Abstract
Embryonic development and normal growth require exquisite control of insulin-like growth factors (IGFs). In mammals the extracellular region of the cation-independent mannose-6-phosphate receptor has gained an IGF-II-binding function and is termed type II IGF receptor (IGF2R). IGF2R sequesters IGF-II; imbalances occur in cancers and IGF2R is implicated in tumour suppression. We report crystal structures of IGF2R domains 11–12, 11–12–13–14 and domains 11–12–13/IGF-II complex. A distinctive juxtaposition of these domains provides the IGF-II-binding unit, with domain 11 directly interacting with IGF-II and domain 13 modulating binding site flexibility. Our complex shows that Phe19 and Leu53 of IGF-II lock into a hydrophobic pocket unique to domain 11 of mammalian IGF2Rs. Mutagenesis analyses confirm this IGF-II ‘binding-hotspot', revealing that IGF-binding proteins and IGF2R have converged on the same high-affinity site.
Keywords: growth factor receptor, IGF-II, IGF system, protein crystallography, tumour suppression
Introduction
The insulin-like growth factor (IGF)-system includes insulin-like growth factors I and II (IGF-I and IGF-II) along with the type I (IGF-1R) and type II (IGF2R) cell-surface receptors, the insulin receptor (IR) and circulating IGF-binding proteins (IGFBPs) (Denley et al, 2005). The biological actions of the IGFs are mediated by IGF-1R and IR, leading to cell growth, differentiation and survival. Their distribution and activity is controlled via high-affinity association with IGFBPs, and the binding sites on IGFs for IGFBPs have been delineated in detail by structural studies (Headey et al, 2004; Carrick et al, 2005; Sitar et al, 2006). In mammals, the activity of IGF-II (but not IGF-I) is further moderated by IGF2R, which sequesters IGF-II for internalization and degradation. IGF2R is classed as a growth inhibitor, with loss of function causing increased growth (Foulstone et al, 2005). In line with this, Igf2r is a putative tumour suppressor gene and mutations have been found in several cancers (reviewed in Falls et al, 1999). Unusually high levels of circulating IGF-II and simultaneous downregulation of IGF2R are correlated with the growth of human and murine tumours (Toretsky and Helman, 1996; Hassan and Howell, 2000). Hence, disruption of IGF-II action is a potential method of tumour control, with the natural scavenging action of IGF2R an obvious tool for such intervention.
IGF2R, also known as the cation-independent mannose-6-phosphate receptor, is found ubiquitously in human tissues with a truncated soluble form of the receptor present in the circulation (Lobel et al, 1988; Oshima et al, 1988). Full-length, 300-kDa, IGF2R comprises a large N-terminal extracellular region of 15 homologous domains, a single membrane-spanning region and a small cytoplasmic tail. In addition to IGF-II binding, major IGF2R functions include sorting newly synthesized lysosomal enzymes and endocytosis of extracellular lysosomal enzymes (reviewed in Ghosh et al, 2003). To perform these disparate functions, the extracellular region contains binding sites for IGF-II and phosphomannosyl residues (Oshima et al, 1988; Schmidt et al, 1995).
Domain 11, the first in the IGF2R extracellular region to be characterized by X-ray crystallography, contains the putative IGF-II-binding site (Schmidt et al, 1995; Brown et al, 2002). Mannosylated proteins are bound by domains 3, 5 and 9 (Reddy et al, 2004). Sequence alignments suggest that all 15 domains share the same β-barrel architecture and the crystal structure of a domain 1–3 fragment supports this hypothesis (Olson et al, 2004). The only large deviation from the canonical repeat occurs in domain 13, where sequence analysis predicts that an insertion forms an independent module with a fibronectin type II fold (FNII). Domain 13 enhances IGF-II binding, an effect believed in someway to be mediated by FNII (Lobel et al, 1988), resulting in a reduction in the rate of IGF-II release (Devi et al, 1998; Linnell et al, 2001).
An Ile1572Thr mutation in IGF2R domain 11 essentially abrogates IGF-II binding (Garmroudi et al, 1996; Linnell et al, 2001). The crystal structure of domain 11 mapped Ile1572 to a hydrophobic pocket, which was therefore proposed as the putative IGF-II-binding site (Brown et al, 2002); subsequently a cluster of residues lining this pocket have been implicated in IGF-II binding by mutational analyses (Zaccheo et al, 2006). In contrast, a series of mutational analyses of IGF-II have highlighted effects on IGF2R binding for a disparate set of residues (Burgisser et al, 1991; Sakano et al, 1991; Delaine et al, 2007). Sequence comparisons across species potentially harbour additional functional insights. IGF2R gained IGF-II-binding activity coincident with the evolution of placental development; the cation-independent mannose-6-phosphate receptors of non-mammalian species, including monotremes such as platypus, as well as the evolutionarily more distant chicken and Xenopus, are unable to bind IGF-II, but those of marsupials and mammals can (Killian et al, 2000). Thus, there is a relative wealth of functional data to relate to the IGF-II/IGF2R interaction; however, the lack of a definitive structural context has hindered development of a robust understanding of this system.
Major questions regarding the IGF-II/IGF2R interaction remain unanswered, including precise definition of the residues forming the interface and understanding the role of domain 13 in raising the affinity for IGF-II. We still do not understand how the structurally homologous domains are arranged in the full-length receptor and whether any rules drive this tertiary assembly. To address these questions, we report the crystal structure of a complex between IGF-II and domains 11–13 of IGF2R, complemented by crystal structures of IGF2R domains 11–12 and 11–12–13–14 in isolation. We have validated our structure-based hypotheses with additional functional analyses of the IGF-II/IGF2R interaction using surface plasmon resonance and mutation of both IGF-II and IGF2R. Our conclusions allow us to analyse the IGF-II/IGF2R interaction as an example of evolutionary gain-of-function, and to uncover features common to IGF-II/IGFBP and IGF-II/IGF2R binding. The latter insights provide potentially important caveats to consider in the development of novel anti-cancer therapies.
Results
The structures of IGF2R fragments and the IGF2R/IGF-II complex
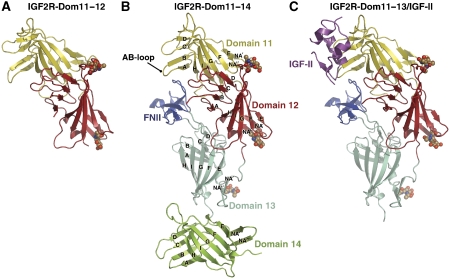
Three multi-domain IGF2R fragments were generated: IGF2R-Dom11–12 (domains 11 and 12), IGF2R-Dom11–13 (domains 11, 12 and 13) and IGF2R-Dom11–14 (domains 11, 12, 13 and 14). Crystal structure determinations were achieved for IGF2R-Dom11–12 (3.2 Å resolution), IGF2R-Dom11–14 (2.9 Å resolution) and for an IGF2R-Dom11–13/IGF-II complex (4.1 Å resolution) (summarized in Materials and methods; Table I and Supplementary Table SI). As expected, the structures of domains 12, 13 and 14 of IGF2R closely resemble that of IGF2R-Dom11 (Figure 1; Supplementary Figure S1A). The root-mean-squared deviation between the domains for which structures are now available (1, 2, 3, 11, 12, 13 and 14) ranges between 1.8 and 2.5 Å for an average of 125 Cα equivalences (Supplementary Table SII). The core domain structure is a flattened β-barrel consisting of nine β-strands, termed βA–βI forming two crossed β-sheets, the first formed by βA–βD and the second by βE–βI. Each of domains 11–14 is labelled with the standard-strand nomenclature in Figure 1B. The N-terminal region preceding βA contains two additional β-strands (βNA′ and βNA″), which form a β-hairpin capping off the β-barrel. Each domain contains four conserved disulphide bonds, with domain 13 having an extra two disulphide bonds in the FNII insert. FNII may be defined as a 48-residue insert spanning Glu1896 to Arg1943, which adopts the typical FNII fold (Figure 1B and C; Supplementary Figure S1B); four β-strands form two sheets of two antiparallel β-strands stabilized by two disulphide bonds. In addition, the first two FNII residues form a third β-strand in the lower β-sheet and a short α-helix packs against the top β-sheet.
Table 1.
Statistics for crystallographic data collection and refinement
| Multiple anomalous dispersion data | Peak | Inflection | Remote |
|---|---|---|---|
| Resolution range (Å) | 30.0–3.2 | 30.0–3.2 | 30.0–3.2 |
| P6122 cell dimensions (Å) | 64.7, 64.7, 269.5 | 64.8, 64.8, 269.9 | 64.8, 64.8, 269.7 |
| Wavelength (Å) | 0.9789 | 0.9791 | 0.8856 |
| Unique reflectionsa | 6204 (592)b | 6183 (594)b | 6147 (581)b |
| Completeness (%)a | 99.9 (100) | 99.1 (100) | 100 (100) |
| I/σIa | 27.0 (3.8) | 40.0 (5.0) | 44.0 (5.2) |
| Redundancya | 14.9 (15.5) | 15.7 (16.7) | 19.7 (20.6) |
| Rmerge (%)a | 9.2 (69.5) | 7.9 (81.3) | 8.4 (79.0) |
| Native data | IGF2R-Dom11–12 | IGF2R-Dom11–14 | Complex |
| Data collection | |||
| Resolution range (Å)a | 20–3.2 (3.3–3.2) | 30–2.9 (3.0–2.9) | 50–4.1 (4.2–4.1) |
| Space group | P6122 | C2 | C2 |
| Cell dimensions (Å) | 64.8, 64.8, 269.6 | 138.8, 69.3, 97.5; β=103.5° | 165.9, 116.9, 116.6; β=123.4° |
| Wavelength (Å) | 0.9789 | 0.931 | 0.933 |
| Unique reflections | 6123 | 19 919 | 14 662 |
| Completeness (%)a | 99.5 (96.6) | 98.6 (92.8) | 99.0 (99.8) |
| I/σIa | 25.5 (4.9) | 13.9 (3.0) | 5.4 (2.5) |
| Redundancya | 19.6 (19.5) | 3.7 (3.6) | 3.7 (3.7) |
| Rmerge (%)a | 9.4 (74.4) | 7.7 (49.9) | 28.8 (54.5) |
| Refinement | |||
| No. of reflections | 5495 | 18 893 | 13 919 |
| Rfactor/Rfree (%) | 25.3/31.7 | 25.7/30.4 | 29.1/32.7 |
| Non-hydrogen protein atoms | 2017 | 4642 | 7880 |
| Non-hydrogen sugar atoms | 28 | 42 | 109 |
| R.m.s.d bonds (Å) | 0.007 | 0.007 | 0.006 |
| R.m.s.d angles (deg) | 1.132 | 1.164 | 0.906 |
| Overall B-factor (Å2) | 97.6 | 65.8 | 90.4 |
| Ramachandran plot (%)c | 80.6/17.6/1.8/0 | 79.7/18.3/1.9/0 | 78.9/19.0/2.1/0 |
| aValues in parentheses correspond to the highest-resolution data shell. | |||
| bFriedel pairs are treated as different reflections. | |||
| cCalculated using PROCHECK (core/allowed/generously allowed/disallowed regions). | |||
Figure 1.
Cartoon representations of IGF2R fragment structures. (A) IGF2R-Dom11–12. (B) IGF2R-Dom11–14. (C) The complex between IGF2R-Dom11–13 and IGF-II. Glycans are shown as spheres and strand labelling is given in panel B.
A unique juxtaposition of domains for IGF-II binding
Domains 11–13 pack into a compact arrangement, which concurs with this section of IGF2R forming the high-affinity IGF-II-binding site (Figure 1; Supplementary Figure S2). The most distinctive feature, the FNII insert, projects out from the FG-loop of domain 13 to nestle beneath the AB-loop of domain 11, making multiple contacts with both domains 11 and 12. Despite its importance in high-affinity IGF-II binding, FNII does not directly contact IGF-II in the complex.
Approximately 4100 Å2 of surface area is buried at interfaces in the IGF2R-Dom11–14 structure. The largest interface buries ∼1330 Å2 between domains 11 and 12, where the domain 12 BC-loop packs against the domain 11 βE–βI sheet. Many residues previously identified as forming a hydrophobic patch on the isolated IGF2R-Dom11 surface are found in this interface (Brown et al, 2002). A further ∼730 Å2 are buried where the domain 11 AB and GH-loops interface with FNII. The domain 12–13 interface is slightly smaller than that between 11 and 12, burying ∼1160 Å2 where FNII packs against the domain 12 NA″A and BC-loops. The smaller interface of ∼890 Å2 between domains 13 and 14 involves mainly the domain 13 NA′–NA″ hairpin loop and the DE-loop and βE of domain 14.
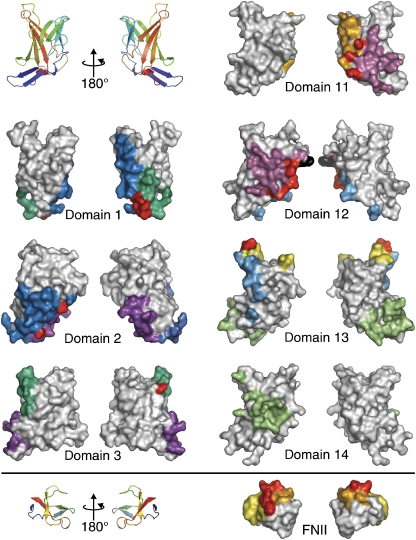
Structural superposition shows the relative orientations of domains 11 and 12 in the three- and four-domain fragments to be conserved from the IGF2R-Dom11–12 structure, implying that this is a comparatively rigid two-domain unit. Furthermore, an overlay of IGF2R-Dom11–13 from the complex with IGF2R-Dom11–14 gives a close match, indicating that this rigidity extends to the tri-domain 11–13 unit. The relative orientations of domains 11–14 as multi-domain assemblies do not resemble the tri-domain motif reported for domains 1–3 (Olson et al, 2004), and there is little commonality of surface use in assembly, despite conservation of the core fold (Figure 2).
Figure 2.
Interface regions in IGF2R. ‘Front' and ‘back' surface views are shown of each IGF2R domain so far crystallized as part of a multi-domain fragment. Cartoon representations are included for orientation purposes. Coloured patches highlight regions involved in interfaces with other domains, with corresponding patches coloured the same. Residues involved in interfaces are defined as those undergoing a change in ASA. Red patches represent residues with ASA buried in more than one interface.
Molecular details of the IGF-II/IGF2R interaction
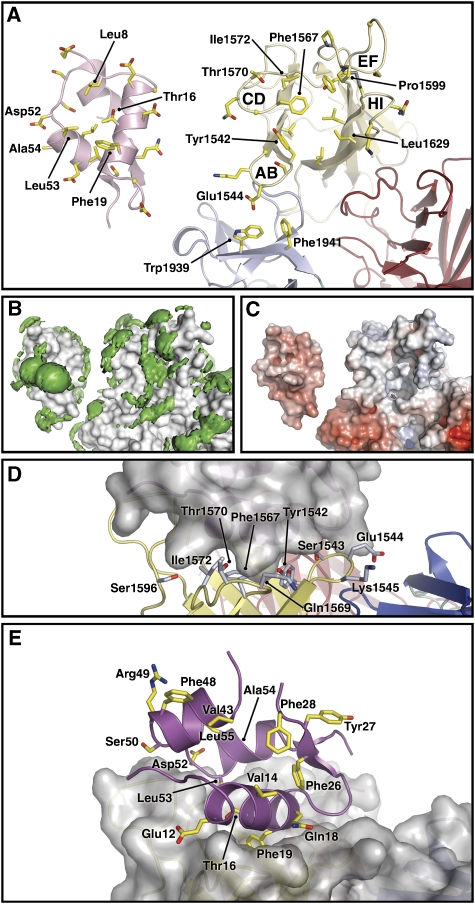
The complex structure described in this paper provides a good ‘coarse-grained' model for analysing the interaction between IGF-II and IGF2R; however, the resolution of 4.1 Å is insufficient for detailed interpretation of side-chain placement and explicit bonding information should not be inferred. IGF-II bound to IGF2R buries ∼750 Å2 of solvent-accessible surface area (ASA) on IGF-II and ∼710 Å2 on IGF2R (Figure 3; Supplementary Figure S3). Analysis of the interface indicates that IGF2R residues burying ASA at the interface are almost all contained in the domain 11 AB-, CD-, FG- and HI-loops (Figure 3A; Supplementary Figure S3). At the core, a hydrophobic cluster (Figure 3B) comprising Tyr1542, Phe1567 and Leu1629 of IGF2R surrounds Phe19 of IGF-II. Only domain 11 directly contacts IGF-II; minor ASA changes for Trp1939 and Phe1941 in FNII reflect their proximity to IGF-II side chains. At the binding site, IGF-II has an overall negatively charged surface and IGF2R an overall positively charged surface (Figure 3C). The involvement of Tyr1542, Glu1544, Phe1567, Thr1570 and Ile1572 is consistent with mutational studies, which implicate these side chains in IGF-II binding (Zaccheo et al, 2006; Figure 3D).
Figure 3.
Views of the IGF2R-Dom11–13/IGF-II complex and summaries of prior mutagenesis studies. (A) An open-book style view in cartoon representation. Side chains undergoing ASA change upon complexation are shown as sticks and key side chains are labelled. (B) Hydrophobic patches (green) on the surface, defined using the programme GRID (Goodford, 1985) and shown here as volumes of pseudo-energies contoured at −2.3 kcal/mole. (C) The electrostatic potential surface, produced using the APBS Tools plug-in for Pymol (Baker et al, 2001) and contoured ±10 kT (blue denotes positive and red negative potential). (D) IGF2R side chains in the proposed IGF-II-binding region, which have been mutagenized (Zaccheo et al, 2006). IGF2R is shown in cartoon representation and IGF-II as surface representation. (E) IGF-II side chains mutagenized before this study (note Ala54 is not visible). IGF-II is shown in cartoon representation and IGF2R as surface representation.
For IGF-II, Phe19 undergoes the largest change in ASA (Supplementary Figure S3) and is the anchor residue of the interaction. This concurs with previous mutagenesis studies of IGF-II, which demonstrated that Thr16, Phe19, Asp52 and Leu53 are critical for IGF2R binding (Delaine et al, 2007). Of these, Thr16 has been highlighted as central to the markedly different binding affinities of the IGFs for IGF2R. Our structure shows that Thr16 is buried in the binding interface where mutation to Ala as in IGF-I most likely causes significant loss of interface interactions.
Of the other interface side chains differing between IGF-II and IGF-I for which mutation data are available, Leu55Arg causes a 3.3-fold drop in affinity while Ala54Arg causes a fourfold drop (Forbes et al, 2001). The complex structure shows that Ala54 does indeed lie within the interface, whereas Leu55 does not appear to be involved. Both Ala54 and Leu55 are close to the crucial Leu53 side chain (Delaine et al, 2007) and so mutation introducing large charged side chains could induce local conformational changes responsible for the observed reduction in IGF2R binding affinity.
Our crystal structure indicates that prior attempts to predict this complex in silico did not provide an accurate model (Roche et al, 2006). The direct crystallographic insights into the interface architecture detailed above provided fresh impetus for us to evaluate the IGF-II/IGF2R interaction by using mutagenesis to probe further IGF-II side-chain involvement and to analyse the contribution of FNII.
IGF2R binding of novel IGF-II mutations
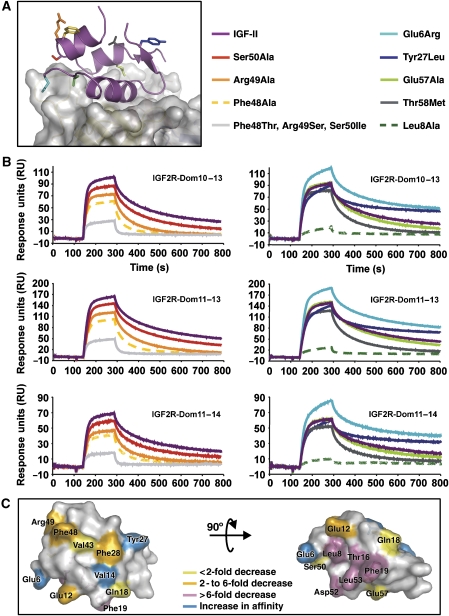
Our complex structure showed that the residues Phe48, Arg49 and Ser50 are not close to the binding interface (Figure 3E). This observation contradicts previous reports suggesting that these residues are critical for IGF2R binding (Burgisser et al, 1991; Sakano et al, 1991). We therefore decided to make single alanine mutations of these residues and to remake the Phe48Thr, Arg49Ser, Ser50Ile IGF-II reported in the previous studies, and performed surface plasmon resonance using our IGF2R multi-domain fragments (IGF2R-Dom10–13, IGF2R-Dom11–13 and IGF2R-Dom11–14). All mutants retained the native IGF-II fold as measured by circular dichroism and were active in IGF-1R binding assays (data not shown), except Tyr27Leu IGF-II, which has previously been characterised (Sakano et al, 1991). Sensorgrams and affinities are presented in Figure 4 and Table II, respectively.
Figure 4.
Novel IGF-II mutations. (A) A view of the IGF-II/IGF2R-Dom11–13 complex with side chains investigated by mutagenesis shown as sticks and coloured according to the legend. (B) BIAcore analyses of IGF-II and analogues (representative curves each at 50 nM) binding to multi-domain IGF2R fragments. Curves are coloured as in the legend to panel A. (C) Summary of all IGF-II mutagenesis studies, which used IGF2R fragments. IGF-II side chains are coloured according to their effect on IGF2R binding (see legend).
Table 2.
BIAcore kinetic analysis of ligand binding by IGF2R fragments
| ka1 (1/Ms) ( × 105) | kd1 (1/s) ( × 10−2) | ka2 (1/Ms) ( × 10−3) | kd2 (1/s) ( × 10−3) | KA (1/M) ( × 108) | Relative KA | χ2 | |
|---|---|---|---|---|---|---|---|
| (A) IGF-II analogues | |||||||
| IGF2R-Dom10–13 | |||||||
| IGF-II | 9.02 | 1.28 | 3.43 | 1.44 | 2.49 | 1.00 | 2.1 |
| Phe48Ala | 6.54 | 3.22 | 2.19 | 0.66 | 0.93 | 0.37 | 0.9 |
| Arg49Ala | 9.55 | 2.08 | 1.62 | 1.76 | 0.91 | 0.37 | 1.2 |
| Ser50Ala | 11.25 | 1.71 | 3.10 | 1.77 | 1.84 | 0.74 | 3.4 |
| Phe48Thr, Arg49Ser, Ser50Ile | 4.66 | 6.02 | 3.61 | 0.52 | 0.58 | 0.23 | 0.6 |
| Glu57Ala | 9.38 | 1.56 | 3.31 | 1.76 | 1.90 | 0.76 | 2.7 |
| Thr58Met | 11.13 | 2.29 | 2.35 | 1.52 | 1.27 | 0.51 | 2.7 |
| Leu8Ala | 2.87 | 6.25 | 6.05 | 0.63 | 0.56 | 0.22 | 0.5 |
| Glu6Arg | 9.16 | 1.20 | 4.47 | 0.76 | 5.35 | 2.15 | 2.5 |
| Tyr27Leu | 5.81 | 1.98 | 5.13 | 0.53 | 3.97 | 1.59 | 1.7 |
| IGF2R-Dom11–13 | |||||||
| IGF-II | 6.85 | 1.28 | 3.85 | 1.48 | 2.01 | 1.00 | 8.1 |
| Phe48Ala | 5.50 | 2.58 | 1.90 | 0.92 | 0.72 | 0.36 | 2.1 |
| Arg49Ala | 6.48 | 1.88 | 2.08 | 2.34 | 0.66 | 0.33 | 6.1 |
| Ser50Ala | 9.43 | 1.67 | 3.61 | 1.82 | 1.77 | 0.88 | 11 |
| Phe48Thr, Arg49Ser, Ser50Ile | 3.84 | 5.30 | 3.20 | 0.58 | 0.53 | 0.26 | 1.2 |
| Glu57Ala | 7.42 | 1.55 | 3.84 | 1.80 | 1.82 | 0.90 | 1 |
| Thr58Met | 8.90 | 1.93 | 2.57 | 1.83 | 1.18 | 0.59 | 4.2 |
| Leu8Ala | 2.36 | 7.01 | 5.75 | 0.69 | 0.31 | 0.15 | 9.7 |
| Glu6Arg | 7.55 | 1.09 | 4.56 | 0.92 | 4.28 | 2.13 | 13 |
| Tyr27Leu | 5.24 | 1.66 | 4.71 | 0.67 | 2.92 | 1.45 | 2.6 |
| IGF2R-Dom11–14 | |||||||
| IGF-II | 6.92 | 1.40 | 3.81 | 1.45 | 1.82 | 1.00 | 1.7 |
| Phe48Ala | 3.51 | 2.82 | 2.22 | 0.87 | 0.51 | 0.28 | 1.1 |
| Arg49Ala | 6.33 | 2.06 | 2.03 | 1.95 | 0.64 | 0.35 | 1.1 |
| Ser50Ala | 8.28 | 1.57 | 3.19 | 1.55 | 1.61 | 0.89 | 2 |
| Phe48Thr, Arg49Ser, Ser50Ile | 1.14 | 4.88 | 3.19 | 0.74 | 0.12 | 0.07 | 0.7 |
| Glu57Ala | 7.72 | 1.63 | 3.71 | 1.73 | 1.65 | 0.90 | 1.8 |
| Thr58Met | 9.66 | 2.28 | 2.71 | 1.53 | 1.18 | 0.65 | 1.5 |
| Leu8Ala | 0.79 | 4.30 | 6.49 | 0.66 | 0.22 | 0.12 | 6.3 |
| Glu6Arg | 6.98 | 1.26 | 5.04 | 0.75 | 4.30 | 2.36 | 4.4 |
| Tyr27Leu | 4.90 | 1.75 | 5.48 | 0.57 | 3.48 | 1.91 | 1.3 |
| (B) IGF2R-Dom10–13 mutants | |||||||
| Wild type | 19.0 | 0.04 | 0.05 | 0.02 | 142 | 1.00 | 2.1 |
| ΔFNII | 15.3 | 4.48 | 6.99 | 0.17 | 14.3 | 0.10 | 0.5 |
| Glu1544Lys | 16.2 | 0.31 | 2.46 | 1.36 | 14.9 | 0.10 | 1.2 |
| Glu1544Lys+ΔFNII | 23.4 | 0.63 | 2.34 | 0.17 | 56.1 | 0.40 | 0.5 |
| FNII, fibronectin type II insert; IGF-II, insulin-like growth factor II; IGF2R, type II IGF receptor. | |||||||
| Association rate constants (ka), dissociation rate constants (kd) and association equilibrium constants (KA=ka/kd) are given. Relative association equilibrium constants form the basis of discussion in the text and are highlighted in bold (either KA IGF-II analogue/KA IGF-II or KA IGF2R mutant/KA IGF2R wild type). χ2 values are an average of values for all experiments with each analogue/mutant. | |||||||
| (A) IGF-II analogues binding to multi-domain IGF2R fragments and (B) IGF2R mutants binding to IGF-II. BIAcore curves were fitted using a two-state conformational change model as outlined in the Materials and methods. | |||||||
Our Phe48Ala and Arg49Ala mutants display a 2.7- to 3.6-fold reduction in affinity for the multi-domain IGF2R fragments compared with wild-type IGF-II, and the Ser50Ala mutant no more than a 1.4-fold reduction in binding affinity. Our triple mutant where the equivalent insulin residues are substituted into IGF-II had a more significant effect, with a 4.3-fold (IGF2R-Dom10–13), 3.8-fold (IGF2R-Dom11–13) and 14.3-fold (IGF2R-Dom11–14) reduction in binding affinity. However, this is a much smaller effect than previously reported for the Phe48Thr, Arg49Ser, Ser50Ile IGF-II mutant (Sakano et al, 1991; Bach et al, 1993), and our new mutagenesis and structural data demonstrate that these side chains are not crucial determinants of IGF2R binding affinity, but perhaps indirectly influence IGF2R binding.
Further mutants predicted to lie within the binding interface were also tested for IGF2R binding. Mutation of Leu8 to Ala has a significant effect, resulting in up to an 8.3-fold reduction in IGF2R binding affinity. Our complex structure reveals that Leu8 is buried in the IGF-II:IGF2R interface in a predominantly hydrophobic environment. Mutation to Ala would lead to a smaller hydrophobic contact area, thus providing an explanation for the observed reduction in binding affinity.
Neither of Glu57Ala IGF-II, Thr58Met IGF-II or Tyr27Leu IGF-II mutants differed greatly from IGF-II in their IGF2R binding affinities. Interestingly, Glu57 is partially buried in the IGF-II/IGF2R complex and there is a significant difference in ASA upon binding (Figure 3; Supplementary Figure S3), but evidently substitution with Ala can be tolerated. Thr58 and Tyr27 are not part of the interface as seen in the complex structure (Figures 3 and 4), and this observation correlates nicely with the mutagenesis data. Tyr27 is crucial for IGF-1R binding and is not situated near the recently described IGF2R-binding surface (Delaine et al, 2007).
Glu6Arg IGF-II shows an increase in IGF2R binding affinity of over twofold compared with IGF-II. The aliphatic portion of the Glu6 side chain is partially buried in the complex close to the FG-loop of domain 11 and mutation to Arg could conserve this interaction, while introducing new hydrogen bonds via its side-chain nitrogen atoms.
Taken together with previous IGF-II mutation studies using the same IGF2R fragments (Delaine et al, 2007), these results give an overall view of the impact of IGF-II mutations on IGF2R binding (Figure 4C). While mutations at the interface drastically affect IGF2R binding, mutating more remote side chains can still influence IGF2R binding affinity, although more subtly than previously reported. The IGFs are relatively small polypeptide hormones and their core structure is perhaps more susceptible to changes in surface-exposed side-chain arrangements than larger proteins. Such changes could be transmitted to other regions of the protein. Although discussion of indirect mechanisms is speculative, it is obvious that mutations of surface-exposed IGF-II residues not directly involved in IGF-II binding can influence binding affinity.
Role of FNII in high-affinity IGF-II binding
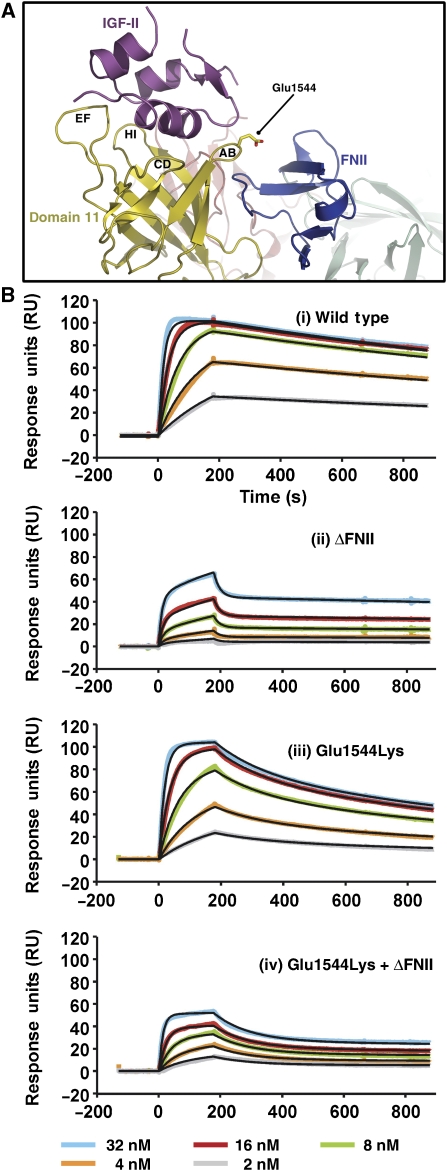
Our structure shows that FNII does not directly contact IGF-II, but rather interfaces with the AB-loop of domain 11 (Figure 5A). Also, an AB-loop mutation in isolated domain 11, Glu1544Lys, increased the IGF-II affinity of domain 11 by sixfold (Zaccheo et al, 2006). The flexibility of this loop is likely to be greater in isolated domain 11, where the interface with FNII is absent. The complex suggests that Glu1544Lys in isolated domain 11 might enhance affinity by introducing an electrostatically favourable interaction with Asp20 of IGF-II.
Figure 5.
Interactions between FNII and the AB-loop of domain 11. (A) A view of the IGF-II/IGF2R-Dom11–13 complex illustrating the proximity of the domain 11 AB-loop to FNII. Glu1544 is in stick form. (B) BIAcore analyses investigating the role of FNII and Glu1544Lys in IGF-II binding. Serial dilutions of IGF-II were passed over immobilized IGF2R-Dom10–13; a wild-type fragment, an FNII deletion fragment (ΔFNII), a Glu1544Lys fragment and an FNII deletion plus Glu1544Lys fragment. Line fits are shown in black.
We have further investigated the effects of FNII and the Glu1544Lys mutation on IGF-II binding by undertaking surface plasmon resonance studies. Sensorgrams are shown in Figure 5 and calculated affinities are in Table II. Four IGF2R-Dom10–13 fragments were used; wild type, Glu1544Lys, FNII deletion (ΔFNII) and ΔFNII with Glu1544Lys. As previously reported (Devi et al, 1998), deletion of FNII markedly reduces IGF-II binding, causing a 10-fold drop in KA (Figure 5B(i) and (ii)). In isolated domain 11, Glu1544Lys causes a significant increase in IGF-II affinity (Zaccheo et al, 2006), whereas in IGF2R-Dom10–13 it has a negative impact, reducing KA 10-fold (Figure 5B(i) and (iii)). However, in the absence of FNII, Glu1544Lys does increase the affinity fourfold compared with ΔFN alone (Figure 5B(ii) and (iv)), confirming the structural observations that FNII interacts with the AB-loop of domain 11.
While not directly involved in IGF-II binding, non-covalent interactions of FNII with domains 11 and 12 contribute indirectly to the formation of the high-affinity IGF-II-binding site by stabilizing the AB-loop of domain 11 (Figure 5A). Loss of high-affinity IGF-II binding upon FNII deletion is probably a consequence of increased AB-loop flexibility raising the entropic barrier to complex formation. FNII is an integral part of the IGF2R structure, but given the lack of apparent similarities in the tertiary assembly of homologous IGF2R domains (Figure 2), it is not clear how FNII absence might affect the overall arrangement. One potential effect would be a similar overall structure, but with increased flexibility in the region spanning domains 11–13.
Using ESCET to compare different X-ray crystallographic structures of the same protein can give insights into flexible and conformationally invariant regions of the molecule (Schneider, 2002). Analysis of isolated IGF2R-Dom11 (PDB Code 1GP0) alongside domain 11 from our two-domain, four-domain and complex structures suggested that residues in the AB, CD, FG and GH-loops are flexible (Supplementary Figure S4A). However, the AB and GH loops appear more rigid in the three- and four-domain structures where they both interface with FNII. Hence, of the loops involved in IGF-II binding, the CD- and FG-loops may have the most, albeit limited, flexibility.
Using ESCET to compare domains 11–13 between the IGF2R-Dom11–14 and IGF-II/IGF2R-Dom11–13 structures reveals a small rigid-body movement between bound and unbound forms of the receptor. The analysis shows that the domain 12–13 unit (including FNII) remains unchanged between the two structures. We therefore superposed domains 12–13 of IGF2R-Dom11–14 onto IGF2R-Dom11–13 from our complex in order to visualize the rigid-body shift. Upon IGF-II-binding, domain 11 undergoes a slight rotation of 5° towards the IGF-II-binding site (Supplementary Figure S4B). The reasonable amount of space around the rigid-body shift region with respect to crystal packing in both crystal structures suggests that the shift is not due to crystal packing forces. Overall, these observations suggest that there is no major conformational change, that is, induced-fit, in this ligand–receptor binding system.
Implications for the overall structure and function of IGF2R
IGF2R forms homodimers at the cell surface independent of ligand binding, with multiple interactions occurring along the extracellular length of the receptor (Byrd et al, 2000; Kreiling et al, 2005). Domain 12 is proposed to be functionally important to dimerization. Inspection of the crystal packing in IGF2R-Dom11–14 and the IGF2R-Dom11–13/IGF-II complex crystals revealed similar arrangements, where two domain 12s in neighbouring molecules interface with each other, suggestive of dimerization. In both structures, the EF-loop of domain 12 packs against the corresponding region of a symmetry-related molecule. This region is a typical protein surface, with hydrophobic character from side chains including Pro1735 and Tyr1741, but also with charged patches from Glu1739 amongst others. This interface overlaps with the dimerization face of the cation-dependent mannose-6-phosphate receptor (Roberts et al, 1998). The equivalent crystal packing interaction is absent from the IGF2R-Dom11–12 crystals; instead, the hydrophobic IGF-II-binding site of domain 11 packs against the domain 12 βE–βI sheet. We used analytical ultracentrifugation to investigate association behaviour in our IGF2R-Dom11–12 and IGF2R-Dom11–13 fragments. Sedimentation velocity experiments modelled with Gaussian fits demonstrated that although the primary species in both cases was a monomer, there was evidence for a protein species of a size consistent with dimerization (Supplementary Figure S5A), corroborating previous results using various IGF2R fragments (Kreiling et al, 2005). The relative amount of dimer varied between 1 and 4% at the concentrations tested, values which correlate well with the 0–15% dimer observed in studies using full-length IGF2R (Byrd et al, 2000). Furthermore, analysis of c(s,fr) distributions (Brown and Schuck, 2006), where s is the sedimentation coefficient and fr is the frictional ratio, clearly demonstrates two species for IGF2R-Dom11–13 at ∼60 and ∼120 kDa, corresponding to monomer and dimer, respectively (Supplementary Figure S5B). This analysis, unlike the more sensitive sedimentation velocity analysis, does not show a clear dimer species for IGF2R-Dom11–12, but does strongly support the identification of the second, smaller peak in the plots shown in Supplementary Figure S5A as a dimer of the major peak in each case. While IGF2R-Dom11–12 and IGF2R-Dom11–13 fragments only weakly dimerize in our AUC experiments, additional interactions in full-length receptors would favour dimerization, potentially via avidity effects.
We and others have previously proposed structures for full-length IGF2R. Our model of alternating domains based on crystal packing of domain 11 was not consistent with the subsequent crystal structure of domains 1–3 (Brown et al, 2002; Olson et al, 2004). Olson and co-workers proposed alternative models where the extracellular region comprises five units of three compact domains (equivalent in architecture to domains 1–3). Our new structures do not support this tri-domain repeat model extending through domains 11–15.
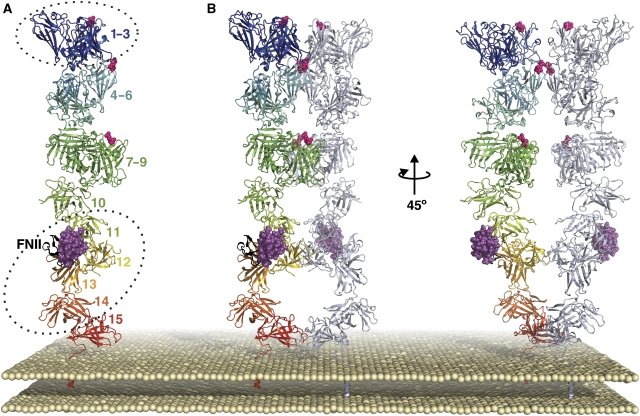
Combining our structures with previous models and biochemical data, we put forward a revised model for the IGF2R monomer/dimer (Figure 6). Like the Olson model, domains 1–9 consist of three tri-domain repeats (based on the domains 1–3 architecture) in an arrangement, which accounts for proteolytic data (Olson et al, 2004; Figure 6A). Each mannose-6-phosphate-binding site (domains 3, 5 and 9) is part of one of these tri-domain ‘sugar-binding' units. The placements have been adjusted such that domain 5 of each monomer can interact, since isolated bovine IGF2R domain 5 can dimerize (Reddy et al, 2004). Domains 11–14 are taken from this study and the crystallographic dimer is maintained in the dimer model (Figure 6B). Individual domains are inserted arbitrarily to represent domains 10 and 15. Since a domain 13–15 IGF2R truncation retained dimerization activity (Kreiling et al, 2005), the two domain 15s are within contact distance in the dimer model. For clarity, this model does not attempt to account for conformational flexibility in IGF2R (a property likely to facilitate diverse multivalent ligand interactions).
Figure 6.
Putative model for the relative arrangement of IGF2R extracellular domains. (A) View of an IGF2R monomer, coloured from blue at the N-terminus to red at the C-terminus, with FNII in black, IGF-II in magenta and mannose-6-phosphate-binding sites indicated by pink spheres. Dotted ellipses indicate regions of the model for which X-ray crystallography structures have been solved (bovine domains 1–3 and human domains 11–14). (B) Views of a tentative IGF2R dimer, based primarily on crystal packing observations for domains 11–14 and domains 11–13. For each dimer, one monomer is coloured as in panel A and the other is coloured gray/blue.
Discussion
Evolution of IGF-II binding by IGF2R
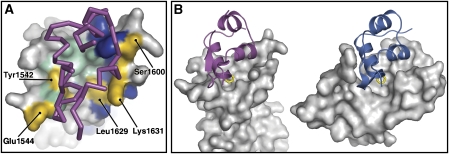
The evolutionary conserved IGF system is maintained in a delicate balance such that IGF-influenced growth and differentiation are tightly controlled. As well as IGF-I and IGF-II, integral components of this system include the signalling IR and IGF-1R cell-surface receptors, the non-signalling IGF2R cell-surface receptor and the extra-cellular IGFBPs. The role of the multifunctional IGF2R in controlling IGF-II supply is not universal and although IGF2R is present across most lineages, IGF-II binding is not a conserved function. Chicken and monotreme IGF2R do not bind IGF-II, whereas that in marsupials and eutherian lineages do and a few amino-acid changes are likely to be responsible for this evolutionary gain-of-function (Killian et al, 2000). From mapping sequence alignment data to the IGF2R/IGF-II interface, we can conclude that acquisition of domain 11 residues Tyr1542 and Leu1629 were particularly crucial in forming the hydrophobic pocket necessary for IGF-II Phe19 binding, and that Glu1544, Ser1600 and Lys1631 were also important to gain-of-function (Figure 7A).
Figure 7.
Evolutionary considerations. (A) Gain of IGF-II binding function. A surface view of IGF2R domain 11 and a ribbon view of bound IGF-II (magenta) are shown. IGF2R side chains identical across all species are coloured blue, while residues conserved across most species are coloured green. Side chains differing between monotremes and therian lineages, which are likely to be crucial in conferring IGF-II binding function are labelled and coloured gold. (B) Convergent evolution of IGF2R and the N-terminal domain of the IGFBPs towards binding the same region of the IGFs. The left panel shows a surface view of IGF2R domain 11, with bound IGF-II in magenta and Phe19 shown as yellow sticks. The right panel shows a surface view of the N-domain of IGFBP4, with bound IGF-I in blue and Phe16 shown as yellow sticks (PDB code 2DSQ).
Control of IGF-II bioavailability
As well as binding to cell-surface receptors, IGFs bind with high affinities to circulating IGFBPs, which control their distribution and activity. IGFBPs comprise N- and C-terminal domains, with a linker domain between which undergoes proteolysis in a mechanism presumed to release IGFs for other functions (Bunn and Fowlkes, 2003). As well as demonstrating IGF-independent functions post-proteolysis, IGFBP N- and C-domains can inhibit IGF activity. The individual domains bind IGFs with affinities in the high nanomolar range, with N-domains generally binding more strongly than C-domains (Sitar et al, 2006). Recent IGFBP complex structures (IGF-I bound to the N- and C-domains of IGFBP-4 and IGF-I bound to the N-domain of IGFBP4 and the C-domain of IGFBP1; PDB codes 2DSQ and 2DSR) suggest a binding mode common to all IGF/IGFBP complexes, with sequence differences giving variation in binding affinities (Sitar et al, 2006). Structural superposition of these complexes onto our complex structure demonstrates that the IGFBP N-domain-binding site closely overlaps with the IGF2R-binding site on IGF-II (Figure 7B). The IGF-II/IGF2R and IGF-I/N-domain interfaces involve many equivalent side chains (including IGF-II Phe19/IGF-I Phe16) and bury ∼730 and ∼800 Å2 ASA per partner, respectively. Conversely, the IGFBP C-domain-binding site on IGFs does not overlap with the IGF2R-binding site; superposition with our complex shows no Cα clashes with the C-domain for the hybrid IGF-I/IGFBP4/IGFBP1 structure and only a small loop overlap for the IGF-I/IGFBP4 structure. This is not surprising since the C-domain-binding site on the IGFs is known to overlap with the IGF-1R-binding site and is likely to inhibit IGF-1R binding (Headey et al, 2004). It is plausible that the tri-domain structure of IGFBPs has evolved such that the major roles of the N- and C-domains are to block the IGF2R- and IGF-1R-binding sites, respectively. IGFs are ‘sticky' peptide hormones, with numerous surface-exposed aromatic and hydrophobic side chains, several of which are important for signalling. However, others may have evolved to engender tight binding to non-signalling partners as a form of internal regulation. In IGF-II, examples of such exposed side chains are Phe19 and Leu53, which are involved in binding to both IGF2R and IGFBPs. The evolution of these two distinct forms of IGF control has converged to target the same binding face on IGF-II, balancing IGF-regulated growth and differentiation via control of IGF bioavailability and by IGF-II sequestration. This has implications for the development of novel anticancer therapies, which must interfere as little as possible with IGF-balance while specifically targeting tumour growth.
Our structure of the IGF2R/IGF-II complex answers many questions hereto outstanding regarding this important biological interaction. Only domain 11 is directly involved in IGF-II binding, with domain 13 (specifically the FNII insert) acting to maintain the IGF-II-binding site conformation in a high-affinity state. Thus, domains 12 and 13, while not directly interacting with IGF-II, act in concert with domain 11 to form the high-affinity ligand-binding region. In particular, FNII provides a crucial yet indirect contribution to binding affinity through modulation of binding site flexibility before ligand binding. The juxtaposition of receptor and ligand along with the molecular details of the interface provide the detailed knowledge required for design of novel therapeutics. Since the requirement of domain 13/FNII for high-affinity IGF-II-binding might be at least partially compensated for with careful mutagenesis (Glu1544) (Zaccheo et al, 2006), it is hoped that further in vivo, in vitro and in silico studies based on this complex structure will lead to the development of effective treatments. Since evolution has conferred IGF-II binding function upon IGF2R as an additional level of control (along with IGFBPs) over IGF-influenced growth and differentiation, such therapeutic design must specifically target growth disorders while guarding against uncontrolled disruption of normal IGF system balance.
Materials and methods
Cloning and expression of IGF2R fragments
IGF2R fragments were generated in Chinese hamster ovary (CHO) cells as previously described (Roche et al, 2006; Delaine et al, 2007), with molecular weights confirmed by electrospray mass spectrometry. Glycosylation details are given in the Supplementary data. Preparation of selenomethionine-labelled IGF2R fragments in CHO cells was as previously described (Davis et al, 2001). Selenium incorporation was confirmed by mass spectrometry.
Crystallization and data collection
Nanolitre-scale sitting-drop crystallization experiments (100 nl protein+100 nl reservoir) were set up at 21°C using a Cartesian Technologies Microsys MIC4000 (Genomic Solutions) (Walter et al, 2005). For complex crystallization setups, lyophilized IGF-II was resuspended in 20 mM Tris–HCl pH 8.0, 150 mM NaCl and mixed immediately with the relevant IGF2R fragment at a molar ratio slightly in excess of the receptor fragment. Crystals which grew to a reasonable size were tested for diffraction directly from the drop. Otherwise, optimizations were performed around promising conditions either using the Cartesian robot or manually in 24-well plates (1 μl protein+1 μl reservoir) using the sitting-drop method (Harlos, 1992). IGF2R-Dom11–14 and IGF2R-Dom11–13/IGF-II complex crystallizations required extensive optimization to obtain diffraction-quality crystals. All X-ray diffraction data were collected at the European Synchrotron Radiation Facility (ESRF, Grenoble, France). Selenomethionine data were processed with the HKL package (Otwinowski and Minor, 1997), while other data were processed using the XDS package (Kabsch, 1993). The programme XPREP (Bruker AXS, Madison, USA) was used to calculate quality indicators and for merging. Crystallization details and data statistics are in Table I and Supplementary Table SI.
Structure determination and refinement
Full details of structure determination and refinement are given in the Supplementary data. Briefly, crystallographic refinement used CNS (Brünger et al, 1998) and REFMAC5 (Murshudov et al, 1997), with O (Jones et al, 1991) and Coot (Emsley and Cowtan, 2004) used for manual checking and building with reference to 2Fo−Fc and Fo−Fc electron density maps. The IGF2R-Dom11–12 structure was solved by selenomethionine phasing. A domain 11 model was placed into the electron density map and domain 12 was built manually. Overall B-factors were refined. The refined structure and a poly-Ala domain based on the main chain of domain 11 were then used as molecular replacement models to phase the IGF2R-Dom11–14 data. Model building and refinement included insertion of the FNII domain and overall B-factor refinement. The complex data were also phased by molecular replacement using domains 11–13 from the IGF2R-Dom11–14 structure, finding two molecules in the asymmetric unit. Further molecular replacement using an IGF-I model (PDB code 1IMX) placed two IGF molecules, yielding two IGF2R-Dom11–13/IGF-II complexes per asymmetric unit. The models were rigid-body refined and the IGF-I side chains mutated to their IGF-II equivalents. Further rounds of positional and normal-mode-based (Poon et al, 2007) refinement followed. Final structures were checked using PROCHECK (Laskowski et al, 1993), which showed no residues in disallowed regions of the Ramachandran Plot, and by WHAT_CHECK (Hooft et al, 1996). Refinement statistics are given in Table I.
Analytical ultracentrifugation
Sedimentation velocity experiments were performed in a Beckman Optima XL-I analytical ultracentrifuge using either IGF2R-Dom11–12 (6 mg/ml) or IGF2R-Dom11–13 (7 mg/ml). Samples were spun at 40 000 r.p.m. and imaged using absorbance optics. Initial analysis was by the g(s) method using SEDFIT (Schuck and Rossmanith, 2000), with further fitting using ProFit software (QuantumSoft, Uetikon Am See, Switzerland). This model-independent mode of analysis is extremely sensitive to polydispersity in samples. Single species display a Gaussian distribution in sedimentation coefficient(s), due to the effects of diffusion, about a central point that is the sedimentation coefficient of the species. Additional species are apparent as additional peaks.
Furthermore, c(s,fr) distributions, two-dimensional plots of the relationship between sedimentation coefficient and frictional ratio (fr) were also determined using SEDFIT (Brown and Schuck, 2006), whereby diffusional broadening of the sedimenting protein boundaries is used to determine the frictional characteristics of the sample. The function c(s,fr) can then be used to derive functions such as c(s,M), dependence of sedimentation coefficient and weight. For the partial specific volumes of the protein, we used weight-average values of 0.7 for IGF2R-Dom11–12 and 0.71 for IGF2R-Dom11–13, based on estimates for the glycosylation level in each case.
Analysis of ligand binding by IGF2R fragments
IGF-II and IGF-II mutants were made in Escherichia coli by previously reported methods (Denley et al, 2004) and were assayed for IGF2R binding by surface plasmon resonance as previously described (Zaccheo et al, 2006; Delaine et al, 2007). The IGF2R-Dom10–13, IGF2R-Dom11–13 and IGF2R-Dom11–14 fragments were coupled directly to a biosensor surface via amine groups to give a final resonance value of 2000 U as described (Delaine et al, 2007). BIAcore (BIAcore, Sweden) analysis of binding was conducted at 25°C in HBS-EP buffer (BIAcore) at a flow rate of 40 μl/min. IGF-II and mutants at concentrations of 100, 50, 25, 12.5 and 6.25 nM were passed over the sensor surfaces in duplicate (3 min association, 15 min dissociation, 1.5 min regeneration with 10 mM HCl) and experiments were performed over two separate sensor chips. Curves were fitted using BIAevaluation 3.2 software (Biacore) to a two-state conformational change model describing a 1:1 binding interaction with a conformational change upon binding as performed previously (Delaine et al, 2007). The affinity constant (KA) derived from six separate experiments on two separate chips for IGF-II on IGF2R-Dom10–13 was 2.49±0.68 × 108/M, and a similar variation between experiments was seen on all surfaces with all mutants analysed.
In parallel, biotinylated IGF2R constructs comprising domains 10–13 with and without FNII and with and without Glu1544Lys were generated in human embryonic kidney 293T cells as previously described (Linnell et al, 2001). The Glu1544Lys mutation and deletion of the FNII region (amino acids 1901–1942) were generated using the Quikchange II site-directed mutagenesis kit (Stratagene) in accordance with the manufacturer's instructions. Surface plasmon resonance to measure binding affinities was conducted as above by passing a serial dilution of IGF-II (32–2 nM) over biotinylated IGF2R fragments immobilised to streptavidin-coated sensor chips (Rmax of ∼100 RU). Each cycle comprised 3 min association, 15 min dissociation and 2 min regeneration with 2 M MgCl2. Curves were fitted using BIAevaluation 4.1 software to a two-state conformational change model describing a 1:1 binding interaction with a conformational change upon binding as performed previously (Zaccheo et al, 2006). The differences in binding affinities observed using the two coupling methods are consistent with our previous observations, which showed that direct amine coupling leads to a lower absolute binding affinity, but the relative binding affinities of analogues are consistent with the literature (Forbes et al, 2002).
Structural analyses and visualization
Secondary structure motifs were confirmed using Promotif (Hutchinson and Thornton, 1996) and surface accessibility evaluated using Naccess (http://wolf.bms.umist.ac.uk/naccess/). Structural superpositions were performed with SHP (Stuart et al, 1979) and protein–protein interfaces were investigated using PDBsum (Laskowski, 2001). Surface hydrophobicity was assessed using GRID (Goodford, 1985). Sequence alignments were formatted using ESPript (http://espript.ibcp.fr/). Figures were produced using Pymol (http://pymol.sourceforge.net/).
Accession codes
Atomic coordinates and structure factors have been deposited in the Protein Data Bank (http://www.pdb.org) under accession codes 2V5N (IGF2R-Dom11–12), 2V5O (IGF2R-Dom11–14) and 2V5P (IGF2R-Dom11–13/IGF-II complex).
Supplementary Material
Supplementary Information
Acknowledgments
We thank the ESRF beamline staff of BM14, ID-14.2 and ID-14.3 and K Harlos for help during data collection; C Alvino and K McNeil (University of Adelaide) and Dr T Mulhern (University of Melbourne) for CD and IGF1R binding assays; J Nettleship for mass spectrometry; N Abrescia, J Dong, RM Esnouf and S Graham for computation assistance; W Lu and L Lyne for help with mammalian tissue culture and D Stuart for helpful discussion. We also thank BK Poon and J Ma (Rice University) for providing, and advising on, normal-mode-based refinement software. We are grateful to the Oxford Protein Production Facility and SPINE-2 (EC Contract Number LSHG-CT-2006-031220) for access to crystallization facilities. This work was supported by Cancer Research UK (C375, EYJ; C429, ABH). AD was funded by an Australian Postgraduate Award and BEF and JCW by the National Health and Medical Research Council of Australia. EYJ is a Cancer Research UK Principal Research Fellow.
References
- Bach LA, Hsieh S, Sakano K, Fujiwara H, Perdue JF, Rechler MM (1993) Binding of mutants of human insulin-like growth factor II to insulin-like growth factor binding proteins 1–6. J Biol Chem 268: 9246–9254 [PubMed] [Google Scholar]
- Baker NA, Sept D, Joseph S, Holst MJ, McCammon JA (2001) Electrostatics of nanosystems: application to microtubules and the ribosome. Proc Natl Acad Sci USA 98: 10037–10041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J, Esnouf RM, Jones MA, Linnell J, Harlos K, Hassan AB, Jones EY (2002) Structure of a functional IGF2R fragment determined from the anomalous scattering of sulfur. EMBO J 21: 1054–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PH, Schuck P (2006) Macromolecular size-and-shape distributions by sedimentation velocity analytical ultracentrifugation. Biophys J 90: 4651–4661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brünger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, Read RJ, Rice LM, Simonson T, Warren GL (1998) Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr D 54: 905–921 [DOI] [PubMed] [Google Scholar]
- Bunn RC, Fowlkes JL (2003) Insulin-like growth factor binding protein proteolysis. Trends Endocrinol Metab 14: 176–181 [DOI] [PubMed] [Google Scholar]
- Burgisser DM, Roth BV, Giger R, Luthi C, Weigl S, Zarn J, Humbel RE (1991) Mutants of human insulin-like growth factor-II with altered affinities for the type-1 and type-2 insulin-like growth-factor receptor. J Biol Chem 266: 1029–1033 [PubMed] [Google Scholar]
- Byrd JC, Park JH, Schaffer BS, Garmroudi F, MacDonald RG (2000) Dimerization of the insulin-like growth factor II/mannose 6-phosphate receptor. J Biol Chem 275: 18647–18656 [DOI] [PubMed] [Google Scholar]
- Carrick FE, Hinds MG, McNeil KA, Wallace JC, Forbes BE, Norton RS (2005) Interaction of insulin-like growth factor (IGF)-I and -II with IGF binding protein-2: mapping the binding surfaces by nuclear magnetic resonance. J Mol Endocrinol 34: 685–698 [DOI] [PubMed] [Google Scholar]
- Davis SJ, Ikemizu S, Collins AV, Fennelly JA, Harlos K, Jones EY, Stuart DI (2001) Crystallization and functional analysis of a soluble deglycosylated form of the human costimulatory molecule B7-1. Acta Crystallogr D 57: 605–608 [DOI] [PubMed] [Google Scholar]
- Delaine C, Alvino CL, McNeil KA, Mulhern TD, Gauguin L, De Meyts P, Jones EY, Brown J, Wallace JC, Forbes BE (2007) A novel binding site for the human IGF-II/mannose 6-phospate receptor (IGF2R) on IGF-II. J Biol Chem 282: 18886–18894 [DOI] [PubMed] [Google Scholar]
- Denley A, Bonython ER, Booker GW, Cosgrove LJ, Forbes BE, Ward CW, Wallace JC (2004) Structural determinants for high-affinity binding of insulin-like growth factor II to insulin receptor (IR)-A, the exon 11 minus isoform of the IR. Mol Endocrinol 18: 2502–2512 [DOI] [PubMed] [Google Scholar]
- Denley A, Cosgrove LJ, Booker GW, Wallace JC, Forbes BE (2005) Molecular interactions of the IGF system. Cytokine Growth Factor Rev 16: 421–439 [DOI] [PubMed] [Google Scholar]
- Devi GR, Byrd JC, Slentz DH, MacDonald RG (1998) An insulin-like growth factor II (IGF-II) affinity-enhancing domain localized within extracytoplasmic repeat 13 of the IGF-II/mannose 6-phosphate receptor. Mol Endocrinol 12: 1661–1672 [DOI] [PubMed] [Google Scholar]
- Emsley P, Cowtan K (2004) Coot: model-building tools for molecular graphics. Acta Crystallogr D 60: 2126–2132 [DOI] [PubMed] [Google Scholar]
- Falls JG, Pulford DJ, Wylie AA, Jirtle RL (1999) Genomic imprinting: implications for human disease. Am J Pathol 154: 635–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes BE, Hartfield PJ, McNeil KA, Surinya KH, Milner SJ, Cosgrove LJ, Wallace JC (2002) Characteristics of binding of insulin-like growth factor (IGF)-I and IGF-II analogues to the type 1 IGF receptor determined by BIAcore analysis. Eur J Biochem 269: 961–968 [DOI] [PubMed] [Google Scholar]
- Forbes BE, McNeil KA, Scott CD, Surinya KH, Cosgrove LJ, Wallace JC (2001) Contribution of residues A54 and L55 of the human insulin-like growth factor-II (IGF-II) a domain to type 2 IGF receptor binding specificity. Growth Factors 19: 163–173 [DOI] [PubMed] [Google Scholar]
- Foulstone E, Prince S, Zaccheo O, Burns JL, Harper J, Jacobs C, Church D, Hassan AB (2005) Insulin-like growth factor ligands, receptors, and binding proteins in cancer. J Pathol 205: 145–153 [DOI] [PubMed] [Google Scholar]
- Garmroudi F, Devi G, Slentz DH, Schaffer BS, MacDonald RG (1996) Truncated forms of the insulin-like growth factor II (IGF-II)/mannose 6-phosphate receptor encompassing the IGF-II binding site: characterization of a point mutation that abolishes IGF-II binding. Mol Endocrinol 10: 642–651 [DOI] [PubMed] [Google Scholar]
- Ghosh P, Dahms NM, Kornfeld S (2003) Mannose 6-phosphate receptors: new twists in the tale. Nat Rev Mol Cell Biol 4: 202–212 [DOI] [PubMed] [Google Scholar]
- Goodford PJ (1985) A computational procedure for determining energetically favorable binding sites on biologically important macromolecules. J Med Chem 28: 849–857 [DOI] [PubMed] [Google Scholar]
- Harlos K (1992) Micro-bridges for sitting drop crystallizations. J Appl Cryst 25: 536–538 [Google Scholar]
- Hassan AB, Howell JA (2000) Insulin-like growth factor II supply modifies growth of intestinal adenoma in Apc(Min/+) mice. Cancer Res 60: 1070–1076 [PubMed] [Google Scholar]
- Headey SJ, Keizer DW, Yao SG, Wallace JC, Bach LA, Norton RS (2004) Binding site for the C-domain of insulin-like growth factor (IGF) binding protein-6 on IGF-II; implications for inhibition of IGF actions. FEBS Lett 568: 19–22 [DOI] [PubMed] [Google Scholar]
- Hooft RWW, Vriend G, Sander C, Abola EE (1996) Errors in protein structures. Nature 381: 272. [DOI] [PubMed] [Google Scholar]
- Hutchinson EG, Thornton JM (1996) PROMOTIF—a program to identify and analyze structural motifs in proteins. Protein Sci 5: 212–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TA, Zou JY, Cowan SW, Kjeldgaard M (1991) Improved methods for building protein models in electron- density maps and the location of errors in these models. Acta Crystallogr A 47: 110–119 [DOI] [PubMed] [Google Scholar]
- Kabsch W (1993) Automatic processing of rotation diffraction data from crystals of initially unknown symmetry and cell constants. J Appl Cryst 26: 795–800 [Google Scholar]
- Killian JK, Byrd JC, Jirtle JV, Munday BL, Stoskopf MK, MacDonald RG, Jirtle RL (2000) M6P/IGF2R imprinting evolution in mammals. Mol Cell 5: 707–716 [DOI] [PubMed] [Google Scholar]
- Kreiling JL, Byrd JC, MacDonald RG (2005) Domain interactions of the mannose 6-phosphate/insulin-like growth factor II receptor. J Biol Chem 280: 21067–21077 [DOI] [PubMed] [Google Scholar]
- Laskowski RA (2001) PDBsum: summaries and analyses of PDB structures. Nucleic Acids Res 29: 221–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski RA, Macarthur MW, Moss DS, Thornton JM (1993) Procheck—a program to check the stereochemical quality of protein structures. J Appl Cryst 26: 283–291 [Google Scholar]
- Linnell J, Groeger G, Hassan AB (2001) Real time kinetics of Insulin-like growth factor II (IGF-II) interaction with the IGF-II/mannose 6-phosphate receptor: the effects of domain 13 and pH. J Biol Chem 276: 23986–23991 [DOI] [PubMed] [Google Scholar]
- Lobel P, Dahms NM, Kornfeld S (1988) Cloning and sequence analysis of the cation-independent mannose 6-phosphate receptor. J Biol Chem 263: 2563–2570 [PubMed] [Google Scholar]
- Murshudov GN, Vagin AA, Dodson EJ (1997) Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D 53: 240–355 [DOI] [PubMed] [Google Scholar]
- Olson LJ, Yammani RD, Dahms NM, Kim JJ (2004) Structure of uPAR, plasminogen, and sugar-binding sites of the 300 kDa mannose 6-phosphate receptor. EMBO J 23: 2019–2028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima A, Nolan CM, Kyle JW, Grubb JH, Sly WS (1988) The human cation-independent mannose 6-phosphate receptor. Cloning and sequence of the full-length cDNA and expression of functional receptor in COS cells. J Biol Chem 263: 2553–2562 [PubMed] [Google Scholar]
- Otwinowski Z, Minor W (1997) Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol 276: 307–326 [DOI] [PubMed] [Google Scholar]
- Poon BK, Chen X, Lu M, Vyas NK, Quiocho FA, Wang Q, Ma J (2007) Normal mode refinement of anisotropic thermal parameters for a supramolecular complex at 3.42-Å crystallographic resolution. Proc Natl Acad Sci USA 104: 7869–7874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy ST, Chai W, Childs RA, Page JD, Feizi T, Dahms NM (2004) Identification of a low affinity mannose 6-phosphate-binding site in domain 5 of the cation-independent mannose 6-phosphate receptor. J Biol Chem 279: 38658–38667 [DOI] [PubMed] [Google Scholar]
- Roberts DL, Weix DJ, Dahms NM, Kim JJ (1998) Molecular basis of lysosomal enzyme recognition: three-dimensional structure of the cation-dependent mannose 6-phosphate receptor. Cell 93: 639–648 [DOI] [PubMed] [Google Scholar]
- Roche P, Brown J, Denley A, Forbes BE, Wallace JC, Jones EY, Esnouf RM (2006) Computational model for the IGF-II/IGF2r complex that is predictive of mutational and surface plasmon resonance data. Proteins 64: 758–768 [DOI] [PubMed] [Google Scholar]
- Sakano K, Enjoh T, Numata F, Fujiwara H, Marumoto Y, Higashihashi N, Sato Y, Perdue JF, Fujita-Yamaguchi Y (1991) The design, expression, and characterization of human insulin-like growth factor II (IGF-II) mutants specific for either the IGF-II/cation-independent mannose 6-phosphate receptor or IGF-I receptor. J Biol Chem 266: 20626–20635 [PubMed] [Google Scholar]
- Schmidt B, Kiecke-Siemsen C, Waheed A, Braulke T, von Figura K (1995) Localization of the insulin-like growth factor II binding site to amino acids 1508–1566 in repeat 11 of the mannose 6-phosphate/insulin-like growth factor II receptor. J Biol Chem 270: 14975–14982 [DOI] [PubMed] [Google Scholar]
- Schneider TR (2002) A genetic algorithm for the identification of conformationally invariant regions in protein molecules. Acta Crystallogr D 58: 195–208 [DOI] [PubMed] [Google Scholar]
- Schuck P, Rossmanith P (2000) Determination of the sedimentation coefficient distribution by least-squares boundary modeling. Biopolymers 54: 328–341 [DOI] [PubMed] [Google Scholar]
- Sitar T, Popowicz GM, Siwanowicz I, Huber R, Holak TA (2006) Structural basis for the inhibition of insulin-like growth factors by insulin-like growth factor-binding proteins. Proc Natl Acad Sci USA 103: 13028–13033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart DI, Levine M, Muirhead H, Stammers DK (1979) Crystal structure of cat muscle pyruvate kinase at a resolution of 2.6 Å. J Mol Biol 134: 109–142 [DOI] [PubMed] [Google Scholar]
- Toretsky JA, Helman LJ (1996) Involvement of IGF-II in human cancer. J Endocrinol 149: 367–372 [DOI] [PubMed] [Google Scholar]
- Walter TS, Diprose JM, Mayo CJ, Siebold C, Pickford MG, Carter L, Sutton GC, Berrow NS, Brown J, Berry IM, Stewart-Jones GB, Grimes JM, Stammers DK, Esnouf RM, Jones EY, Owens RJ, Stuart DI, Harlos K (2005) A procedure for setting up high-throughput nanolitre crystallization experiments. Crystallization workflow for initial screening, automated storage, imaging and optimization. Acta Crystallogr D 61: 651–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccheo OJ, Prince SN, Miller DM, Williams C, Kemp CF, Brown J, Jones EY, Catto LE, Crump MP, Hassan AB (2006) Kinetics of insulin-like growth factor II (IGF-II) interaction with domain 11 of the human IGF-II/mannose 6-phosphate receptor: function of CD and AB loop solvent-exposed residues. J Mol Biol 359: 403–421 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information