function, localization of cohesin to both sites suggested that cin-4 may directly interact with cohesin either to induce or to prevent a protein modification affecting cohesin localization.
In conclusion, we have identified an essential gene, cin-4, which appears to be a partial duplication of the C. elegans topoisomerase II gene top-2. The cin-4 gene is required to prevent aberrant cohesin localization and inhibition of centromere resolution. As no cin-4-like gene is present in other organisms, we speculate that both these functions may be performed by topoisomerase II in other organisms. In most organisms, a single topoisomerase II is all that is needed. However, in mammals, two topoisomerase II proteins, α and β, with distinct biochemical and localization properties are present, suggesting a division of function (Chung et al. 1989; Drake et al. 1989). The topoisomerase II α isoform is primarily responsible for passage through mitosis, but can be partially substituted by topoisomerase II β (Sakaguchi and Kikuchi 2004). Topoisomerase II β appears to function in regulating developmental gene expression (Ju et al. 2006; Lyu et al. 2006). Similarly, CIN-4 may represent a division of function for topoisomerase II in C. elegans that represents a unique opportunity to investigate the different roles of topoisomerase II in centromere function.
Acknowledgments
The authors thank M. Roth for the temperature-sensitive embryonic lethal mutant bank, J. Loidl for the gift of SCC1/COH-2 antibody, and P. Padilla for critical reading of the manuscript. Some strains used here were provided by the Caenorhabditis Genetics Center. This work was supported by departmental funds to L.L.M.
References
- Albertson, D. G., and J. N. Thomson, 1982. The kinetochores of Caenorhabditis elegans. Chromosoma 86: 409–428. [DOI] [PubMed] [Google Scholar]
- Bachant, J., A. Alcasabas, Y. Blat, N. Kleckner and S. J. Elledge, 2002. The SUMO-1 isopeptidase Smt4 is linked to centromeric cohesion through SUMO-1 modification of DNA topoisomerase II. Mol. Cell 9: 1169–1182. [DOI] [PubMed] [Google Scholar]
- Baumann, C., R. Korner, K. Hofmann and E. A. Nigg, 2007. PICH, a centromere-associated SNF2 family ATPase, is regulated by Plk1 and required for the spindle checkpoint. Cell 128: 101–114. [DOI] [PubMed] [Google Scholar]
- Blat, Y., and N. Kleckner, 1999. Cohesins bind to preferential sites along yeast chromosome III, with differential regulation along arms versus the centric region. Cell 98: 249–259. [DOI] [PubMed] [Google Scholar]
- Blower, M. D., B. A. Sullivan and G. H. Karpen, 2002. Conserved organization of centromeric chromatin in flies and humans. Dev. Cell 2: 319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner, S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner, S., D. Pepper, M. W. Berns, E. Tan and B. R. Brinkley, 1981. Kinetochore structure, duplication, and distribution in mammalian cells: analysis by human autoantibodies from scleroderma patients. J. Cell Biol. 91: 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchwitz, B. J., K. Ahmad, L. L. Moore, M. B. Roth and S. Henikoff, 1999. A histone-H3-like protein in C. elegans. Nature 401: 547–548. [DOI] [PubMed] [Google Scholar]
- Chan, R. C., A. Chan, M. Jeon, T. F. Wu, D. Pasqualone et al., 2003. Chromosome cohesion is regulated by a clock gene paralogue TIM-1. Nature 423: 1002–1009. [DOI] [PubMed] [Google Scholar]
- Chung, T. D., F. H. Drake, K. B. Tan, S. R. Per, S. T. Crooke et al., 1989. Characterization and immunological identification of cDNA clones encoding two human DNA topoisomerase II isozymes. Proc. Natl. Acad. Sci. USA 86: 9431–9435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimini, D., B. Howell, P. Maddox, A. Khodjakov, F. Degrassi et al., 2001. Merotelic kinetochore orientation is a major mechanism of aneuploidy in mitotic mammalian tissue cells. J. Cell Biol. 153: 517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciosk, R., M. Shirayama, A. Shevchenko, T. Tanaka, A. Toth et al., 2000. Cohesin's binding to chromosomes depends on a separate complex consisting of Scc2 and Scc4 proteins. Mol. Cell 5: 243–254. [DOI] [PubMed] [Google Scholar]
- Davis, L. I., and G. Blobel, 1986. Identification and characterization of a nuclear pore complex protein. Cell 45: 699–709. [DOI] [PubMed] [Google Scholar]
- Drake, F. H., G. A. Hofmann, H. F. Bartus, M. R. Mattern, S. T. Crooke et al., 1989. Biochemical and pharmacological properties of p170 and p180 forms of topoisomerase II. Biochemistry 28: 8154–8160. [DOI] [PubMed] [Google Scholar]
- Gandhi, R., P. J. Gillespie and T. Hirano, 2006. Human Wapl is a cohesin-binding protein that promotes sister-chromatid resolution in mitotic prophase. Curr. Biol. 16: 2406–2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshima, G., and M. Yanagida, 2000. Establishing biorientation occurs with precocious separation of the sister kinetochores, but not the arms, in the early spindle of budding yeast. Cell 100: 619–633. [DOI] [PubMed] [Google Scholar]
- Gregson, H. C., J. A. Schmiesing, J. S. Kim, T. Kobayashi, S. Zhou et al., 2001. A potential role for human cohesin in mitotic spindle aster assembly. J. Biol. Chem. 276: 47575–47582. [DOI] [PubMed] [Google Scholar]
- Gruber, S., C. H. Haering and K. Nasmyth, 2003. Chromosomal cohesin forms a ring. Cell 112: 765–777. [DOI] [PubMed] [Google Scholar]
- He, D., and B. R. Brinkley, 1996. Structure and dynamic organization of centromeres/prekinetochores in the nucleus of mammalian cells. J. Cell Sci. 109: 2693–2704. [DOI] [PubMed] [Google Scholar]
- He, X., S. Asthana and P. K. Sorger, 2000. Transient sister chromatid separation and elastic deformation of chromosomes during mitosis in budding yeast. Cell 101: 763–775. [DOI] [PubMed] [Google Scholar]
- Howman, E. V., K. J. Fowler, A. J. Newson, S. Redward, A. C. MacDonald et al., 2000. Early disruption of centromeric chromatin organization in centromere protein A (Cenpa) null mice. Proc. Natl. Acad. Sci. USA 97: 1148–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, J., and D. Moazed, 2006. Sister chromatid cohesion in silent chromatin: each sister to her own ring. Genes Dev. 20: 132–137. [DOI] [PubMed] [Google Scholar]
- Jensen, S., A. H. Andersen, E. Kjeldsen, H. Biersack, E. H. Olsen et al., 1996. Analysis of functional domain organization in DNA topoisomerase II from humans and Saccharomyces cerevisiae. Mol. Cell. Biol. 16: 3866–3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju, B. G., V. V. Lunyak, V. Perissi, I. Garcia-Bassets, D. W. Rose et al., 2006. A topoisomerase IIbeta-mediated dsDNA break required for regulated transcription. Science 312: 1798–1802. [DOI] [PubMed] [Google Scholar]
- Kamath, R. S., and J. Ahringer, 2003. Genome-wide RNAi screening in Caenorhabditis elegans. Methods 30: 313–321. [DOI] [PubMed] [Google Scholar]
- Kamath, R. S., M. Martinez-Campos, P. Zipperlen, A. G. Fraser and J. Ahringer, 2001. Effectiveness of specific RNA-mediated interference through ingested double-stranded RNA in Caenorhabditis elegans. Genome Biol. 2: RESEARCH0002.0001–0002.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katju, V., and M. Lynch, 2003. The structure and early evolution of recently arisen gene duplicates in the Caenorhabditis elegans genome. Genetics 165: 1793–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kueng, S., B. Hegemann, B. H. Peters, J. J. Lipp, A. Schleiffer et al., 2006. Wapl controls the dynamic association of cohesin with chromatin. Cell 127: 955–967. [DOI] [PubMed] [Google Scholar]
- Laloraya, S., V. Guacci and D. Koshland, 2000. Chromosomal addresses of the cohesin component mcd1p. J. Cell Biol. 151: 1047–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengauer, C., K. W. Kinzler and B. Vogelstein, 1998. Genetic instabilities in human cancers. Nature 396: 643–649. [DOI] [PubMed] [Google Scholar]
- Lindsley, J. E., and J. C. Wang, 1991. Proteolysis patterns of epitopically labeled yeast DNA topoisomerase II suggest an allosteric transition in the enzyme induced by ATP binding. Proc. Natl. Acad. Sci. USA 88: 10485–10489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link, C. D., A. Taft, V. Kapulkin, K. Duke, S. Kim et al., 2003. Gene expression analysis in a transgenic Caenorhabditis elegans Alzheimer's disease model. Neurobiol. Aging 24: 397–413. [DOI] [PubMed] [Google Scholar]
- Losada, A., M. Hirano and T. Hirano, 1998. Identification of Xenopus SMC protein complexes required for sister chromatid cohesion. Genes Dev. 12: 1986–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losada, A., M. Hirano and T. Hirano, 2002. Cohesin release is required for sister chromatid resolution, but not for condensin-mediated compaction, at the onset of mitosis. Genes Dev. 16: 3004–3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu, Y. L., C. P. Lin, A. M. Azarova, L. Cai, J. C. Wang et al., 2006. Role of topoisomerase IIbeta in the expression of developmentally regulated genes. Mol. Cell. Biol. 26: 7929–7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuinness, B. E., T. Hirota, N. R. Kudo, J. M. Peters and K. Nasmyth, 2005. Shugoshin prevents dissociation of cohesin from centromeres during mitosis in vertebrate cells. PLoS Biol. 3: 433–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mito, Y., A. Sugimoto and M. Yamamoto, 2003. Distinct developmental function of two Caenorhabditis elegans homologs of the cohesin subunit Scc1/Rad21. Mol. Biol. Cell 14: 2399–2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, L. L., and M. B. Roth, 2001. HCP-4, a CENP-C-like protein in Caenorhabditis elegans, is required for resolution of sister centromeres. J. Cell Biol. 153: 1199–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, L. L., M. Morrison and M. B. Roth, 1999. HCP-1, a protein involved in chromosome segregation, is localized to the centromere of mitotic chromosomes in Caenorhabditis elegans. J. Cell Biol. 147: 471–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, L. L., G. Stanvitch, M. B. Roth and D. Rosen, 2005. HCP-4/CENP-C promotes the prophase timing of centromere resolution by enabling the centromere association of HCP-6 in Caenorhabditis elegans. Mol. Cell. Biol. 25: 2583–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasmyth, K., 2001. Disseminating the genome: joining, resolving, and separating sister chromatids during mitosis and meiosis. Annu. Rev. Genet. 35: 673–745. [DOI] [PubMed] [Google Scholar]
- Oegema, K., A. Desai, S. Rybina, M. Kirkham and A. A. Hyman, 2001. Functional analysis of kinetochore assembly in Caenorhabditis elegans. J. Cell Biol. 153: 1209–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasierbek, P., M. Jantsch, M. Melcher, A. Schleiffer, D. Schweizer et al., 2001. A Caenorhabditis elegans cohesion protein with functions in meiotic chromosome pairing and disjunction. Genes Dev. 15: 1349–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praitis, V., E. Casey, D. Collar and J. Austin, 2001. Creation of low-copy integrated transgenic lines in Caenorhabditis elegans. Genetics 157: 1217–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattner, J. B., M. J. Hendzel, C. S. Furbee, M. T. Muller and D. P. Bazett-Jones, 1996. Topoisomerase II alpha is associated with the mammalian centromere in a cell cycle- and species-specific manner and is required for proper centromere/kinetochore structure. J. Cell Biol. 134: 1097–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder, C. L., and E. D. Salmon, 1998. The vertebrate cell kinetochore and its roles during mitosis. Trends Cell Biol. 8: 310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca, J., 2004. The path of the DNA along the dimer interface of topoisomerase II. J. Biol. Chem. 279: 25783–25788. [DOI] [PubMed] [Google Scholar]
- Roca, J., J. M. Berger and J. C. Wang, 1993. On the simultaneous binding of eukaryotic DNA topoisomerase II to a pair of double-stranded DNA helices. J. Biol. Chem. 268: 14250–14255. [PubMed] [Google Scholar]
- Roca, J., J. M. Berger, S. C. Harrison and J. C. Wang, 1996. DNA transport by a type II topoisomerase: direct evidence for a two-gate mechanism. Proc. Natl. Acad. Sci. USA 93: 4057–4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi, A., and A. Kikuchi, 2004. Functional compatibility between isoform alpha and beta of type II DNA topoisomerase. J. Cell Sci. 117: 1047–1054. [DOI] [PubMed] [Google Scholar]
- Salic, A., J. C. Waters and T. J. Mitchison, 2004. Vertebrate shugoshin links sister centromere cohesion and kinetochore microtubule stability in mitosis. Cell 118: 567–578. [DOI] [PubMed] [Google Scholar]
- Stear, J. H., and M. B. Roth, 2002. Characterization of HCP-6, a C. elegans protein required to prevent chromosome twisting and merotelic attachment. Genes Dev. 16: 1498–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strome, S., 1993. Determination of cleavage planes. Cell 72: 3–6. [DOI] [PubMed] [Google Scholar]
- Sumara, I., E. Vorlaufer, P. T. Stukenberg, O. Kelm, N. Redemann et al., 2002. The dissociation of cohesin from chromosomes in prophase is regulated by Polo-like kinase. Mol. Cell 9: 515–525. [DOI] [PubMed] [Google Scholar]
- Tanaka, T., J. Fuchs, J. Loidl and K. Nasmyth, 2000. Cohesin ensures bipolar attachment of microtubules to sister centromeres and resists their precocious separation. Nat. Cell Biol. 2: 492–499. [DOI] [PubMed] [Google Scholar]
- Toth, A., R. Ciosk, F. Uhlmann, M. Galova, A. Schleiffer et al., 1999. Yeast cohesin complex requires a conserved protein, Eco1p(Ctf7), to establish cohesion between sister chromatids during DNA replication. Genes Dev. 13: 320–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trigueros, S., J. Salceda, I. Bermudez, X. Fernandez and J. Roca, 2004. Asymmetric removal of supercoils suggests how topoisomerase II simplifies DNA topology. J. Mol. Biol. 335: 723–731. [DOI] [PubMed] [Google Scholar]
- West, K. L., and C. A. Austin, 1999. Human DNA topoisomerase IIbeta binds and cleaves four-way junction DNA in vitro. Nucleic Acids Res. 27: 984–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worland, S. T., and J. C. Wang, 1989. Inducible overexpression, purification, and active site mapping of DNA topoisomerase II from the yeast Saccharomyces cerevisiae. J. Biol. Chem. 264: 4412–4416. [PubMed] [Google Scholar]
- Zalevsky, J., A. J. MacQueen, J. B. Duffy, K. J. Kemphues and A. M. Villeneuve, 1999. Crossing over during Caenorhabditis elegans meiosis requires a conserved MutS-based pathway that is partially dispensable in budding yeast. Genetics 153: 1271–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X., and C. E. Walczak, 2006. Chromosome segregation: correcting improperly attached chromosomes. Curr. Biol. 16: R677–R679. [DOI] [PubMed] [Google Scholar]