Abstract
Apical dendrites of pyramidal neurons in the neocortex have a stereotypic orientation that is important for neuronal function. Neural recognition molecule Close Homolog of L1 (CHL1) has been shown to regulate oriented growth of apical dendrites in the mouse caudal cortex. Here we show that CHL1 directly associates with NB-3, a member of the F3/contactin family of neural recognition molecules, and enhances its cell surface expression. Similar to CHL1, NB-3 exhibits high-caudal to low-rostral expression in the deep layer neurons of the neocortex. NB-3-deficient mice show abnormal apical dendrite projections of deep layer pyramidal neurons in the visual cortex. Both CHL1 and NB-3 interact with protein tyrosine phosphatase α (PTPα) and regulate its activity. Moreover, deep layer pyramidal neurons of PTPα-deficient mice develop misoriented, even inverted, apical dendrites. We propose a signaling complex in which PTPα mediates CHL1 and NB-3-regulated apical dendrite projection in the developing caudal cortex.
Keywords: adhesion, dendrite development, p59fyn
Introduction
Dendrite development is a fundamental process in the formation of a functional nervous system. Communication between neurons requires proper synaptic formation between axons and dendrites. The extent and pattern of dendritic branching determine the range and scope of synaptic inputs and, to a large extent, the output of a neuron (Jan and Jan, 2003). Compared with our current understanding of axon outgrowth and guidance, the control of dendrite development is much less well understood and has proven to be difficult to tackle. However, some progress has been made in understanding certain aspects of dendrite development, such as the oriented growth of apical dendrites of pyramidal neurons in the neocortex. Pyramidal neurons are a major cell type in the neocortex with a single apical dendrite growing toward the pial surface and several highly branched basal dendrites emanating from the cell body. A diffusible chemoattractant present at high levels near the marginal zone can orient apical dendrites toward the pial surface. Semaphorin 3A (Sema3A), acting through its receptor neuropilin-1, is a good candidate for such a factor (Polleux et al, 2000; Whitford et al, 2002). Genetic and biochemical evidence suggests that p59fyn and Cdk5 mediate Sema3A signaling in the orientation of apical dendrites (Sasaki et al, 2002).
Recently, another cell surface molecule, Close Homolog of L1 (CHL1), has been shown to regulate apical dendrite orientation in the neocortex. CHL1 is a type I transmembrane protein that belongs to L1 family of cell adhesion molecules (CAMs), a subclass of neural recognition molecules of the immunoglobulin superfamily. In the developing mouse cortex, CHL1 is expressed in deep layer pyramidal neurons in a low-rostral to high-caudal gradient. Accordingly, in the caudal cortex (somatosensory and visual cortices) of Chl1−/− mice, a significant proportion (40–60%) of deep layer pyramidal neurons exhibit misoriented apical dendrites (Demyanenko et al, 2004). Loss of CHL1 also decreases the rate of radial migration and causes the shift of some neurons to lower laminar positions in the caudal cortex (Demyanenko et al, 2004). Interestingly, CHL1-deficient mice display alterations in emotional reactivity and motor coordination (Montag-Sallaz et al, 2002; Pratte et al, 2003). Mutations in call (the chl1 ortholog in human) have been associated with mental retardation and schizophrenia (Angeloni et al, 1999; Chen et al, 2005). Abnormality in radial migration and apical dendrite development may underlie brain malfunction in mice and humans that carry mutations in the chl1 gene. However, the incomplete loss-of-function phenotype in apical dendrite projections in the Chl1−/− neocortex suggests that other molecules are involved in the same process. The mechanism of CHL1 signaling in apical dendrite projections is not clear either.
In search for proteins associated with CHL1, we found that NB-3/contactin-6, a member of the F3/contactin family of neural recognition molecules, exists in a complex with CHL1. The F3/contactin family consists of six structurally related proteins: F3/contactin, TAG-1, BIG-1, BIG-2, NB-2, and NB-3. NB-3 lacks an intracellular domain and is anchored at the cell surface via a glycosylphosphatidylinositol (GPI) link. NB-3 has a similar extracellular domain structure as CHL1, consisting of six Ig-like domains and four fibronectin type III (FNIII) repeats (CHL1 has 4.5 FNIII repeats). Interestingly, genomic mapping reveals that the nb-3 and chl1 genes are closely linked. In the mouse genome, they are located on chromosome 6, only 0.8 Mb apart without any other gene in between. In the human genome, these two genes are located on chromosome 3p26. The distance is 0.5 Mb, with only one hypothetical gene in between. NB-3 is expressed exclusively in the nervous system. NB-3 expression in the mouse cortex is maximal at postnatal day 7 (P7), and declines to a lower level thereafter (Lee et al, 2000). At P7, NB-3 is expressed in a graded pattern in the neocortex, with higher expression levels observed in the caudal cortex (Takeda et al, 2003). These findings suggest a potential role for NB-3 in cortical development and function. However, cortical abnormalities, especially in dendrite development, of Nb-3−/− mice have not been investigated.
Early work indicated that L1 family members function not only as adhesion molecules, but are also capable of transducing signals into cells upon activation (Maness and Schachner, 2007). CHL1 has a short cytoplasmic domain that binds ankyrin, a spectrin adapter that couples CHL1 to the subcortical actin cytoskeleton. CHL1 acts as a cooperative partner for integrins in promoting migration (Buhusi et al, 2003; Demyanenko et al, 2004). However, the signal transducers for CHL1 that regulate apical dendrite projection are not clear. Several lines of evidence suggest that protein tyrosine phosphatase α (PTPα), a receptor-like protein phosphatase, may be a potential signal mediator of CHL1 and/or NB-3 in the developing cortical neurons. PTPα engages in cis-interactions with neural cell adhesion molecule (NCAM) and F3/contactin, and mediates their signaling to the intracellular tyrosine kinase p59fyn (Zeng et al, 1999; Bodrikov et al, 2005). The kinase activity of p59fyn is inhibited through intramolecular interaction between phosphorylated Tyr-531 and its SH2 domain, which stabilizes a noncatalytic conformation. PTPα activates p59fyn via dephosphorylation of the Tyr-531 site, and in PTPα−/− mouse brains the phosphorylation of p59fyn at Tyr531 is increased and results in reduced p59fyn activity (Bhandari et al, 1998; Ponniah et al, 1999; Su et al, 1999). PTPα is expressed in deep layers of the developing mouse neocortex and hippocampus. Moreover, radial migration of neurons is impaired in both the hippocampus and neocortex of Ptpα−/− mice (Petrone et al, 2003). Interestingly, inversion of apical dendrites of cortical pyramidal neurons was also observed in mice lacking p59fyn (Sasaki et al, 2002). These findings, taken together, prompted us to investigate possible interaction of PTPα with CHL1 and the role of PTPα in dendrite development in neocortex.
Here, we investigated the molecular mechanism of neural recognition molecule-regulated dendrite development of pyramidal neurons in the mouse neocortex. We provide evidence indicating that NB-3 also participates in regulation of apical dendrite development in the caudal cortex. Both CHL1 and NB-3 interact with PTPα and regulate its activity. PTPα is also required for correct apical dendrite projections in the neocortex. These results indicate that PTPα mediates NB-3- and CHL1-regulated apical dendrite development in the deep layer of caudal neocortex.
Results
CHL1 interacts with NB-3 and enhances its cell surface expression
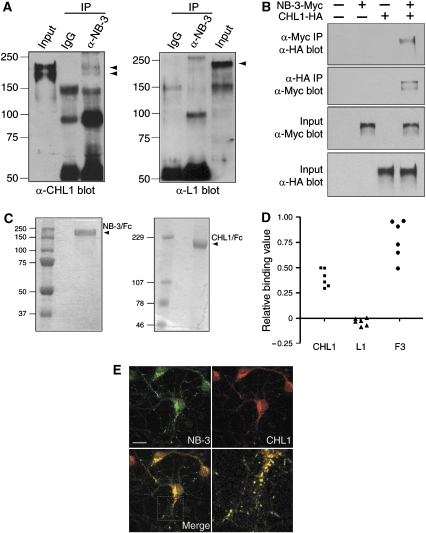
To identify proteins that associate with CHL1, we performed co-immunoprecipitation from mouse brain membrane fractions. CHL1 was detected in the precipitates prepared using a rabbit NB-3 antibody (Figure 1A). In contrast, nonspecific rabbit IgG did not precipitate detectable CHL1 (Figure 1A). This co-immunoprecipitation appeared specific since L1, a family member of CHL1 with high homology, was not precipitated by the same NB-3 antibody (Figure 1A). To confirm the interaction between CHL1 and NB-3, we next performed co-immunoprecipitation from transfected HEK293T cells. Cells expressing either NB-3-Myc or CHL1-HA, or both proteins were immunoprecipitated with an anti-Myc antibody. CHL1-HA was detected in the precipitates from the NB-3-Myc/CHL1-HA-coexpressing cells, but not from mock- or single-transfected cells (Figure 1B). Reciprocal experiments were carried out in which NB-3-Myc was detected in the anti-HA immunoprecipitates from the NB-3-Myc/CHL1-HA-coexpressing cells (Figure 1B). Next, to check if NB-3 directly binds to CHL1, purified NB-3/Fc protein was added to wells coated with purified CHL1/Fc, L1/Fc, or F3/Fc proteins. Binding of NB-3/Fc to the coated wells was detected using the rabbit anti-NB-3 antibody. NB-3/Fc showed strong binding to immobilized CHL1/Fc and F3/Fc, but not to L1/Fc (Figure 1D), indicating that there is direct binding between NB-3 and CHL1. Colocalization of NB-3 and CHL1 was also observed in the soma and neurites of cultured cortical neurons (Figure 1E). Together, these results indicate that NB-3 and CHL1 engage in physical interactions.
Figure 1.
NB-3 interacts with CHL1. (A) CHL1 was co-immunoprecipitated with NB-3 from mouse brains. P7 mouse brain membrane fractions immunoprecipitated (IP) with either rabbit anti-NB-3 or nonspecific rabbit IgG were probed for the presence of CHL1 or L1 as indicated. Input, lysates before immunoprecipitation; IgG, nonspecific rabbit IgG. (B) Association of NB-3-Myc and CHL1-HA in HEK293T cells. Transfected HEK293T cells were precipitated with anti-Myc or anti-HA antibodies and were probed with anti-HA or anti-Myc antibodies, respectively. (C) Purity of recombinant mouse NB-3/Fc and CHL1/Fc proteins used in ELISA as determined by SDS–PAGE and Coomassie blue stain. (D) Binding of NB-3/Fc (50 μg/ml) to immobilized CHL1/Fc (▪), L1/Fc (▴), and F3/Fc (•) (100 μg/ml) in ELISA plates. Background was estimated in BSA-coated (1 mg/ml) wells. Readings for wells coated with NB-3/Fc (5 μg/ml) followed by ELISA were given the value as 1.0. Relative binding values for each well were calculated accordingly. Values are from two independent experiments performed in triplicate. (E) Colocalization of endogenous NB-3 and CHL1 on soma and neurites of cultured cortical neurons. E17 cortical culture (5–10 DIV) were fixed and stained with rabbit anti-NB-3 and mouse anti-CHL1 antibodies. Scale bars, 30 μm.
Neural recognition molecules are clustered and activated when binding to their ligands (Bodrikov et al, 2005). To check whether NB-3 and CHL1 could engage in a trans-interaction, we added purified NB-3/Fc protein to live cortical neurons. Unlike the antibody against CHL1, NB-3/Fc did not induce clustering of the cell surface CHL1; neither did CHL1/Fc protein change the surface distribution of NB-3 (Supplementary Figure 1). Moreover, NB-3/Fc-coated microspheres did not bind to CHL1-expressing COS1a cells (data not shown). These results suggest that NB-3 and CHL1 do not function as ligand for each other, and they may engage in a cis-interaction.
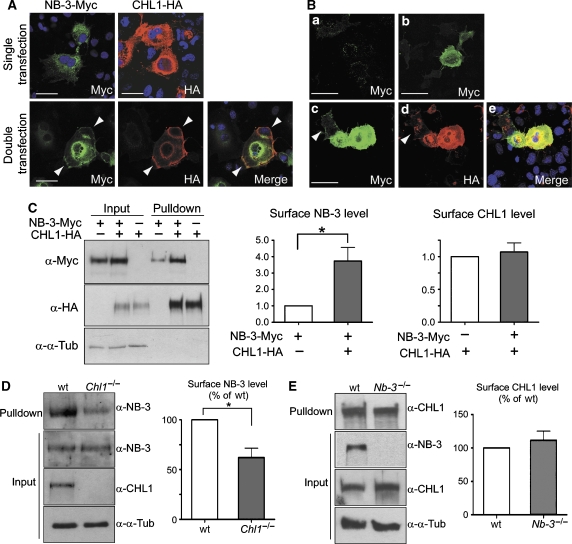
In COS1a cells expressing only NB-3-Myc, NB-3-Myc protein was mostly located inside the cells (Figure 2A), and only a very faint cell surface staining for NB-3-Myc was detected (Figure 2B(a)). Interestingly, when coexpressed with CHL1-HA, which was mainly located on the cell surface, some NB-3-Myc proteins moved to the cell periphery and colocalized with cell surface CHL1-HA (Figure 2A). We also observed a strong cell surface staining for NB-3-Myc in these cells (Figure 2B(b–e)). Notably, CHL1-negative cells, although in contact with CHL1-positive cells, had a low level of surface NB-3-Myc (Figure 2B(c–e), arrowheads).
Figure 2.
CHL1 enhances cell surface expression of NB-3. (A) Transfected COS1a cells were fixed and stained with anti-Myc and/or anti-HA antibodies to reveal the localization of NB-3-Myc and CHL1-HA proteins. When expressed alone, NB-3-Myc was mainly located inside the cells. When coexpressed with CHL1-HA, some NB-3-Myc protein moved to the cell periphery and colocalized with CHL1-HA (arrowheads). Scale bars, 50 μm. (B) Increase of cell surface NB-3-Myc when coexpressed with CHL1-HA in COS1a cells. After transfected with NB-3-Myc alone (a) or with both NB-3-Myc and CHL1-HA cDNAs (b–e), live COS1a cells were incubated with anti-Myc antibody followed by fixation and staining for cell surface NB-3-Myc protein alone (a and b), or double staining with anti-HA for CHL1-HA proteins (c–e). Note the cells with high surface NB-3-Myc level also expressed high level of CHL1-HA; CHL1-negative cells (arrowheads) had low levels of surface NB-3-Myc. Scale bars, 50 μm. (C) Analysis of surface expression of NB-3-Myc and CHL1-HA in single- and double-transfected COS1a cells. Cell surface proteins were biotinylated and precipitated using NeutrAvidin Gel. Pull-down samples were blotted with anti-Myc or anti-HA antibodies to assess the level of cell surface NB-3-Myc and CHL1-HA proteins. (D, E) Analysis of surface expression of NB-3 protein in cortical neurons from Chl1−/− mice (D) and CHL1 protein in neurons from Nb-3−/− mice (E). Cell surface proteins of E17 cortical cultures (7 DIV) were biotinylated. Pull-down samples using NeutrAvidin Gel were blotted with antibodies as indicated. For quantification in panels C–E, levels of cell surface proteins were normalized to corresponding input proteins. Results from four independent experiments (n=4) are presented as mean±s.e.m. for each panel. *P<0.05; one-sample t-test. α-α-Tub, anti-α-tubulin antibody.
To quantitatively analyze surface expression of NB-3-Myc in transfected COS1a cells, cell surface proteins were biotinylated and precipitated using a NeutrAvidin™ Gel. Probing of precipitates with anti-Myc antibody revealed the amount of cell surface NB-3-Myc. We found an average of 3.7±0.8-fold increase in the amount of cell surface NB-3-Myc in cotransfected cells, compared with that of the NB-3-Myc single-transfected cells, while the cell surface CHL1-HA level was not affected by the coexpressing of NB-3-Myc (Figure 2C). The increase in cell surface NB-3-Myc appeared to be a specific result of coexpression with CHL1-HA, as coexpression with L1 did not significantly change the surface expression of NB-3-Myc in transfected COS1a cells (Supplementary Figure 2).
NB-3 and CHL1 are normally expressed in neurons that are very different from transfected COS1a cells. To assess whether CHL1 also affects surface expression of NB-3 in neurons, the surface level of endogenous NB-3 was analyzed in cultured cortical neurons from wild-type and Chl1−/− mice. We detected a significant reduction of cell surface NB-3 in the Chl1−/− cortical neurons (62.1±9.4% of the wild type, Figure 2D). In the Nb-3−/− cortical neurons, however, the level of cell surface CHL1 protein was similar to the wild-type neurons (111.7±13.5% of the wild type, Figure 2E). Together, these results indicate that CHL1 interacts with NB-3 and positively regulates its surface expression in neurons, while NB-3 has no effect on the surface expression of CHL1.
Graded expression of NB-3 in developing cortical pyramidal neurons
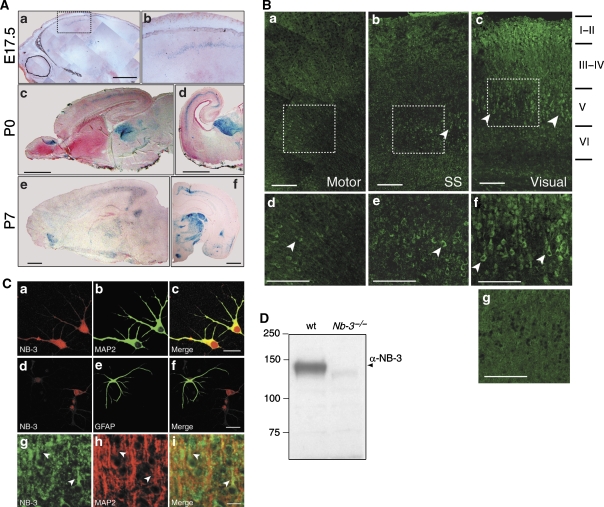
To assess the potential role of NB-3 in cortical development, the spatiotemporal expression of NB-3 was analyzed in the developing mouse neocortex. X-gal staining of the Nb-3+/− mouse, which has a LacZ gene inserted into the Nb-3 locus, could reflect the normal NB-3 expression pattern (Takeda et al, 2003). A weak X-gal signal in the intermediate zone of neocortex was detected as early as embryonic day 15.5 (E15.5) (data not shown). At around E17, deep layer pyramidal neurons in the neocortex begin to extend axonal and dendritic processes. At this stage, X-gal signal was observed in the deeper layer of the caudal cortex (Figure 3A(a and b)). At postnatal day 0 (P0), X-gal signal was mainly present in the layer V of the caudal cortex (Figure 3A(c)), and was higher in the visual cortex than in the auditory cortex (Figure 3A(d)). High-caudal to low-rostral pattern of X-gal signal in the cortex was maintained at P7 (Figure 3A(e and f)) (Takeda et al, 2003) and as late as P17 (data not shown).
Figure 3.
Expression of NB-3 in the developing mouse neocortex. (A) Localization of cells expressing NB-3 monitored by LacZ expression in the medial sagittal (a, c, and e) and coronal (d and f) sections of the Nb-3+/− brains at various developmental stages. (b) Higher magnification of the selected area in (a). Scale bars, 1 mm. (B) Immunostaining of the medial sagittal sections of P7 wild-type mouse neocortex using rabbit anti-NB-3 antibody. Arrowheads point to NB-3-positive cells. (d–f) Higher magnification of the selected areas in (a–c), respectively. (g) Control staining of Nb-3−/− layer V visual cortex (P7) with the same NB-3 antibody. Motor, motor cortex; SS, somatosensory cortex; Visual, visual cortex. Scale bars, 100 μm. (C) E17 cortical culture (a–f) or P7 mouse brain (g–i) were stained with rabbit anti-NB-3 antibody and monoclonal antibodies against MAP2 or GFAP. NB-3 is expressed in dendrites and soma of neurons in cortical culture (a–c) and brain tissues (g–i; arrowheads points to proximal apical dendrites) but not in astrocytes (d–f). Scale bars, 25 μm. (D) Specificity of the rabbit anti-NB-3 antibody. Whole brain homogenates from wild-type and Nb-3−/− mice were resolved by SDS–PAGE, followed by immunoblotting with the anti-NB-3 antibody.
Immunofluorescent staining of NB-3 protein in the wild-type mouse cortex (P7) also showed the strongest signal in the visual cortex, with many deep layer pyramidal neurons stained (Figure 3B(c and f)). Staining in layer I–II may be due to NB-3 protein present in the apical dendritic tufts of deep layer neurons, although the signal was also present on some cell bodies. A moderate number of layer V cells were stained in the somatosensory cortex (Figure 3B(b and e)), and even less in the motor cortex (Figure 3B(a and d)). Staining of the Nb-3−/− cortex with the same NB-3 antibody was minimal (P7 littermate; Figure 3B(g)). The pattern of NB-3 protein expression was largely consistent with the X-gal signal in the Nb-3+/− cortex. Together, these results indicate that NB-3 is expressed in a low-rostral to high-caudal gradient, with the highest level in the visual cortex during the time when deep layer pyramidal neurons develop axonal and dendritic processes.
In cultured mouse cortical neurons, only a small percentage of cells (<10%) exhibited higher than background signals when stained with the NB-3 antibody, consistent with the fact that NB-3 is predominantly expressed in layer V of the caudal cortex. NB-3 was expressed in the soma and processes of cortical neurons, including dendrites (Figure 3C(a–c)) and axons (data not shown). Little NB-3 signal was observed in astrocytes (Figure 3C(d–f)). Double staining of P7 visual cortex for NB-3 and MAP2 also showed NB-3 expression in the dendrites of layer V pyramidal neurons (Figure 3C(g–i)).
Misorientation of apical dendrites in layer V pyramidal neurons of the Nb-3−/− mouse neocortex
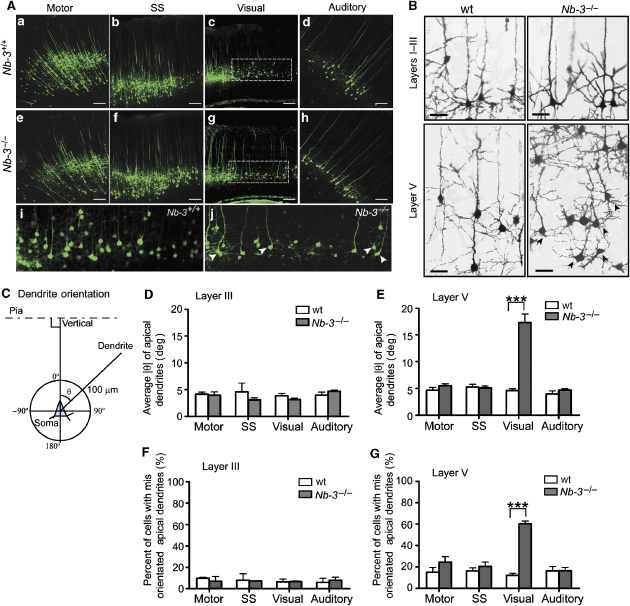
The physical interaction between NB-3 and CHL1 and their similar expression pattern suggested that NB-3 functions together with CHL1 in the cortical development. We therefore investigated the positioning and morphology of pyramidal neurons in the Nb-3−/− cortex. The organization of the Nb-3−/− neocortex was indistinguishable from that of the wild-type littermates, with all layers present in the same order (Takeda et al, 2003). To investigate if Nb-3−/− mice exhibit altered layer V pyramidal neuronal distribution as observed in the Chl1−/− mice, NB-3 mutant mice were crossed with a reporter strain in which layer V pyramidal neurons are intrinsically labeled with enhanced yellow fluorescent protein (YFP) (Thy1/YFP transgenic mice line H) (Feng et al, 2000; Demyanenko et al, 2004). In all cortical areas examined, the distribution of layer V pyramidal neurons in the Nb-3−/− mice was indistinguishable from that of the wild-type littermates (Figure 4A and Supplementary Figure 3), suggesting that, unlike CHL1, NB-3 does not regulate migration of deep layer pyramidal neurons.
Figure 4.
Misorientation of apical dendrites of layer V pyramidal neurons in the visual cortex of Nb-3−/− mice. (A) Morphology of layer V pyramidal neurons in the motor, somatosensory (SS), visual and auditory cortices of Nb-3+/+ (a–d, i) and Nb-3−/− (e–h, j) littermates. To visualize the morphology of layer V neurons in neocortex, NB-3 mutant mice were crossed to the Thy1-YFPH transgenic mice, which express YFP in layer V cells in the neocortex. (i) and (j) are higher magnification of the selected areas in (c) and (g), respectively. Arrowheads point to neurons displaying misoriented apical dendrites. Scale bar, 100 μm. (B) Golgi-impregnated visual cortex of wild-type (wt) and Nb-3−/− mice (1-month-old littermates). Arrows point to neurons with misoriented apical dendrites. Scale bars, 30 μm. (C) Scoring method for apical dendrite orientation (see Supplementary data). (D, E) Average ∣θ∣ of layer III (D) and layer V (E) pyramidal neurons of motor, somatosensory, visual and auditory cortices from wild-type and Nb-3−/− littermates. (F, G) Proportion of pyramidal neurons with misoriented apical dendrites was significantly increased in layer V but not in the layer III visual cortex of Nb-3−/− mice. Misoriented apical dendrites were defined as those with ∣θ∣>8°. For the above quantification, three to six pairs of wild-type and Nb-3−/− littermates (1-month-old) were analyzed. The number for layer V neurons ranged from 80 to 445 for each cortical region of respective genotypes, and ranged from 40 to 145 for layer III neurons. Results are presented as mean±s.e.m. ***P<0.001; two-way ANOVA with repeated measures followed by Bonferroni post-tests.
However, in the Nb-3−/− visual cortex, we observed abnormal apical dendrites oriented sideways in many layer V pyramidal neurons, although they ultimately reached layer I and formed apical tufts (Figure 4A(g and j)). Golgi impregnation of Nb-3−/− brains also revealed misoriented apical dendrites in layer V pyramidal neurons of the visual cortex (Figure 4B). This abnormality was not observed in the motor, somatosensory, and auditory cortices (Figure 4A), or in the upper layer of the visual cortex (Figure 4B).
To quantitatively compare the apical dendrite orientation of pyramidal neurons in the Nb-3−/− and wild-type cortices (1-month-old), Golgi impregnation was carried out and the direction of apical dendrites was measured as an angle of orientation relative to the pial surface (θ) (Figure 4C). Among all the cortical areas examined, only in the layer V visual cortex was the average ∣θ∣ significantly increased in the Nb-3−/− mice (17.1±1.3°) compared with the wild-type littermates (4.7±0.2°; Figure 4E). For the upper layer pyramidal neurons, the average ∣θ∣ in the Nb-3−/− mice was not significant different from that of the wild-type littermates in all cortical areas (Figure 4D). In each area of the wild-type cortex, 80–90% of neurons had apical dendrites with ∣θ∣⩽8°. Neurons with apical dendrites that projected outside this normal range (∣θ∣>8°) were significantly increased (60.4±2.5%) in layer V of Nb-3−/− visual cortex compared with those in the wild-type littermates (12.2±2.0%) (Figure 4G). This increase was not observed in layer V of other cortical areas (Figure 4G), or in layer III of all cortical regions including the visual cortex (Figure 4F). However, unlike Chl1−/− mice, the number of layer V pyramidal neurons with inverted dendrites (∣θ∣>90°) was not significantly increased in the Nb-3−/− visual cortex (data not shown). Thus, Nb-3−/− mice exhibit a similar, yet less severe, loss-of-function phenotype in apical dendrite projections in the caudal cortex as compared to Chl1−/− mice.
Owing to the close linkage of nb-3 and chl1 genes on the chromosome, there is a possibility that disruption of one gene changes the expression level of the other, resulting in the similar phenotypes observed in these two types of knockout mice. However, we found that in the Nb-3−/− brains, the total CHL1 protein level was similar to the wild-type littermates, and vice versa (Supplementary Figure 4), suggesting that the defects observed in the Nb-3−/− mice were not due to changes in CHL1 expression level in the cortex of the mutant mice.
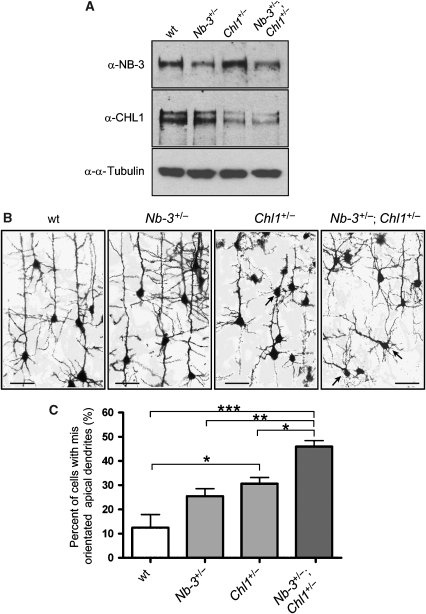
The interaction of NB-3 and CHL1 proteins, the similarity of extracellular domain structures, and the similar phenotypes in the apical dendrites projection in these two types of knockout mice prompted us to examine whether eliminating both genes would result in a more severe phenotype. However, close linkage of these two genes rules out the possibility of generating double-knockout mice. Therefore, we analyzed the apical dendrite orientation of layer V pyramidal neurons in the compound heterozygous (Nb-3+/−; Chl1+/−) mice. The Nb-3+/−; Chl1+/− mouse brains had reduced NB-3 and CHL1 protein levels compared with those of the wild-type littermates (Figure 5A). Layer V pyramidal neurons in the visual cortex of the compound heterozygous mice showed a more severe misoriented dendrite phenotype (46.0±2.4% abnormal cells) than the single-heterozygous mice (Nb-3+/−, 25.5±3.1%; Chl1+/−, 30.7±2.5%) and their wild-type littermates (12.5±5.4%; Figure 5B and C). The increase in abnormal neurons in the compound heterozygous mice is more or less equivalent to the additive deficits of two single-heterozygous mice, suggesting that NB-3 and CHL1 partially compensate each other's function and that both are required for correct apical dendrite projections.
Figure 5.
More severe loss-of-function phenotype of the compound heterozygous (Nb-3+/−; Chl1+/−) mice in apical dendrite orientation in the visual cortex. (A) Reduced expression of NB-3 and CHL1 proteins in the brains of Nb-3+/−; Chl1+/− mice compared to the wild-type littermates (P7). (B) Golgi-impregnated layer V visual cortex of wild-type, Nb-3+/−, Chl1+/−, and Nb-3+/−; Chl1+/− mice (1-month-old littermates). Arrows point to pyramidal neurons with misoriented apical dendrites. Scale bars, 50 μm. (C) Proportion of pyramidal neurons with misoriented apical dendrites (∣θ∣>8°) in the layer V visual cortex. Four groups of littermates (1-month-old) were analyzed (n=4). The number of neurons ranged from 238 to 462 for each genotype. Results are presented as mean±s.e.m. ***P<0.001; **P<0.01; *P<0.05; one-way ANOVA with repeated measures followed by Tukey's post-test.
CHL1 and NB-3 interact with PTPα and regulate PTPα signaling
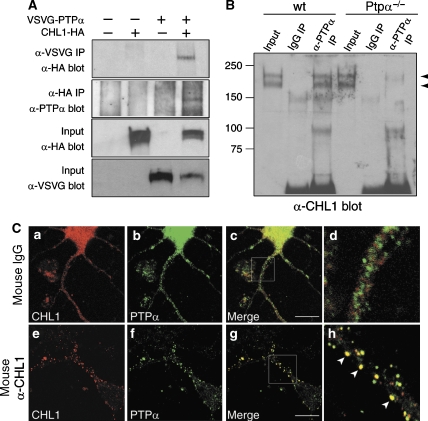
To investigate if PTPα mediates signaling of CHL1 and NB-3 in dendrite development, we first examined whether CHL1 and/or NB-3 interact with PTPα. In lysates from CHL1-HA/VSVG-PTPα coexpressing HEK293T cells, we observed co-immunoprecipitation of CHL1-HA using an anti-VSVG antibody, and also reciprocal co-immunoprecipitation of VSVG-PTPα using an anti-HA antibody (Figure 6A). Similarly, in NB-3-Myc/VSVG-PTPα coexpressing cells, anti-VSVG antibody could precipitate NB-3-Myc, and anti-Myc antibody brought down VSVG-PTPα levels (Supplementary Figure 5A). Antibody against PTPα could precipitate CHL1 protein from wild-type, but not Ptpα−/−, mouse brain extracts, confirming that PTPα interacts with CHL1 in vivo (Figure 6B).
Figure 6.
CHL1 interacts with PTPα. (A) Association of ectopically expressed CHL1-HA and VSVG-PTPα in HEK293T cells. Transfected HEK293T cells were lysed and immunoprecipitated (IP) with anti-VSVG or anti-HA antibodies and were blotted with anti-HA or anti-PTPα antibodies, respectively. (B) PTPα associates with CHL1 in the mouse brain. Wild-type or Ptpα−/− mouse (P0) brain membrane fractions were immunoprecipitated with either rabbit anti-PTPα or nonspecific rabbit IgG, and were blotted for the presence of CHL1 protein. (C) Live cortical neurons were treated with antibodies at 37°C for 2 h as indicated, followed by fixation and immunostaining for PTPα and CHL1. Note that CHL1 antibody induced clustering of CHL1 at the cell surface and redistribution of PTPα to the CHL1 clusters (arrowheads in h). (d and h) Higher magnification of selected areas in (c) and (g), respectively. Scale bars, 15 μm.
To examine the distribution of CHL1 and PTPα proteins in cells, we performed immunostaining in cultured mouse cortical neurons. CHL1 and PTPα showed a relatively diffused expression pattern, with some CHL1 signal partially overlapping with that of PTPα (Figure 6C(a–d)). Neural recognition molecules are clustered and activated when binding to their ligands (Bodrikov et al, 2005). Ligands for CHL1 on developing neurons have not been identified. Instead, we used a CHL1 antibody to induce its clustering on the surface of live cortical neurons to verify whether CHL1 interacts with PTPα upon its activation. Clustering of cell surface CHL1 induced accumulation of PTPα protein, and the overlap between CHL1 and PTPα clusters was significantly increased (mean correlation between CHL1 and PTPα localization: 0.34±0.02 for neurons treated with nonspecific mouse IgG; 0.62±0.03 for neurons treated with the CHL1 antibody; Figure 6C), indicating that PTPα redistributes to CHL1 clusters upon CHL1 activation. Similar to CHL1, clustering of NB-3 protein on the surface of cortical neurons with an NB-3 antibody also induced accumulation of PTPα with NB-3 clusters (Supplementary Figure 5C). Together, these results indicate that CHL1 and NB-3 interact with PTPα; PTPα is recruited to CHL1 and/or NB-3 upon their clustering.
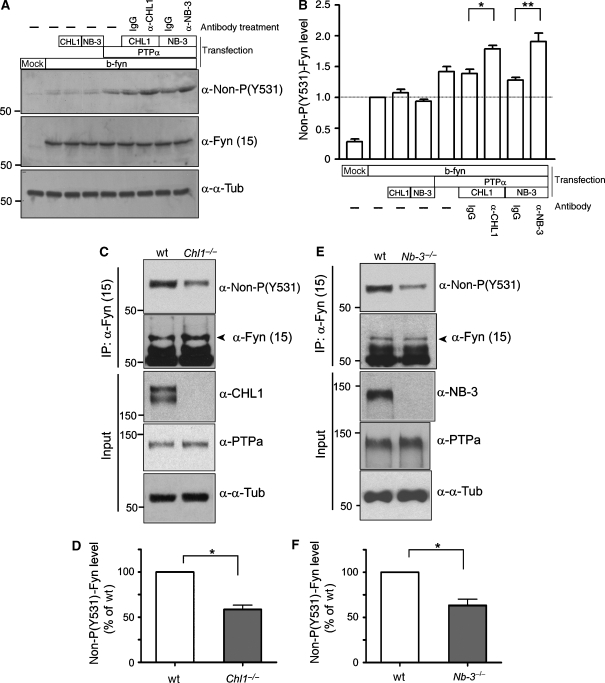
Next, to determine whether CHL1 and NB-3 regulate PTPα signaling, we expressed VSVG-PTPα and p59fyn (brain isoform) with CHL1-HA or NB-3-Myc in HEK293T cells, and examined the dephosphorylation status of p59fyn at Tyr-531 (non-P-Y531), a targeting site of PTPα. Consistent with previous studies (Bhandari et al, 1998), coexpression with VSVG-PTPα increased the level of non-P-Y531 p59fyn, while CHL1-HA and NB-3-Myc had no such effect (Figure 7A and B). However, when cell surface CHL1 or NB-3 were clustered using corresponding antibodies, the levels of non-P-Y531 p59fyn were significantly increased compared with cells treated with nonspecific mouse IgG (Figure 7A and B), suggesting that clustering of either CHL1 or NB-3 independently leads to PTPα activation and p59fyn dephosphorylation at Y531. In the brains of PTPα−/− mice, p59fyn activity is reduced to ∼30–40% of that in the wild-type mouse brain, consistent with the absence of PTPα activity toward p59fyn at Y531 (Ponniah et al, 1999; Su et al, 1999; Le et al, 2006). In the brains of wild-type and Chl1−/− mice, the levels of PTPα and p59fyn proteins were similar; however, non-P-Y531 p59fyn was reduced in the Chl1−/− brains (58.6±4.8% of the wild type; Figure 7C and D). Similarly, non-P-Y531 p59fyn in the Nb-3−/− brain was reduced to 63.4±6.8% of the wild-type brain (Figure 7E and F). Together, these results suggest that the impaired dephosphorylation of p59fyn in the Chl1−/− and Nb-3−/− brains is due to the lack of upstream stimulation by CHL1 and NB-3 of PTPα signaling to p59fyn.
Figure 7.
CHL1 and NB-3 regulate PTPα signaling to p59fyn. (A, B) Transfected HEK293T cells were incubated with nonspecific mouse IgG or clustering antibodies at 37°C for 30 min as indicated. Cells were lysed, and levels of p59fyn dephosphorylated at Tyr-531 (non-P-Y531) were measured and normalized to total p59fyn protein. b-fyn, brain isoform of p59fyn. Results from four independent experiments (n=4) are presented as mean±s.e.m. *P<0.05; **P<0.01; one-way ANOVA with repeated measures followed by Tukey's post-test. (C–F) Non-P-Y531 p59fyn is reduced in the Chl1−/− and the Nb-3−/− brains. Brains of Chl1−/− (P2-7) and Nb-3−/− (P5-7) mice and the wild-type littermates were homogenized and immunoprecipitated using monoclonal anti-p59fyn antibody (α-Fyn(15)), followed by immunoblotting as indicated. (D, F) Levels of non-P-Y531 p59fyn were normalized to total p59fyn levels. Results from four independent experiments (n=4) are presented as mean±s.e.m. for each panel. *P<0.05; one-sample t-test.
Aberrant apical dendrite projection in the Ptpα−/− cortex
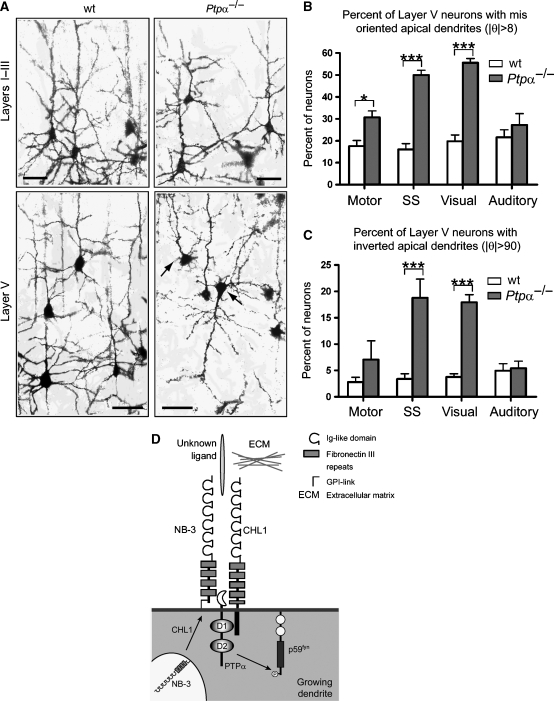
To investigate if PTPα mediates CHL1/NB-3 signaling and plays a role in dendrite development, we examined trajectories of apical dendrites in the neocortex of wild-type and Ptpα−/− mice. In the medial cortex of Ptpα−/− mice, some deep layer pyramidal neurons showed abnormal apical dendrites oriented sideways or even in an inverted direction (Figure 8A). The dendritic abnormality was less severe in the lateral (auditory) cortex, and was not observed in the upper layer in all cortical areas (Figure 8A, data not shown). To analyze the phenotype quantitatively, we measured the direction of apical dendrites (θ) relative to the pial surface. In layer V of Ptpα−/− caudal cortices, the proportion of neurons with misoriented apical dendrites (∣θ∣>8°) was significantly higher (50.0±2.2% for somatosensory; 55.6±1.9% for visual) than that in the wild-type littermates (16.1±2.6% for somatosensory; 19.8±2.9% for visual) (Figure 8B). In the motor cortex, this increase was less dramatic (17.6±2.6% for wild type; 30.7±2.9% for Ptpα−/−) yet still statistically significant (Figure 8B). In the auditory cortex, we observed a trend toward an increase in the number of abnormal neurons in Ptpα−/− mice, although this was not statistically significant (21.6±3.4% for wild type; 27.3±5.1% for Ptpα−/−) (Figure 8B). The number of neurons with inverted dendrites (∣θ∣>90°) was also significantly higher in the Ptpα−/− somatosensory (3.4±1.0% for wild type; 18.8±3.6% for Ptpα−/−) and the visual cortices (3.8±0.6% for wild type; 18.0±1.4% for Ptpα−/−), but not in the motor (3.4±1.0% for wild type; 8.8±3.6% for Ptpα−/−) and auditory cortices (5±1.3% for wild type; 5.4±1.3% for Ptpα−/−) (Figure 8C). These data suggest that PTPα regulates the orientated growth of apical dendrites of deep layer pyramidal neurons in medial cortex, and in the caudal cortex it most likely acts downstream of CHL1 and NB-3.
Figure 8.
Aberrant apical dendrite projection of layer V pyramidal neurons in the Ptpα−/− cortex. (A) Golgi-impregnated somatosensory cortex of wild-type (wt) and Ptpα−/− mice (1-month-old littermates). Arrows point to pyramidal neurons with misoriented apical dendrites. Scale bars, 50 μm. (B, C) Proportion of layer V pyramidal neurons with misoriented (∣θ∣>8°; B) or inverted (∣θ∣>90°; C) apical dendrites. Four pairs of wt and Ptpα−/− littermates (1-month-old) were analyzed (n=4). The number of layer V neurons ranged from 204 to 652 for each cortical region in respective genotypes. Results are presented as mean±s.e.m. ***P<0.001; *P<0.05; two-way ANOVA with repeated measures followed by Bonferroni post-tests. (D) A model depicting molecular pathway of CHL1- and NB-3-regulated apical dendrite orientation. CHL1 enhances the surface expression of NB-3. Upon activation, CHL1 and NB-3 recruit PTPα into this complex and result in subsequent activation of p59fyn to mediate oriented growth of apical dendrites of layer V pyramidal neurons in the caudal cortex.
Discussion
Previous work has shown that CHL1 regulates apical dendrite development of deep layer pyramidal neurons in the mouse caudal cortex. Here, we identified another neural recognition molecule, NB-3, that is required for region-specific apical dendrite development in vivo. Both CHL1 and NB-3 interact with PTPα and regulate its activity in the brain. PTPα is also required for correct apical dendrite projection in vivo. Our results support a model in which PTPα mediates CHL1- and NB-3-regulated apical dendrite projection in the caudal cortex.
CHL1 and NB-3 in cortical development
Neural recognition molecules NB-3 and CHL1 exhibit a close relationship in many aspects. Close linkage of these two genes is conserved from the mouse to human. They both exhibit high-caudal to low-rostral expression in the cortex (Demyanenko et al, 2004) (Figure 3). Consistently, both knockout mice show similar defects in apical dendrite projection in the visual cortex (Demyanenko et al, 2004) (Figure 4). CHL1 directly interacts with NB-3 and they colocalize in cultured cortical neurons (Figure 1). More interestingly, CHL1 facilitates surface expression of NB-3 (Figure 2). NB-3 surface expression is compromised in Chl1−/− neurons, which makes Chl1−/− mice a ‘hypomorph' for Nb-3. This may be one reason why Chl1−/− mice showed a more severe loss-of-function phenotype than the Nb-3−/− mice.
What is their relation in signaling? Our current data suggest that NB-3 and CHL1 do not function as ligand and receptor, as NB-3 or CHL1 protein cannot induce clustering of endogenous CHL1 or NB-3 on neuronal surface (Supplementary Figure 1), and NB-3-coated microspheres do not bind to CHL1-expressing cells (data not shown). On the neuronal surface, NB-3 and CHL1 may bind to their respective ligands and function independently to regulate dendrite development, and their signaling can partially compensate for one another's absence. Because NB-3 directly binds to CHL1, they may also engage in a cis-interaction and form a coreceptor/adhesion complex on the neuronal surface. Such a coreceptor complex has the potential of binding to more ligands and adhesion molecules with different kinetics, adding flexibility and stability to the system. For example, L1 serves as its own receptor with slow binding kinetics, while L1 and NCAM, via a cis-interaction on neurons, form a more potent receptor complex for L1 expressed on other cells (trans-binding) (Kadmon et al, 1990). Either way, both NB-3 and CHL1 are indispensable for deep layer apical dendrite development in the caudal cortex. A recent study provided in vitro evidence for a homophilic CHL1–CHL1 interaction (Jakovcevski et al, 2007). When ligands for NB-3 and CHL1 in developing neurons are identified and confirmed, one can reveal the nature of their signaling in dendrite development at the ligand–receptor level.
Although the neocortex has a common organization, it can be divided into distinct areas with different patterns of neural connectivity and functions. A fascinating question is what molecules and mechanisms govern region-specific cortical development. In the CHL1- and NB-3-deficient mice, deficits in dendrite development are restricted to the deep layer caudal cortex. Interestingly, in the L1−/y mice, a small percentage of pyramidal neurons in the motor cortex have apical dendrites projecting sideways, while those in the caudal cortex develop relatively normal apical dendrites (Demyanenko et al, 1999). Based on these results, one can postulate that neural recognition molecules, each with different expression patterns in the neocortex, participate in cortical area differentiation and formation. Genes encoding BIG-1 and BIG-2 are located close to the nb-3 and chl1 genes on mouse chromosome 6. It will be interesting to examine whether BIG-1 and BIG-2 exhibit slightly different expression patterns from that of CHL1 and NB-3, and regulate migration and dendrite development in other regions of the neocortex. Careful study of the expression profiles of neural recognition molecules will yield interesting information about region-specific regulation of cortical development.
Mechanism of apical dendrite misorientation of deep layer pyramidal neurons
Besides abnormal apical dendrites of layer V pyramidal neurons, Chl1−/− mice also exhibit deficits in the radial migration of neurons in the developing neocortex. Many neurons with aberrant apical dendrite projection are also shifted to lower cortical layers. The displaced neurons may be less responsive to guidance cues from the upper cortical region, such as Sema3A, thus resulting in defects in dendrite projection. However, in Nb-3−/− mice, no migration defects were observed, and many cells in the right laminar location in the Chl1−/− and Ptpα−/− mice also exhibit misoriented apical dendrites, suggesting that the apical dendrite deficits observed in these knockouts are independent of neuronal migration, at least to a large extent. However, one cannot completely rule out the possibility of cross talk between neural recognition molecules and Sema3A signaling in apical dendrite development. It has been shown that L1 modulates Sema3A–neuropilin-1 signaling in axonal guidance. Trans-binding of L1 to a cis-interacting L1–neuropilin-1 complex converts Sema3A-mediated axon repulsion to attraction (Castellani et al, 2000, 2002). Further studies are needed to assess if CHL1 and/or NB-3 could also modulate attraction/repulsion responses generated from Sema3A/neuropilin-1 signaling in the growing apical dendrites.
Neural recognition molecules have been found to engage in trans- and cis-interactions with many molecules, either in the extracellular matrix (ECM) or on the cell surface, to mediate cell contact and adhesion. CHL1 and NB-3 may mediate contact and adhesion of migrating neurons and growing apical dendrites with ECM or other cells, providing them a permissive environment or directions for movement and growth. For instance, via direct or indirect cis-interaction with β1 integrins, CHL1 potentiates integrin-dependent haptotactic migration to ECM proteins of embryonic cortical neurons (Buhusi et al, 2003; Demyanenko et al, 2004). A similar mechanism may also underlie the CHL1/NB-3-regulated growth of apical dendrites. Identification of ligands for CHL1 and NB-3 would shed light on the molecular mechanism of neural recognition molecule-mediated apical dendrite growth.
PTPα mediates signal transduction of CHL1 and NB-3
Our results indicate that PTPα is a signal mediator for CHL1 and NB-3 in the apical dendrite development. CHL1 binds to PTPα both in vitro and in vivo (Figure 6). Co-immunoprecipitation of NB-3 and PTPα was observed in transfected HEK293T cells (Supplementary Figure 5), but was not successful in vivo. This may be due to the NB-3 antibody's low efficiency in immunoprecipitation (as seen in Figure 1) and in immunoblotting. PTPα redistributes to CHL1 or NB-3 clusters induced by their corresponding antibodies (Figure 6 and Supplementary Figure 5). Clustering of either CHL1 or NB-3 leads to increased dephosphorylation of p59fyn at Y531 by PTPα in HEK293T cells (Figure 7), suggesting that CHL1 and NB-3 are able to signal independently through PTPα. Also, in the Chl1−/− and Nb-3−/− mouse brains, dephosphorylation of p59fyn at Y531 is impaired (Figure 7).
Ptpα−/− mice exhibit defects in neuronal migration in the neocortex (Petrone et al, 2003), which are also observed in the caudal cortex of Chl1−/− mice (Demyanenko et al, 2004). Also, we found misoriented, even inverted, apical dendrites in layer V pyramidal neurons in the medial cortex of Ptpα−/− mice (Figure 8). The Ptpα−/− motor cortex has increased numbers of pyramidal neurons with aberrant apical dendrites, which was not seen in the Chl1−/− and Nb-3−/− mice, suggesting that PTPα mediates signaling of other molecules in this region. p59fyn may be one of the downstream effectors of CHL1/NB-3 and PTPα signaling in dendrite development, as Fyn−/− mice also have inverted apical dendrites in the deep layer pyramidal neurons in the medial cortex (Sasaki et al, 2002), resembling the phenotype of Ptpα−/− mice. Therefore, CHL1 and NB-3 may regulate the activity of p59fyn via PTPα, eventually leading to microtubule reorganization and actin dynamics in developing neurons.
In summary, we show that neural recognition molecules NB-3 and CHL1 signal through PTPα to regulate apical dendrite development in the deep layer of the caudal neocortex. In the developing cortex, Sema3A acts as a chemoattractant guidance cue; CHL1 and NB-3 may function as modulators of cellular contact and adhesion. Together they regulate apical dendrite development of deep layer pyramidal neurons in specific areas of the neocortex.
Materials and methods
Mice
CHL1- (Montag-Sallaz et al, 2002), NB-3- (Takeda et al, 2003), and PTPα-deficient (Ponniah et al, 1999) mice and Thy1/YFP transgenic mice (line H) (Feng et al, 2000) were described. PTPα-deficient mice are on 129J background, while all other mice are on C57BL/6 background. Heterozygous mice were mated to generate wild-type and knockout pairs. Chl1+/− mice were crossed with Nb-3+/− mice to obtain wild-type, single-heterozygous and compound-heterozygous mice in the same litters. All procedures were approved and monitored by the Institutional Animal Care and Use Committee (IACUC) in Biological Resource Center (BRC) of Singapore.
Antibodies
Rabbit polyclonal antibodies (pAB) against NB-3 (Takeda et al, 2003; Cui et al, 2004), CHL1 (Holm et al, 1996; Demyanenko et al, 2004), L1 (Rathjen and Schachner, 1984), and PTPα (Bhandari et al, 1998) were used in immunoprecipitation, blotting, and staining. The pAB anti-NB-3 recognizes a major band (∼135 kDa, correlates with NB-3) in the wild-type brain extracts, while a faint band with a slightly lower molecular weight is also present in the Nb-3−/− brain extracts (Figure 3D), which may explain the background staining of NB-3 in the brain slices and cell culture. Mouse pAB anti-CHL1 (Abnova) and mAB anti-NB-3 were used in cortical neuron clustering assay. The mAB anti-NB-3 was made against mouse NB-3 (aa. 691–879) and the specificity was tested (Supplementary Figure 5B). Rabbit pAB against Y527–dephosphorylated src kinase that cross-reacts with Y531–dephosphorylated p59fyn (Cell Signaling) was used. Other antibodies were: HA (mAB, Cell Signaling; rabbit pAB, Sigma), Myc (Cell Signaling), VSVG (Roche), Fyn (15) (Santa Cruz), MAP2, GFAP and α-tubulin (all from Sigma).
Golgi impregnation and analysis of dendrite orientation
Golgi impregnation was done using FD Rapid GolgiStain™ Kit (FD NeuroTechnologies) according to the manual. After staining, images were taken and analyzed using Carl Zeiss AIM LSM5 Image Browser. Detailed information about analysis of dendrite orientation is provided in Supplementary data.
Supplementary Material
Supplementary Information
Supplementary Figures
Acknowledgments
We thank Dr DD Ginty and R Kuruvilla from Johns Hopkins University, and Dr C Gu from Harvard Medical School for helpful comments on the manuscript. This work was supported by grants to ZC Xiao from the National Medical Research Council of Singapore, Singapore Health Services Pte Ltd, Department of Clinical Research, Singapore General Hospital, Institute of Molecular and Cell Biology, A*STAR, Singapore; grants to K Watanabe from Grant-in-Aid for Scientific Research (B) (no. 18300120) and Grant-in-Aid for Scientific Research on Priority Areas (no. 16047208); a grant to CJ Pallen (MOP-62759) from the Canadian Institutes of Health Research; grants to SQ Wang from Ministry of Science and Technology (973 no. 2004CB720007) and National Science Foundation of China (no. 30421004). Schachner is New Jersey Professor for Spinal Cord Research.
References
- Angeloni D, Lindor NM, Pack S, Latif F, Wei MH, Lerman MI (1999) CALL gene is haploinsufficient in a 3p− syndrome patient. Am J Med Genet 86: 482–485 [DOI] [PubMed] [Google Scholar]
- Bhandari V, Lim KL, Pallen CJ (1998) Physical and functional interactions between receptor-like protein-tyrosine phosphatase α and p59fyn. J Biol Chem 273: 8691–8698 [DOI] [PubMed] [Google Scholar]
- Bodrikov V, Leshchyns'ka I, Sytnyk V, Overvoorde J, den Hertog J, Schachner M (2005) RPTPα is essential for NCAM-mediated p59fyn activation and neurite elongation. J Cell Biol 168: 127–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhusi M, Midkiff BR, Gates AM, Richter M, Schachner M, Maness PF (2003) Close homolog of L1 is an enhancer of integrin-mediated cell migration. J Biol Chem 278: 25024–25031 [DOI] [PubMed] [Google Scholar]
- Castellani V, Chedotal A, Schachner M, Faivre-Sarrailh C, Rougon G (2000) Analysis of the L1-deficient mouse phenotype reveals cross-talk between Sema3A and L1 signaling pathways in axonal guidance. Neuron 27: 237–249 [DOI] [PubMed] [Google Scholar]
- Castellani V, De Angelis E, Kenwrick S, Rougon G (2002) Cis and trans interactions of L1 with neuropilin-1 control axonal responses to semaphorin 3A. EMBO J 21: 6348–6357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen QY, Chen Q, Feng GY, Lindpaintner K, Chen Y, Sun X, Chen Z, Gao Z, Tang J, He L (2005) Case–control association study of the close homologue of L1 (CHL1) gene and schizophrenia in the Chinese population. Schizophr Res 73: 269–274 [DOI] [PubMed] [Google Scholar]
- Cui XY, Hu QD, Tekaya M, Shimoda Y, Ang BT, Nie DY, Sun L, Hu WP, Karsak M, Duka T, Takeda Y, Ou LY, Dawe GS, Yu FG, Ahmed S, Jin LH, Schachner M, Watanabe K, Arsenijevic Y, Xiao ZC (2004) NB-3/Notch1 pathway via Deltex1 promotes neural progenitor cell differentiation into oligodendrocytes. J Biol Chem 279: 25858–25865 [DOI] [PubMed] [Google Scholar]
- Demyanenko GP, Schachner M, Anton E, Schmid R, Feng G, Sanes J, Maness PF (2004) Close homolog of L1 modulates area-specific neuronal positioning and dendrite orientation in the cerebral cortex. Neuron 44: 423–437 [DOI] [PubMed] [Google Scholar]
- Demyanenko GP, Tsai AY, Maness PF (1999) Abnormalities in neuronal process extension, hippocampal development, and the ventricular system of L1 knockout mice. J Neurosci 19: 4907–4920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR (2000) Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 28: 41–51 [DOI] [PubMed] [Google Scholar]
- Holm J, Hillenbrand R, Steuber V, Bartsch U, Moos M, Lubbert H, Montag D, Schachner M (1996) Structural features of a close homologue of L1 (CHL1) in the mouse: a new member of the L1 family of neural recognition molecules. Eur J Neurosci 8: 1613–1629 [DOI] [PubMed] [Google Scholar]
- Jakovcevski I, Wu J, Karl N, Leshchyns'ka I, Sytnyk V, Chen J, Irintchev A, Schachner M (2007) Glial scar expression of CHL1, the close homolog of the adhesion molecule L1, limits recovery after spinal cord injury. J Neurosci 27: 7222–7233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan YN, Jan LY (2003) The control of dendrite development. Neuron 40: 229–242 [DOI] [PubMed] [Google Scholar]
- Kadmon G, Kowitz A, Altevogt P, Schachner M (1990) The neural cell adhesion molecule N-CAM enhances L1-dependent cell–cell interactions. J Cell Biol 110: 193–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le HT, Maksumova L, Wang J, Pallen CJ (2006) Reduced NMDA receptor tyrosine phosphorylation in PTPα-deficient mouse synaptosomes is accompanied by inhibition of four src family kinases and Pyk2: an upstream role for PTPα in NMDA receptor regulation. J Neurochem 98: 1798–1809 [DOI] [PubMed] [Google Scholar]
- Lee S, Takeda Y, Kawano H, Hosoya H, Nomoto M, Fujimoto D, Takahashi N, Watanabe K (2000) Expression and regulation of a gene encoding neural recognition molecule NB-3 of the contactin/F3 subgroup in mouse brain. Gene 245: 253–266 [DOI] [PubMed] [Google Scholar]
- Maness PF, Schachner M (2007) Neural recognition molecules of the immunoglobulin superfamily: signaling transducers of axon guidance and neuronal migration. Nat Neurosci 10: 19–26 [DOI] [PubMed] [Google Scholar]
- Montag-Sallaz M, Schachner M, Montag D (2002) Misguided axonal projections, neural cell adhesion molecule 180 mRNA upregulation, and altered behavior in mice deficient for the close homolog of L1. Mol Cell Biol 22: 7967–7981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrone A, Battaglia F, Wang C, Dusa A, Su J, Zagzag D, Bianchi R, Casaccia-Bonnefil P, Arancio O, Sap J (2003) Receptor protein tyrosine phosphatase α is essential for hippocampal neuronal migration and long-term potentiation. EMBO J 22: 4121–4131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polleux F, Morrow T, Ghosh A (2000) Semaphorin 3A is a chemoattractant for cortical apical dendrites. Nature 404: 567–573 [DOI] [PubMed] [Google Scholar]
- Ponniah S, Wang DZ, Lim KL, Pallen CJ (1999) Targeted disruption of the tyrosine phosphatase PTPα leads to constitutive downregulation of the kinases Src and Fyn. Curr Biol 9: 535–538 [DOI] [PubMed] [Google Scholar]
- Pratte M, Rougon G, Schachner M, Jamon M (2003) Mice deficient for the close homologue of the neural adhesion cell L1 (CHL1) display alterations in emotional reactivity and motor coordination. Behav Brain Res 147: 31–39 [DOI] [PubMed] [Google Scholar]
- Rathjen FG, Schachner M (1984) Immunocytological and biochemical characterization of a new neuronal cell surface component (L1 antigen) which is involved in cell adhesion. EMBO J 3: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Cheng C, Uchida Y, Nakajima O, Ohshima T, Yagi T, Taniguchi M, Nakayama T, Kishida R, Kudo Y, Ohno S, Nakamura F, Goshima Y (2002) Fyn and Cdk5 mediate semaphorin-3A signaling, which is involved in regulation of dendrite orientation in cerebral cortex. Neuron 35: 907–920 [DOI] [PubMed] [Google Scholar]
- Semba K, Nishizawa M, Miyajima N, Yoshida MC, Sukegawa J, Yamanashi Y, Sasaki M, Yamamoto T, Toyoshima K (1986) yes-related protooncogene, syn, belongs to the protein-tyrosine kinase family. Proc Natl Acad Sci USA 83: 5459–5463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J, Muranjan M, Sap J (1999) Receptor protein tyrosine phosphatase α activates Src-family kinases and controls integrin-mediated responses in fibroblasts. Curr Biol 9: 505–511 [DOI] [PubMed] [Google Scholar]
- Takeda Y, Akasaka K, Lee S, Kobayashi S, Kawano H, Murayama S, Takahashi N, Hashimoto K, Kano M, Asano M, Sudo K, Iwakura Y, Watanabe K (2003) Impaired motor coordination in mice lacking neural recognition molecule NB-3 of the contactin/F3 subgroup. J Neurobiol 56: 252–265 [DOI] [PubMed] [Google Scholar]
- Whitford KL, Dijkhuizen P, Polleux F, Ghosh A (2002) Molecular control of cortical dendrite development. Annu Rev Neurosci 25: 127–149 [DOI] [PubMed] [Google Scholar]
- Zeng L, D'Alessandri L, Kalousek MB, Vaughan L, Pallen CJ (1999) Protein tyrosine phosphatase α (PTPα) and contactin form a novel neuronal receptor complex linked to the intracellular tyrosine kinase fyn. J Cell Biol 147: 707–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Supplementary Figures