Abstract
Major histocompatibility complex class II (MHC II) molecules are targeted to endocytic compartments, known as MIIC, by the invariant chain (Ii) that is degraded upon arrival in these compartments. MHC II acquire antigenic fragments from endocytosed proteins for presentation at the cell surface. In a unique and complex series of reactions, MHC II succeed in exchanging a remaining fragment of Ii for other protein fragments in subdomains of MIIC before transport to the cell surface. Here, the mechanisms regulating loading and intracellular trafficking of MHC II are discussed.
Keywords: intracellular transport, MHC class II, MIIC, motor proteins, Rab7
Role of MHC II in the immune system
Major histocompatibility complex class II molecules (MHC II) are expressed by immune cells like B cells, dendritic cells (DCs), and monocytes/macrophages and designed to stably bind and present fragments from exogenous proteins to the immune system. MHC II present antigens to CD4+ T-helper cells and then control differentiation of B cells in antibody-producing B-cell blasts. Patients or mice failing to produce proper MHC II–peptide complexes will not produce efficient antibody responses to infection (Viville et al, 1993). MHC II are also important to control cytotoxic T-cell activation, autoimmune responses and other responses to pathogens or the environment.
MHC II are polymorphic and various MHC II alleles show linkage disequilibrium to a variety of autoimmune diseases. These cannot be linked entirely to the MHC II allele implying further involvement of genetic and/or environmental factors. For example, 95% of patients with Celiac Disease express an MHC II molecule, HLA-DQ2, present in 25% of the population. The gliadin peptide (a gluten fragment) is selectively presented by HLA-DQ2, which, in addition to unknown factors, causes this disorder (www.enabling.org/ia/celiac). Studying the cell biology of antigen presentation by MHC II is of crucial importance to identify these factors or reveal modes for controlling MHC II antigen presentation.
How MHC II acquire peptides in the endocytic route?
Antigen loading of MHC II occurs in the endocytic pathway at a site that is commonly known as MIIC (for ‘MHC class II-containing compartment') (Neefjes et al, 1990). MHC II assemble as heterodimers in the endoplasmic reticulum (ER) to form a peptide-binding groove (Brown et al, 1993). Efficient ER egress of MHC II is assisted by the invariant chain (Ii) (Bikoff et al, 1993; Viville et al, 1993). An Ii region called CLIP occupies the peptide-binding groove, thereby preventing premature peptide binding (Roche and Cresswell, 1990). Ii also contains a cytosolic di-leucine-targeting motif that directs MHC II complexes into the endocytic pathway, either directly from the trans-Golgi network or—if this fails—via rapid internalization (Bakke and Dobberstein, 1990; Roche et al, 1993). After having guided MHC II to MIIC, Ii is degraded by various late endosomal proteases, including cathepsin S and L, to prepare MHC II for peptide loading. Inhibition of these proteases will prevent MHC II antigen presentation, immune responses (Riese and Chapman, 2000) and also cell surface expression (Neefjes and Ploegh, 1992). Consequently, inhibitors for cathepsin S are currently developed for the treatment of autoimmune diseases (Vasiljeva et al, 2007). The proteases degrade Ii in a stepwise fashion leaving the CLIP fragment occupying the peptide-binding groove. The resulting MHC II complex does not contain relevant antigenic information for the immune system. Exchange of CLIP for such antigenic fragments is facilitated by low pH, proteolytic trimming of the CLIP peptide, and by a unique chaperone called HLA-DM, which is surprisingly an MHC II look-alike (Mosyak et al, 1998). HLA-DM is a dedicated chaperone (only target known is MHC II) in a compartment where other proteins are usually degraded. HLA-DM stabilizes MHC II devoid of peptides, preventing aggregation and supporting peptide exchange until a high-affinity-binding peptide is acquired (Sloan et al, 1995; Denzin et al, 1996). HLA-DM is thus editing the MHC II peptide repertoire (Kropshofer et al, 1996). But the reaction is more complicated. The interaction between MHC II and HLA-DM occurs in subdomains of the MIIC (the intraluminal vesicles) and not at the limiting membrane as determined by FRET studies (Zwart et al, 2005). Consequently, MHC II fails to acquire antigenic peptides in phagosomes containing intracellular bacteria as these lack intraluminal vesicles (Zwart et al, 2005). Possibly, microdomains like those formed by members of the tetraspanin family of proteins (the tetraspanin web) residing in the intraluminal vesicles of the MIIC and interacting with MHC II, HLA-DM, and other proteins (Hammond et al, 1998) play an additional role in efficient peptide loading of MHC II.
Whether loading of MHC II with high-affinity peptides is a prerequisite for transport from MIIC to the plasma membrane is unlikely. Endosomes may not have a sophisticated ‘quality control system' like the ER that allows the egress of properly folded proteins only, since CLIP exchange by HLA-DM is not required for cell surface expression of MHC II (Fung-Leung et al, 1996; Martin et al, 1996). Proper expression levels of HLA-DM, transport of MHC II and HLA-DM to internal vesicles in MVB, transit time of MHC II through the MIIC, proteolysis of antigen and Ii, and delivery of antigenic fragments (by diffusion?) to MHC II probably ensure that the system suffices to efficiently load MHC II in transit through the MIIC (Figure 1).
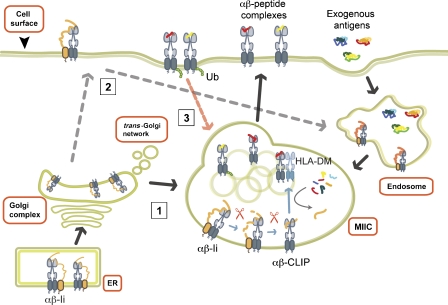
Figure 1.
The cell biology of antigen presentation by MHC II. MHC II αβ heterodimers are assembled in the endoplasmic reticulum (ER) and form a peptide-binding groove that is occupied by Ii. Ii chaperones MHC II often directly (route 1; black solid arrows) and sometimes indirectly after internalization from the cell surface (route 2; gray dashed arrows) into MIIC where Ii is degraded by a series of endosomal proteases with the CLIP fragment remaining (orange). HLA-DM assists exchange of CLIP for relevant exogenous antigenic fragments (red or yellow) in subdomains of MIIC (the internal vesicles) prior to transport for stable integration in the plasma membrane (blue arrows in MIIC) unless internalization is induced by processes like ubiquitination (Ub) of the MHC II β-chain cytoplasmic tail (route 3; pink dashed arrow).
Definition of the MIIC
The exact definition of THE MIIC as the site of MHC II peptide loading has been a matter of debate. Originally, the MIIC was defined based on immuno-electronmicroscopy studies as a late endosome (LE) with multilamellar morphology containing MHC II (Peters et al, 1991). MHC II was subsequently found in many different compartments with distinct morphologies and its expression in HEK 293 cells even induced the multilamellar morphology (Calafat et al, 1994). Thus, neither morphology nor the presence of MHC II can define THE MIIC. Other factors required for efficient loading of MHC II include acidic pH (Ziegler and Unanue, 1982), HLA-DM and proteases like cathepsin S and L (Honey and Rudensky, 2003). Electronmicroscopy showed that these locate in LEs that label for the conventional markers Lamp-1 and CD63.
Is the MIIC then a unique compartment or an LE expressing additional proteins for MHC II antigen presentation? Eliminating MHC II, cathepsin S or HLA-DM still shows LEs labeling for the conventional markers, indicating that MHC II-related proteins are not critical in this compartment. In addition, LEs lacking MHC II are difficult to detect in cells expressing MHC II. MIIC appears to be an LE with the components for efficient MHC II loading. Still, loading of MHC II at nearly every location of the endocytic route is reported. Since HLA-DM is transported in the MIIC to the plasma membrane along with MHC II (Wubbolts et al, 1996), loading may even be supported by HLA-DM at the plasma membrane (Moss et al, 2007), albeit at neutral pH and without proteases for antigen preparation. Moreover, HLA-DM contains a classical tyrosine-based internalization motif and will be internalized, thus entering early endosomal compartments in transit to MIIC. In principle, HLA-DM support in MHC II loading can occur whenever protein fragments are present, although the late endosomal MIIC likely is the primary site for antigen loading of MHC II, since it congregates all known components for efficient peptide loading.
Further control of MHC II antigen presentation
The complex process of MHC II antigen presentation is further complicated by additional factors. Immature B cells express an HLA-DM homolog called HLA-DO (Liljedahl et al, 1996). This non-polymorphic MHC II-like molecule stably interacts with HLA-DM and acts as a pH sensor to preferentially stimulate presentation of antigens entering the more acidic LEs at the cost of normal HLA-DM functioning, paradoxically resulting in MHC II–CLIP complexes and reduced immune responses (Denzin et al, 1997; van Ham et al, 1997). Other factors involved in MHC II presentation are more related to the control of protein targeting to MIIC or the control of proteolysis. Antibody-bound proteins can be recognized by Fc receptors for uptake, transfer to MIIC, and degradation. Analogously, surface Ig receptors on B cells can specifically recognize and target antigens to LEs for degradation, which also affects the specificity of antigen proteolysis (Davidson and Watts, 1989). Alterations in proteolytic conditions contribute to the success of MHC II antigen presentation as well. In classic experiments, neutralization of acidic compartments inhibited MHC II antigen presentation, implying lysosomal proteases in antigen presentation (Ziegler and Unanue, 1982).
Some late endosomal proteases are critical in MHC II antigen presentation. Cathepsin S- and L-deficient mice have reduced Ii degradation and antigen presentation (Nakagawa et al, 1998; Shi et al, 1999). To complicate matters, naturally occurring inhibitors of lysosomal proteases, called cystatins, can also exert a regulatory role. Overexpression of cystatin C inhibits the activity of cathepsin S, and consequently, Ii degradation and MHC II cell surface expression in DC (Pierre and Mellman, 1998).
Finally, control of MHC II antigen presentation by interleukins and Toll-like receptors (Blander and Medzhitov, 2006) occurs in particular cell types. The ‘immunosuppressive' interleukin IL-10 prevents MHC II cell surface expression in human monocytes (Koppelman et al, 1997), whereas interferon-γ enhances MHC II expression and presentation.
Proteases, protease inhibitors, protease conditions, and substrate delivery are all factors contributing to the efficiency and specificity of MHC II antigen presentation and therefore represent attractive targets for manipulating immune responses. In addition, motor proteins, kinases, GTPases, and possibly other signaling systems control MHC II presentation. These include the actin-based motor protein myosin II that interacts with Ii following B-cell receptor activation and is essential for antigen presentation (Vascotto et al, 2007), and GTPases of the families Rab and Rho (Ghittoni et al, 2006). We are only beginning to grasp the complexity of regulating MHC II antigen presentation.
How to move MHC II to the plasma membrane?
Trafficking of late endosomal proteins, including MHC II, to the plasma membrane is poorly understood. LEs may not have the machinery for the selective sorting of molecules and the appearance of many late endosomal proteins at the plasma membrane is followed by efficient internalization and transport back to LEs. Ii contains the targeting motif for MHC II. Since degradation of this motif occurs in the MIIC, MHC II remains stable at the plasma membrane upon delivery, unless internalization is supported for example by its ubiquitination (Shin et al, 2006; van Niel et al, 2006).
Transport of GFP-tagged MHC II has been studied in tissue culture cells (Wubbolts et al, 1996), B cells, and mouse DCs (Boes et al, 2002; Chow et al, 2002). We visualized MIIC with GFP-tagged MHC II exhibiting the canonical motility of LEs. These two similar compartments move in a so-called bidirectional manner and in a stop-and-go fashion along microtubules to the plasma membrane (Wubbolts et al, 1996). This required the activities of oppositely directed motor proteins, dynein (powers transport to the microtubule-organizing center) and kinesin (powers outward transport) (Wubbolts et al, 1999). Ultimately, MIIC fuses to the plasma membrane (Raposo et al, 1996; Wubbolts et al, 1996).
An additional route for the transport of MHC II to the plasma membrane has been observed in activated DC. Upon activation, DCs upregulate surface expression of MHC II from intracellular storages and tubular structures emanating from the MIIC and containing MHC II are formed (Kleijmeer et al, 2001; Boes et al, 2002; Chow et al, 2002). Live imaging revealed that these tubules exhibit dynamics similar to MIIC, including bidirectional microtubule-based movement in a stop-and-go fashion (Vyas et al, 2007). Since immature DCs, B cells, and melanoma do not show these tubules but do express MHC II at the plasma membrane, tubules may be an activated DC-selective route for the transport of MHC II to the cell surface.
How MIIC (and possibly tubules) fuses to the plasma membrane is unclear. It probably requires the activities of Rab GTPases, actin-based motor proteins, and actin depolymerizing factors, analogously to the situation for other specialized lysosome-related organelles such as cytolytic granules and melanosomes (Jordens et al, 2006; Raposo et al, 2007).
Two collaborating receptors for one or more motor proteins on MIIC
Rab7 is a small Rab GTPase decorating membranes of MIIC and other late endocytic structures (Chavrier et al, 1990; Meresse et al, 1995; Wubbolts et al, 1996). Activated Rab7 specifies the target membrane for dynein recruitment through an interaction of its effector Rab7-interacting lysosomal protein (RILP) with the p150Glued subunit of dynactin, a critical component of the dynein motor complex (Johansson et al, 2007). RILP expression promotes inward-directed dynein-mediated transport of MIIC/LEs to the microtubule minus-end (Jordens et al, 2001).
The Rab7-RILP complex interacts with a second effector protein—OSBP-related protein 1L (ORP1L)—to form a tripartite complex on lysosomal membranes. ORP1L is required to transfer the dynein/dynactin motor complex from the specific lysosomal receptor Rab7-RILP to a general receptor termed βIII spectrin (Johansson et al, 2007). βIII spectrin is located on the cytosolic side of multiple compartments and can interact, via its actin-binding domain, with actin-related protein 1 (Arp1) at the base of dynactin (Karki and Holzbaur, 1999). The dynein motor only becomes active after consecutive interactions with these two membrane-associated receptors: the LE-specific receptor Rab7-RILP and the general receptor βIII spectrin (Johansson et al, 2007) (Figure 2).
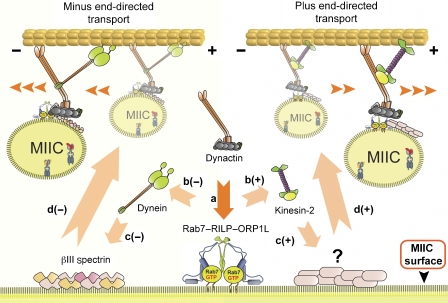
Figure 2.
Reciprocal coordination of motor proteins for bidirectional microtubule-based MIIC transport. Left: control of inward transport of MIIC toward the microtubule minus-end. Right: control of plus end-directed transport of MIIC to the cell periphery. Activation of Rab7 precedes formation of the tripartite Rab7-RILP-ORP1L complex. RILP interacts with the dynactin subunit p150Glued (a). Dynactin then interacts either with dynein (b(−)) or kinesin-2 (KIF3) (b(+)) motor proteins, specifying the direction of vesicle transport. Motor activity requires binding to a second LE membrane receptor, βIII spectrin (c(−)). Full activation of kinesin-2 may require a similar interaction with a general receptor on MIIC (c(+)). In this model, the p150Glued-associated type of motor specifies the direction of MIIC transport (d).
The bidirectional nature of vesicle movement implies that, in addition to the inward-directed dynein motor, at least one outward-directed motor is involved. Two members of the kinesin superfamily of motors may be involved in outward-directed motility of LEs along microtubules. Kinesin-1 (conventional kinesin or KIF5) but also kinesin-2 (heterotrimeric kinesin or KIF3) have been implicated (Hollenbeck and Swanson, 1990; Wubbolts et al, 1999).
How do motors of opposite polarity cooperate to achieve bidirectional motility? They may be reciprocally coordinated and not act simultaneously on one individual vesicle. Xenopus melanophores as well as Drosophila fast axonal cargoes and lipid droplets use dynactin (or its subunit p150Glued) to interact with dynein and KIF3 motors in a mutually exclusive manner (Deacon et al, 2003). Furthermore, disruption of the dynactin complex by overexpressing p50dynamitin (Burkhardt et al, 1997) inhibits both minus- and plus-end motility (Deacon et al, 2003). The dynactin subunit p150Glued may be the adaptor for KIF3 and dynein on LEs (Deacon et al, 2003; Brown et al, 2005). Thus, the bidirectionality of MIIC movement may be accomplished by alternating interactions of p150Glued-dynein and p150Glued-KIF3 motor complexes with a single Rab7-RILP receptor on MIIC that likely employs βIII spectrin in both cases (Figure 2). The interaction of Rab7-RILP with p150Glued (the common motor adaptor for dynein and kinesin) would then be at the heart of the bidirectionality of MIIC motility.
The control of motor activities and motor-receptor binding may involve kinases, lipids, the Rab7 GTPase cycle, IL-10 signaling, JNK-interacting proteins (JIPs), and undoubtedly many other factors. How these factors control the motility of MIIC and how these factors are subsequently controlled remains to be determined.
Antigen presentation by MHC II incorporates activities like late endosomal proteolysis of Ii and antigen, regulation of late endosomal morphology and pH, and intracellular transport. Further identification of molecules involved in controlling these processes should provide targets for further manipulation of MHC II-restricted immune responses, particularly those resulting in autoimmune responses.
References
- Bakke O, Dobberstein B (1990) MHC class II-associated invariant chain contains a sorting signal for endosomal compartments. Cell 63: 707–716 [DOI] [PubMed] [Google Scholar]
- Bikoff EK, Huang LY, Episkopou V, van Meerwijk J, Germain RN, Robertson EJ (1993) Defective major histocompatibility complex class II assembly, transport, peptide acquisition, and CD4+ T cell selection in mice lacking invariant chain expression. J Exp Med 177: 1699–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blander JM, Medzhitov R (2006) Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature 440: 808–812 [DOI] [PubMed] [Google Scholar]
- Boes M, Cerny J, Massol R, Op den Brouw M, Kirchhausen T, Chen J, Ploegh HL (2002) T-cell engagement of dendritic cells rapidly rearranges MHC class II transport. Nature 418: 983–988 [DOI] [PubMed] [Google Scholar]
- Brown CL, Maier KC, Stauber T, Ginkel LM, Wordeman L, Vernos I, Schroer TA (2005) Kinesin-2 is a motor for late endosomes and lysosomes. Traffic 6: 1114–1124 [DOI] [PubMed] [Google Scholar]
- Brown JH, Jardetzky TS, Gorga JC, Stern LJ, Urban RG, Strominger JL, Wiley DC (1993) Three-dimensional structure of the human class II histocompatibility antigen HLA-DR1. Nature 364: 33–39 [DOI] [PubMed] [Google Scholar]
- Burkhardt JK, Echeverri CJ, Nilsson T, Vallee RB (1997) Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J Cell Biol 139: 469–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat J, Nijenhuis M, Janssen H, Tulp A, Dusseljee S, Wubbolts R, Neefjes J (1994) Major histocompatibility complex class II molecules induce the formation of endocytic MIIC-like structures. J Cell Biol 126: 967–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavrier P, Parton RG, Hauri HP, Simons K, Zerial M (1990) Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell 62: 317–329 [DOI] [PubMed] [Google Scholar]
- Chow A, Toomre D, Garrett W, Mellman I (2002) Dendritic cell maturation triggers retrograde MHC class II transport from lysosomes to the plasma membrane. Nature 418: 988–994 [DOI] [PubMed] [Google Scholar]
- Davidson HW, Watts C (1989) Epitope-directed processing of specific antigen by B lymphocytes. J Cell Biol 109: 85–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon SW, Serpinskaya AS, Vaughan PS, Lopez Fanarraga M, Vernos I, Vaughan KT, Gelfand VI (2003) Dynactin is required for bidirectional organelle transport. J Cell Biol 160: 297–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denzin LK, Hammond C, Cresswell P (1996) HLA-DM interactions with intermediates in HLA-DR maturation and a role for HLA-DM in stabilizing empty HLA-DR molecules. J Exp Med 184: 2153–2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denzin LK, Sant'Angelo DB, Hammond C, Surman MJ, Cresswell P (1997) Negative regulation by HLA-DO of MHC class II-restricted antigen processing. Science 278: 106–109 [DOI] [PubMed] [Google Scholar]
- Fung-Leung WP, Surh CD, Liljedahl M, Pang J, Leturcq D, Peterson PA, Webb SR, Karlsson L (1996) Antigen presentation and T cell development in H2-M-deficient mice. Science 271: 1278–1281 [DOI] [PubMed] [Google Scholar]
- Ghittoni R, Napolitani G, Benati D, Ulivieri C, Patrussi L, Laghi Pasini F, Lanzavecchia A, Baldari CT (2006) Simvastatin inhibits the MHC class II pathway of antigen presentation by impairing Ras superfamily GTPases. Eur J Immunol 36: 2885–2893 [DOI] [PubMed] [Google Scholar]
- Hammond C, Denzin LK, Pan M, Griffith JM, Geuze HJ, Cresswell P (1998) The tetraspan protein CD82 is a resident of MHC class II compartments where it associates with HLA-DR, -DM, and -DO molecules. J Immunol 161: 3282–3291 [PubMed] [Google Scholar]
- Hollenbeck PJ, Swanson JA (1990) Radial extension of macrophage tubular lysosomes supported by kinesin. Nature 346: 864–866 [DOI] [PubMed] [Google Scholar]
- Honey K, Rudensky AY (2003) Lysosomal cysteine proteases regulate antigen presentation. Nat Rev Immunol 3: 472–482 [DOI] [PubMed] [Google Scholar]
- Johansson M, Rocha N, Zwart W, Jordens I, Janssen L, Kuijl C, Olkkonen VM, Neefjes J (2007) Activation of endosomal dynein motors by stepwise assembly of Rab7-RILP-p150Glued, ORP1L, and the receptor betalll spectrin. J Cell Biol 176: 459–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordens I, Fernandez-Borja M, Marsman M, Dusseljee S, Janssen L, Calafat J, Janssen H, Wubbolts R, Neefjes J (2001) The Rab7 effector protein RILP controls lysosomal transport by inducing the recruitment of dynein–dynactin motors. Curr Biol 11: 1680–1685 [DOI] [PubMed] [Google Scholar]
- Jordens I, Westbroek W, Marsman M, Rocha N, Mommaas M, Huizing M, Lambert J, Naeyaert JM, Neefjes J (2006) Rab7 and Rab27a control two motor protein activities involved in melanosomal transport. Pigment Cell Res 19: 412–423 [DOI] [PubMed] [Google Scholar]
- Karki S, Holzbaur EL (1999) Cytoplasmic dynein and dynactin in cell division and intracellular transport. Curr Opin Cell Biol 11: 45–53 [DOI] [PubMed] [Google Scholar]
- Kleijmeer M, Ramm G, Schuurhuis D, Griffith J, Rescigno M, Ricciardi-Castagnoli P, Rudensky AY, Ossendorp F, Melief CJ, Stoorvogel W, Geuze HJ (2001) Reorganization of multivesicular bodies regulates MHC class II antigen presentation by dendritic cells. J Cell Biol 155: 53–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppelman B, Neefjes JJ, de Vries JE, de Waal Malefyt R (1997) Interleukin-10 down-regulates MHC class II alphabeta peptide complexes at the plasma membrane of monocytes by affecting arrival and recycling. Immunity 7: 861–871 [DOI] [PubMed] [Google Scholar]
- Kropshofer H, Vogt AB, Moldenhauer G, Hammer J, Blum JS, Hammerling GJ (1996) Editing of the HLA-DR-peptide repertoire by HLA-DM. EMBO J 15: 6144–6154 [PMC free article] [PubMed] [Google Scholar]
- Liljedahl M, Kuwana T, Fung-Leung WP, Jackson MR, Peterson PA, Karlsson L (1996) HLA-DO is a lysosomal resident which requires association with HLA-DM for efficient intracellular transport. EMBO J 15: 4817–4824 [PMC free article] [PubMed] [Google Scholar]
- Martin WD, Hicks GG, Mendiratta SK, Leva HI, Ruley HE, Van Kaer L (1996) H2-M mutant mice are defective in the peptide loading of class II molecules, antigen presentation, and T cell repertoire selection. Cell 84: 543–550 [DOI] [PubMed] [Google Scholar]
- Meresse S, Gorvel JP, Chavrier P (1995) The rab7 GTPase resides on a vesicular compartment connected to lysosomes. J Cell Sci 108 (Part 11): 3349–3358 [DOI] [PubMed] [Google Scholar]
- Moss CX, Tree TI, Watts C (2007) Reconstruction of a pathway of antigen processing and class II MHC peptide capture. EMBO J 26: 2137–2147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosyak L, Zaller DM, Wiley DC (1998) The structure of HLA-DM, the peptide exchange catalyst that loads antigen onto class II MHC molecules during antigen presentation. Immunity 9: 377–383 [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Roth W, Wong P, Nelson A, Farr A, Deussing J, Villadangos JA, Ploegh H, Peters C, Rudensky AY (1998) Cathepsin L: critical role in Ii degradation and CD4 T cell selection in the thymus. Science 280: 450–453 [DOI] [PubMed] [Google Scholar]
- Neefjes JJ, Ploegh HL (1992) Inhibition of endosomal proteolytic activity by leupeptin blocks surface expression of MHC class II molecules and their conversion to SDS resistance alpha beta heterodimers in endosomes. EMBO J 11: 411–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neefjes JJ, Stollorz V, Peters PJ, Geuze HJ, Ploegh HL (1990) The biosynthetic pathway of MHC class II but not class I molecules intersects the endocytic route. Cell 61: 171–183 [DOI] [PubMed] [Google Scholar]
- Peters PJ, Neefjes JJ, Oorschot V, Ploegh HL, Geuze HJ (1991) Segregation of MHC class II molecules from MHC class I molecules in the Golgi complex for transport to lysosomal compartments. Nature 349: 669–676 [DOI] [PubMed] [Google Scholar]
- Pierre P, Mellman I (1998) Developmental regulation of invariant chain proteolysis controls MHC class II trafficking in mouse dendritic cells. Cell 93: 1135–1145 [DOI] [PubMed] [Google Scholar]
- Raposo G, Marks MS, Cutler DF (2007) Lysosome-related organelles: driving post-Golgi compartments into specialisation. Curr Opin Cell Biol 19: 394–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ (1996) B lymphocytes secrete antigen-presenting vesicles. J Exp Med 183: 1161–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riese RJ, Chapman HA (2000) Cathepsins and compartmentalization in antigen presentation. Curr Opin Immunol 12: 107–113 [DOI] [PubMed] [Google Scholar]
- Roche PA, Cresswell P (1990) Invariant chain association with HLA-DR molecules inhibits immunogenic peptide binding. Nature 345: 615–618 [DOI] [PubMed] [Google Scholar]
- Roche PA, Teletski CL, Stang E, Bakke O, Long EO (1993) Cell surface HLA-DR-invariant chain complexes are targeted to endosomes by rapid internalization. Proc Natl Acad Sci USA 90: 8581–8585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi GP, Villadangos JA, Dranoff G, Small C, Gu L, Haley KJ, Riese R, Ploegh HL, Chapman HA (1999) Cathepsin S required for normal MHC class II peptide loading and germinal center development. Immunity 10: 197–206 [DOI] [PubMed] [Google Scholar]
- Shin JS, Ebersold M, Pypaert M, Delamarre L, Hartley A, Mellman I (2006) Surface expression of MHC class II in dendritic cells is controlled by regulated ubiquitination. Nature 444: 115–118 [DOI] [PubMed] [Google Scholar]
- Sloan VS, Cameron P, Porter G, Gammon M, Amaya M, Mellins E, Zaller DM (1995) Mediation by HLA-DM of dissociation of peptides from HLA-DR. Nature 375: 802–806 [DOI] [PubMed] [Google Scholar]
- van Ham SM, Tjin EP, Lillemeier BF, Gruneberg U, van Meijgaarden KE, Pastoors L, Verwoerd D, Tulp A, Canas B, Rahman D, Ottenhoff TH, Pappin DJ, Trowsdale J, Neefjes J (1997) HLA-DO is a negative modulator of HLA-DM-mediated MHC class II peptide loading. Curr Biol 7: 950–957 [DOI] [PubMed] [Google Scholar]
- van Niel G, Wubbolts R, Ten Broeke T, Buschow SI, Ossendorp FA, Melief CJ, Raposo G, van Balkom BW, Stoorvogel W (2006) Dendritic cells regulate exposure of MHC class II at their plasma membrane by oligoubiquitination. Immunity 25: 885–894 [DOI] [PubMed] [Google Scholar]
- Vascotto F, Lankar D, Faure-Andre G, Vargas P, Diaz J, Le Roux D, Yuseff MI, Sibarita JB, Boes M, Raposo G, Mougneau E, Glaichenhaus N, Bonnerot C, Manoury B, Lennon-Dumenil AM (2007) The actin-based motor protein myosin II regulates MHC class II trafficking and BCR-driven antigen presentation. J Cell Biol 176: 1007–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasiljeva O, Reinheckel T, Peters C, Turk D, Turk V, Turk B (2007) Emerging roles of cysteine cathepsins in disease and their potential as drug targets. Curr Pharm Des 13: 385–401 [DOI] [PubMed] [Google Scholar]
- Viville S, Neefjes J, Lotteau V, Dierich A, Lemeur M, Ploegh H, Benoist C, Mathis D (1993) Mice lacking the MHC class II-associated invariant chain. Cell 72: 635–648 [DOI] [PubMed] [Google Scholar]
- Vyas JM, Kim YM, Artavanis-Tsakonas K, Love JC, Van der Veen AG, Ploegh HL (2007) Tubulation of class II MHC compartments is microtubule dependent and involves multiple endolysosomal membrane proteins in primary dendritic cells. J Immunol 178: 7199–7210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wubbolts R, Fernandez-Borja M, Jordens I, Reits E, Dusseljee S, Echeverri C, Vallee RB, Neefjes J (1999) Opposing motor activities of dynein and kinesin determine retention and transport of MHC class II-containing compartments. J Cell Sci 112: 785–795 [DOI] [PubMed] [Google Scholar]
- Wubbolts R, Fernandez-Borja M, Oomen L, Verwoerd D, Janssen H, Calafat J, Tulp A, Dusseljee S, Neefjes J (1996) Direct vesicular transport of MHC class II molecules from lysosomal structures to the cell surface. J Cell Biol 135: 611–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler HK, Unanue ER (1982) Decrease in macrophage antigen catabolism caused by ammonia and chloroquine is associated with inhibition of antigen presentation to T cells. Proc Natl Acad Sci USA 79: 175–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwart W, Griekspoor A, Kuijl C, Marsman M, van Rheenen J, Janssen H, Calafat J, van Ham M, Janssen L, van Lith M, Jalink K, Neefjes J (2005) Spatial separation of HLA-DM/HLA-DR interactions within MIIC and phagosome-induced immune escape. Immunity 22: 221–233 [DOI] [PubMed] [Google Scholar]