Abstract
The liver is frequently challenged by surgery-induced metabolic overload, viruses or toxins, which induce the formation of reactive oxygen species. To determine the effect of oxidative stress on liver regeneration and to identify the underlying signaling pathways, we studied liver repair in mice lacking the Nrf2 transcription factor. In these animals, expression of several cytoprotective enzymes was reduced in hepatocytes, resulting in oxidative stress. After partial hepatectomy, liver regeneration was significantly delayed. Using in vitro and in vivo studies, we identified oxidative stress-mediated insulin/insulin-like growth factor resistance as an underlying mechanism. This deficiency impaired the activation of p38 mitogen-activated kinase, Akt kinase and downstream targets after hepatectomy, resulting in enhanced death and delayed proliferation of hepatocytes. Our results reveal novel roles of Nrf2 in the regulation of growth factor signaling and in tissue repair. In addition, they provide new insight into the mechanisms underlying oxidative stress-induced defects in liver regeneration. These findings may provide the basis for the development of new strategies to improve regeneration in patients with acute or chronic liver damage.
Keywords: insulin, liver regeneration, Nrf2, oxidative stress, PI3 kinase
Introduction
The liver is the only organ in the mammalian body that can fully regenerate after injury. Upon loss of functional liver tissue, for example, by surgery, toxin-induced necrosis or viral infections, quiescent hepatocytes re-enter the cell cycle and proliferate to restore the original liver mass. This is essential to the body, as the liver fulfills central roles in metabolic homeostasis, detoxification of various compounds, and in the synthesis, storage and secretion of nutrients. Impairment of regeneration contributes to the pathogenesis of liver failure or development of fibrosis/cirrhosis (Diehl, 2002).
Liver regeneration after partial hepatectomy can be divided into three phases: priming, proliferation and cessation (Fausto, 2000; Diehl, 2002; Taub, 2004). Priming is mediated by pro-inflammatory cytokines like tumor necrosis factor α (TNF-α) and interleukin 6 (IL-6) (Fausto, 2000; Diehl, 2002; Taub, 2004). The subsequent activation of nuclear factor κB (NF-κB), activator protein 1 (AP-1), and signal transducer and activator of transcription 3 (STAT3) is responsible for G0 to G1 transition and survival of hepatocytes (Cressman et al, 1995; FitzGerald et al, 1995; Heim et al, 1997). Hepatocyte proliferation is regulated by different mitogens, including hepatocyte growth factor (HGF), and ligands of the epidermal growth factor (EGF) and fibroblast growth factor receptors (Mead and Fausto, 1989; Steiling et al, 2003; Borowiak et al, 2004; Huh et al, 2004; Mitchell et al, 2005). In addition, recent studies revealed a role of insulin-like growth factor 1 (IGF-1) and its receptor in hepatocyte proliferation after hepatectomy (Pennisi et al, 2004; Desbois-Mouthon et al, 2006). Once the original liver mass is restored, hepatocytes are rendered quiescent, most likely through transforming growth factor β (TGF-β) and activin signaling (Oe et al, 2004).
The liver often encounters oxidative stress, which affects liver function, induces hepatocyte cell death and disturbs the regeneration process after injury (Fausto, 2000; Kamata et al, 2005; Schwabe and Brenner, 2006). Therefore, a tight regulation of the cellular redox balance in the liver is essential. A crucial player in the defense against oxidative stress is the NF-E2-related factor 2 (Nrf2). This transcription factor controls the expression of antioxidant proteins and enzymes involved in the detoxification of harmful compounds, such as glutathione S-transferase (GST)-ya, GST-π, glutamate-cysteine ligase catalytic subunit (GCLC) and NAD(P)H quinone oxidoreductase 1 (NQO1) (reviewed by Jaiswal, 2004). Nrf2 is a member of the ‘cap“n”collar' family of transcription factors, which also includes the related Nrf1 and Nrf3 proteins, as well as p45 NF-E2, Bach1 and Bach2 (reviewed by Motohashi et al, 2002). These transcription factors bind to cis-acting elements in the promoters of their target genes, called antioxidant response element (ARE) (reviewed by Nguyen et al, 2003).
The crucial role of Nrf2 in the cellular stress response is reflected by the phenotype of Nrf2 knockout mice. Upon ageing they develop an autoimmune disorder resembling lupus erythematosus (Li et al, 2004). Even young animals are more susceptible to various toxins (Chan and Kan, 1999; Chan et al, 2001), and the lack of Nrf2-mediated gene expression enhances the susceptibility to cancer as shown for the liver (Ramos-Gomez et al, 2001) and the skin (auf dem Keller et al, 2006). Nrf2 was also identified as a regulator of inflammation in cutaneous wound repair, although the lack of Nrf2 did not result in impaired healing (Braun et al, 2002). Here, we demonstrate a novel role of Nrf2 in liver regeneration and insulin resistance.
Results
Reduced liver/body weight ratio but lack of liver damage in Nrf2 knockout mice
A histological analysis of the livers of Nrf2-deficient mice at 8–10 weeks of age did not reveal apparent abnormalities (Supplementary Figure 1A). The liver/body weight ratio of these animals was reduced (Supplementary Figure 1B), possibly as a result of reduced hepatocyte proliferation during development. To test whether this affects general liver functions, a serum analysis was performed. Levels of total protein and albumin were comparable between animals of both genotypes, and glucose levels were also unaltered. An increase in total bilirubin (normalized to liver weight) was observed in the Nrf2 knockout mice (Supplementary Figure 1C), but the activities of aspartate aminotransferase and alanine aminotransferase in the serum (normalized to the liver weight) were similar in mice of both genotypes (Supplementary Figure 1D and E). Since excessive release of these liver-specific enzymes into the serum is a hallmark of liver dysfunction and damage, it seems likely that liver function is not severely impaired in the knockout animals.
Nrf2 is crucial for liver regeneration
To determine the role of Nrf2 in liver regeneration, we applied the model of two-third hepatectomy to Nrf2 knockout mice and wild-type littermates. As shown by RNase protection assay (RPA), Nrf2 mRNA levels were similar in normal and injured livers of wild-type mice and undetectable in the knockout mice. Nrf1 was also expressed in normal liver and after hepatectomy, but was not upregulated in the absence of Nrf2. Nrf3 mRNA was undetectable in mice of both genotypes (Supplementary Table 1).
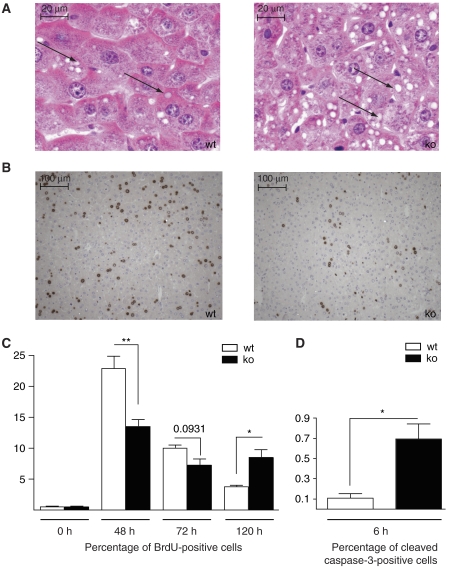
A histological analysis as well as staining for adipophilin revealed that the transient steatosis, which is normally observed after hepatectomy and which is important for regeneration (Shteyer et al, 2004), was aggravated in the Nrf2 knockout mice 48 h after hepatectomy (Figure 1A). Using 5-bromo-2′-deoxyuridine (BrdU) incorporation studies, we found a significant delay in liver cell proliferation in the Nrf2 knockout animals (Figure 1B and C). In mice of both genotypes, only very few proliferating hepatocytes were detected in non-injured liver and 6 h after hepatectomy. Even 24 h after injury, less than 1% of the hepatocytes had incorporated BrdU (data not shown). Proliferation then peaked 48 h after hepatectomy, but the peak was much lower in Nrf2 knockout mice compared to wild-type animals (21.3% BrdU-positive cells versus 13.36% in the knockout mice; Figure 1B and C; P=0.0057, n⩾8). At 72 h after injury, the number of BrdU-labeled cells remained lower in the knockout mice compared to wild-type animals (Figure 1C; P=0.0931, n=6), although the difference was no longer statistically significant. At day 5, proliferation was almost completed in the wild-type mice, whereas Nrf2-deficient hepatocytes continued to proliferate at this time point (Figure 1C; P=0.0111, n=7). The percentage of BrdU-positive hepatocytes was less than 1% at day 7 after injury in mice of both genotypes (data not shown). Consistent with this finding, the liver/body weight ratio had almost returned to the levels seen before injury in both wild-type and knockout mice at day 7 post-hepatectomy. These findings demonstrate that livers of Nrf2-deficient mice can regenerate, albeit with a significant delay.
Figure 1.
Liver regeneration is impaired in Nrf2-deficient mice. (A) Liver sections of Nrf2 knockout mice (ko) and wild-type (wt) littermates 48 h after hepatectomy were stained with hematoxylin/eosin. Arrows indicate lipid droplets in hepatocytes. (B) Proliferation was assessed by BrdU incorporation. Representative sections from injured liver (48 h after hepatectomy) are shown. (C) The percentage of proliferating cells was determined by counting 3–5 independent microscopic fields per liver at × 200 magnification, n (number of mice) >6 per genotype and time point. (D) Cryosections were stained for cleaved caspase-3. The percentage of stained cells 6 h after hepatectomy was determined by counting 3–5 independent microscopic fields ( × 200 magnification, n=4 per genotype). Bars represent mean±s.e.m.; *P<0.05; **P<0.01.
Impaired liver regeneration in Nrf2 knockout mice is not due to reduced expression of major hepatocyte mitogens
To determine if the delayed hepatocyte proliferation in Nrf2 knockout mice results from reduced expression of growth factors and cytokines involved in liver repair, we analyzed their expression by RPA. HGF, TGF-α and TGF-β1 mRNA levels increased transiently during the regeneration process with different kinetics, but no difference was observed between knockout and wild-type mice (Supplementary Table 1). Consistent with these findings, phosphorylation of the EGF and the HGF receptor (c-Met), which reflects their activation in response to ligand binding, was comparable in wild-type and knockout mice at all stages after hepatectomy (Supplementary Figure 2A and B). IGF-1 and vascular endothelial growth factor were highly expressed in normal and injured livers, but the mRNA levels of these growth factors were not affected by hepatectomy or genotype (Supplementary Figure 1). IGF-1 levels in the serum were generally high in wild-type and knockout mice at all time points. Serum levels of knockout mice were slightly lower compared to wild-type mice, but this difference was not statistically significant and may also reflect the lower liver weight (Supplementary Figure 2C). Finally, IL-6 and TNF-α mRNA levels were slightly induced after injury in mice of both genotypes (Supplementary Table 1).
Enhanced hepatocyte apoptosis in injured liver of Nrf2 knockout mice
Under pathological conditions, enhanced hepatocyte apoptosis can occur after hepatectomy (Malhi et al, 2006; Schwabe and Brenner, 2006). Staining for cleaved caspase-3 revealed only few apoptotic cells in non-injured liver and in the liver of sham-operated mice of both genotypes (data not shown). However, 6 h after hepatectomy, the number of apoptotic cells was five-fold increased in Nrf2 knockout mice (P=0.0159, n=4) compared to wild-type controls (Figure 1D). Necrotic areas were never detected in hepatectomized liver, independent of the genotype (data not shown).
Reduced expression of Nrf2 target genes in normal and hepatectomized livers of Nrf2 knockout mice
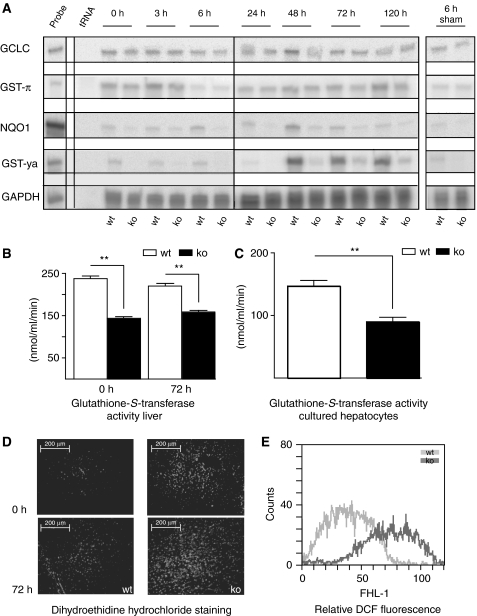
We next analyzed the mRNA levels of major Nrf2 target genes in normal and injured livers of Nrf2 knockout mice. Expression of GST-ya, NQO1 and to a lesser extent GST-π was reduced in resting livers of Nrf2 knockout mice. Induction of GST-ya and NQO1 expression, and a slight increase in GST-π mRNA levels were observed within 48–120 h after hepatectomy in livers of wild-type animals, but to a much lesser extent in Nrf2-deficient mice (Figure 2A and Supplementary Table 1). RNA levels encoding the catalytic subunit of the rate-limiting enzyme for glutathione biosynthesis, GCLC, were slightly induced in wild-type livers 48 h after hepatectomy, but not in Nrf2 knockout mice (Figure 2A and Supplementary Table 1). Expression of heme oxygenase-1 (HO-1), and of peroxiredoxins (Prdx) 1 and 6 was not affected by the loss of Nrf2 (Supplementary Table 1).
Figure 2.
Reduced mRNA levels of Nrf2 target genes and enhanced oxidative stress in normal and hepatectomized livers of Nrf2 knockout mice. (A) Total cellular RNA (10 μg from pooled livers of at least four mice per time point and genotype) was analyzed by RPA for transcripts encoding Nrf2 target genes. The time after hepatectomy or sham surgery is indicated on top of each lane; 0 h indicates resting liver, which was removed during hepatectomy; 20 μg tRNA served as a negative control and 1000 c.p.m. of the hybridization probes were used as a size marker. Hybridization with a GAPDH riboprobe served as a loading control. Representative autoradiograms of two independent experiments are shown. (B) Total lysates from resting and injured livers 72 h after partial hepatectomy were assayed for GST activity. The formation of glutathione/1-chloro-2,4-dinitrobenzene conjugates was measured spectrophotometrically. Bars represent mean±s.e.m.; **P<0.01 (n=6). (C) Hepatocytes were analyzed for GST activity. Bars represent mean±s.e.m.; *P<0.05; **P<0.01 (n=6). (D) Freshly prepared cryosections (7 μm) from non-injured and injured livers (72 h after hepatectomy) were stained with DEH and analyzed by fluorescence microscopy (n⩾5). (E) Primary hepatocytes from Nrf2 knockout and wild-type mice were treated with H2DCFH-DA and analyzed by flow cytometry for the levels of intracellular ROS.
To identify the full spectrum of Nrf2 target genes in the liver, we performed microarray analysis of RNAs from non-injured livers of young wild-type and knockout mice. In the liver of Nrf2 knockout mice, we found reduced expression of several GSTs and NQO1, whereas GCLC, HO-1, and Prdx 1 and 6 were expressed at similar levels. Therefore, the differential expression of known Nrf2 target genes was shown by two independent methods. In addition, the microarray analysis identified the differential expression of several enzymes involved in drug metabolism and detoxification in the liver of Nrf2 knockout mice (Supplementary Table 2). The complete microarray data can be downloaded from the GEO repository: (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=tvwphcsycaqgcti&acc=GSE8969).
Consistent with the mRNA data, GST activity was reduced by 36% in resting livers of Nrf2 knockout mice compared to controls and by 26% 72 h after hepatectomy (Figure 2B). Reduced GST activity was also observed in cultured primary hepatocytes from Nrf2-deficient mice (Figure 2C), demonstrating that hepatocytes are directly affected.
Increased oxidative stress in the liver of Nrf2-deficient mice
We subsequently stained the livers with dihydroethidine, a dye that intercalates into DNA upon oxidation, and therefore allows monitoring of oxidative stress in vivo. Nuclear staining was observed in livers of Nrf2 knockout mice, whereas livers of wild-type animals showed only weak fluorescence (Figure 2D). Numbers of stained nuclei increased 72 h after hepatectomy in animals of both genotypes (Figure 2D lower panels), with stronger staining in Nrf2-deficient mice.
This finding was verified biochemically with freshly isolated hepatocytes. Cells were incubated with H2DCFH-DA that is converted by intracellular ROS to the fluorescent derivative DCF. The latter was detected by flow cytometry. Hepatocytes from Nrf2 knockout mice displayed strongly increased fluorescence, reflecting an increase in intracellular ROS levels (Figure 2E). These results identify Nrf2 as an important regulator of the cellular redox balance in normal and hepatectomized livers and demonstrate that deficiency in Nrf2 results in chronic oxidative stress in hepatocytes, which is further aggravated upon liver injury.
MAPK signaling is altered in regenerating livers of Nrf2 knockout mice
To elucidate the mechanisms underlying the impaired liver regeneration in Nrf2-deficient mice, we first analyzed the injury-induced activation of NF-κB, STAT3 and AP-1 transcription factor complexes. In resting liver, cytoplasmic staining for the NF-κB p65 subunit was observed in animals of both genotypes (Supplementary Figure 3A). At 3 h after hepatectomy NF-κB was predominantly localized in the nuclei of hepatocytes, and no difference was observed between wild-type and Nrf2-deficient mice (Supplementary Figure 3A). In addition, IKK-β, which is at least in part responsible for NF-κB activation, was expressed at similar levels in wild-type and knockout mice (Supplementary Figure 3B). However, EMSA experiments revealed that the extent of NF-κB activation was stronger in the liver of Nrf2-deficient mice 3 and 6 h after hepatectomy (Supplementary Figure 3C). This finding is consistent with the enhanced oxidative stress, which was shown to result in NF-κB activation (Schreck et al, 1991). Since NF-κB enhances survival of hepatocytes after hepatectomy (Luedde et al, 2006), its activation is most likely a compensatory effect to reduce the extent of cell death in Nrf2 knockout mice.
A strong increase in Tyr705 phosphorylation of STAT3, which reflects its activation, was observed 3 and 6 h after hepatectomy in animals of both genotypes in comparison to resting liver, which was removed upon hepatectomy (Supplementary Figure 4). In contrast, total STAT3 levels were not affected (Supplementary Figure 4). Thus, STAT3 activity is apparently not altered in Nrf2 knockout mice after hepatectomy. As an additional control, we analyzed the activation of STAT3 in sham-operated mice. The latter underwent abdominal surgery and manipulation of the liver, but not hepatectomy. In sham-operated mice, activation of STAT3 was also observed to a similar extent in mice of both genotypes, most likely reflecting the stress response (Supplementary Figure 4).
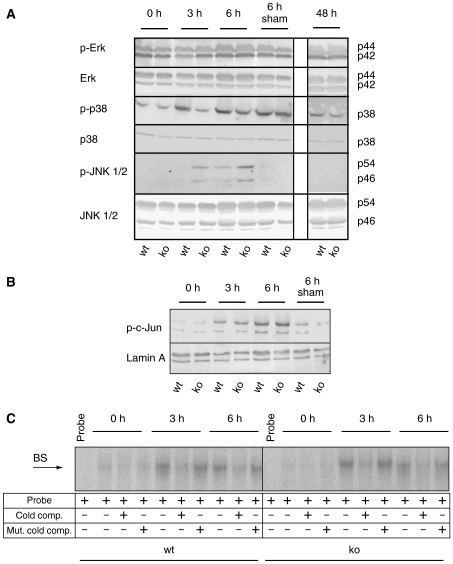
Phosphorylation of p38 was apparent 3 and 6 h after hepatectomy in wild-type mice. Interestingly, the levels of phosphorylated p38 were significantly lower in hepatectomized liver of Nrf2 knockout mice compared to wild-type mice within 3 and 6 h after injury in three independent experiments (Figure 3A) (average reduction 39% (3 h after injury) and 43% (6 h after injury)). No difference in Erk phosphorylation was detectable (Figure 3A). Using an antibody that recognizes the phosphorylated forms of the 54 and 46 kDa variants of both JNK1 and JNK2, we found hyperphosphorylation of both variants within 3 and 6 h after hepatectomy compared to control littermates (Figure 3A). However, this did not result in abnormal AP-1 activation, as suggested by the similar levels of phosphorylated c-Jun in mice of both genotypes (Figure 3B) and by the similar induction of AP-1 DNA-binding activity within 3 and 6 h after hepatectomy as demonstrated by EMSA (Figure 3C). This may be due to differential activities of MAPK phosphatases in the nucleus and the cytoplasm (Wu et al, 2006). Sham operation did not affect the phosphorylation status of Erk, JNK and c-Jun. Although p38 was also activated by sham surgery, the activation was independent of the genotype.
Figure 3.
Altered JNK and p38 activation but normal AP-1 activity in injured liver of Nrf2 knockout mice. (A, B) Total protein (60 μg) from liver lysates of Nrf2 knockout and wild-type mice (from pooled livers of at least four mice per time point after hepatectomy and genotype) was analyzed by immunoblotting for the levels of phosphorylated and total Erk, p38 and JNK (A), and phosphorylated c-Jun (B). Staining of the membrane with antibodies to GAPDH or Lamin A was used as a loading control. Representative blots from two independent experiments with lysates from different hepatectomy experiments are shown. (C) Radiolabeled oligonucleotides containing AP-1-binding sites were incubated with total protein lysates (20 μg) from normal and hepatectomized livers, and EMSAs were performed. Addition of an excess of non-labeled oligonucleotides (lanes labeled: cold comp.) inhibited mobility shifts, whereas addition of non-labeled oligonucleotides with mutated AP-1-binding sites had no effect (lanes labeled: mut. cold comp.).
Reduced PI3 kinase/Akt signaling in Nrf2 knockout mice after partial hepatectomy
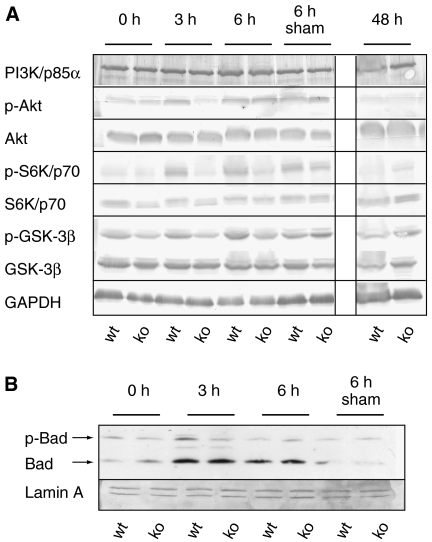
Expression of the regulatory subunit of phosphoinositide 3-kinase (PI3K), p85α, and its target Akt was unaltered in Nrf2-deficient mice. However, phosphorylation of Akt (Ser473), which results in its activation, occurred within 3 h after hepatectomy in wild-type mice. At this time point, however, levels of phosphorylated Akt were significantly lower in the knockout mice (average reduction 48% in four independent experiments) (Figures 4A, 5A and 7D). Consistent with these findings, phosphorylation of the Akt target glycogen synthase kinase-3β (GSK-3β) at Ser9 was reduced (56% reduction 3 h after hepatectomy; 63% reduction 6 h after hepatectomy) (Figure 4A). In addition, p70/S6K and Bad, two major targets of PI3K signaling, displayed reduced phosphorylation in injured liver of the Nrf2 knockout mice (Figure 4A and B) (pS6K: 34 and 60% reduction after 3 or 6 h, respectively; pBad: 56% reduction after 3 h). Since PI3K, Akt and their downstream targets are crucial for cell survival and proliferation (Lawlor and Alessi, 2001; Song et al, 2005), the reduced activation of this pathway provides a likely explanation for the observed defect in liver regeneration in Nrf2-deficient mice and could also account for the increased cell death after hepatectomy.
Figure 4.
Reduced activation of the PI3K/Akt signaling pathway in injured liver of Nrf2 knockout mice. Total protein (60 μg) from liver lysates of Nrf2 knockout and wild-type mice (from pooled livers of at least four mice per time point after hepatectomy and genotype) was analyzed by immunoblotting for the levels of the PI3K p85α subunit, phosphorylated and total Akt, GSK-3β and S6 kinase (A), non-phosphorylated and phosphorylated Bad, GAPDH and Lamin A (B). Representative blots from two to four experiments with lysates from different hepatectomy experiments are shown.
Figure 5.
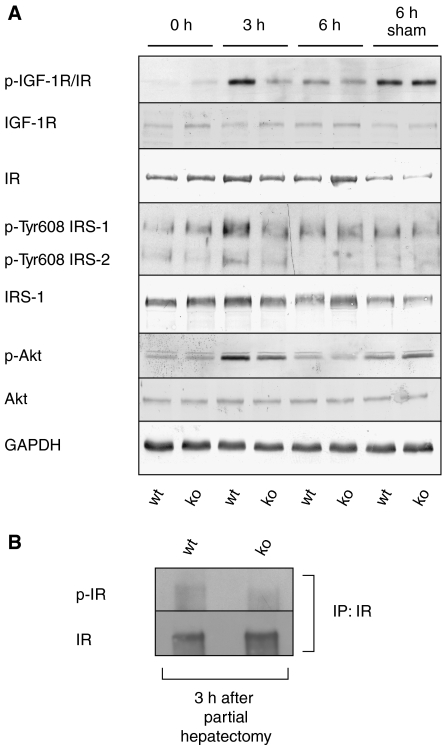
Reduced IGF-1R/IR signaling in injured liver of Nrf2 knockout mice. (A) Liver lysates (60 μg protein) of Nrf2 knockout and wild-type mice at different time points after hepatectomy were analyzed by immunoblotting for the levels of the total IGF-1R and IR, phosphorylated IGF-1R/IR, phosphorylated and total Akt, phosphorylated IRS-1/2 (Tyr608) and total IRS-1, and GAPDH. Representative blots from two to three experiments with lysates from different hepatectomy experiments are shown. (B) Total liver lysates from injured liver (3 h after hepatectomy) of Nrf2-deficient and wild-type mice were subjected to immunoprecipitation using antibodies against IR. Precipitates were analyzed by immunoblotting using antibodies against IR or pIR/IGF-1R.
Insulin/IGF-I receptor signaling is impaired in injured liver of Nrf2-deficient mice
We next wondered about the upstream signals that are responsible for the reduced activation of PI3K and p38. Since most hepatocyte mitogens are only expressed at later stages of regeneration (Supplementary Table 1), we speculated that IGF-1 expression or signaling might be affected. IGF-1 is known for its potent activation of the PI3K/Akt signaling pathway, and it can also activate p38 (Cheng and Feldman, 1998). Using an antibody, which recognizes the phosphorylated forms of the IGF-1 receptor (IGF-1R) and the insulin receptor (IR), we found strongly enhanced levels of the phosphorylated form(s) within 3 h after hepatectomy of wild-type mice and to a much lesser extent in Nrf2 knockout mice (71 and 73% reduction in two independent experiments). Phosphorylation of IGF-1R/IR strongly correlated with the levels of pAkt. High levels of IR and much lower levels of IGF-1R were detected in normal and hepatectomized livers, and their levels were similar at all stages of the repair process (Figure 5A). This indicates that either ligand-dependent activation or dephosphorylation, in particular of the IR, is affected by the lack of Nrf2 (Figure 5A). Consistent with this assumption, immunoprecipitation with antibodies against the IR or the IGF-1R, respectively, and subsequent western blotting with an antibody against pIR/IGF-1R revealed that it is predominantly the IR, which is expressed in the liver and activated in response to hepatectomy, and that the activation of this receptor is reduced in the absence of Nrf2 (Figure 5B). In contrast, only very low levels of IGF-1R could be precipitated, although the same antibody efficiently precipitated the IGF-1R from cultured human hepatoma cells (data not shown).
Consistent with the reduced IR activation, levels of phosphorylated IR substrate 1 (IRS-1) (Tyr608 corresponding to Tyr612 in human IRS-1) and pIRS-2 were reduced in knockout animals (Figure 5A). Phosphorylation of Tyr608 in IRS-1 and the corresponding residue in IRS-2 reflects their activation (Bloch-Damti et al, 2006). These findings suggest that IR signaling is impaired, resulting in reduced PI3K activity.
Oxidative stress reduces insulin responsiveness in liver cells
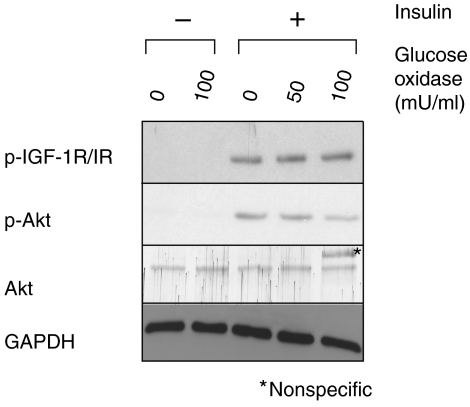
To determine if the abnormalities in IGF-1R/IR and PI3K signaling are a consequence of the enhanced oxidative stress or related to a different activity of Nrf2, we generated oxidative stress in primary murine hepatocytes from wild-type mice by a 2 h treatment with glucose oxidase (GO), which continuously generates intracellular ROS. Cells were subsequently treated with insulin. In response to insulin treatment, phosphorylation of IGF-1R/IR and Akt was strongly activated (Figure 6). GO treatment reduced the Akt activation in a dose-dependent manner. However, phosphorylation of the IGF-1R/IR receptor was not affected by GO, further suggesting that the ROS-mediated insulin resistance is mediated at a level downstream of the receptor, most likely at the level of IRS.
Figure 6.
Oxidative stress impairs insulin-induced Akt activation in hepatocytes. Primary murine hepatocytes from wild-type mice were pretreated for 2 h with 50 or 100 mU/ml GO and subsequently stimulated for 15 min with 100 nM insulin. Activation of IGF-1R/IR signaling was monitored by immunoblotting of 30 μg of total protein with phosphospecific antibodies to IGF-1R/IR and Akt. Staining of the membrane with antibodies against total Akt and GAPDH served as controls. Representative blots from three experiments are shown.
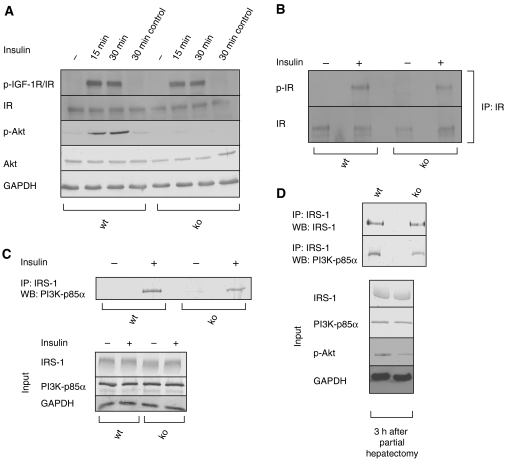
These in vitro findings strongly suggest that the enhanced oxidative stress in hepatocytes of Nrf2-deficient mice is directly responsible for the inhibition of the IGF-1R/IR—PI3K-Akt signaling pathway. To further test this hypothesis, primary hepatocytes from wild-type and Nrf2 knockout mice were cultured, serum-starved and subsequently treated with insulin. Consistent with the enhanced oxidative stress in the Nrf2-deficient cells (see Figure 2E), phosphorylation of Akt was almost completely abolished in cells from knockout mice (Figure 7A; 82.7% reduction compared to cells from wild-type mice after 15 min treatment with insulin and 91.3% reduction after 30 min, n=4). In addition, the association of IRS-1 with PI3K (p85α subunit) that occurs upon insulin treatment was reduced in Nrf2-deficient cells (50% reduction compared to cells from wild-type mice) (Figure 7C). In contrast, IR phosphorylation was not affected (Figure 7A and B). IGF-1R expression was hardly detectable in primary hepatocytes by western blotting or immunoprecipitation followed by western blotting (data not shown). These results were obtained in two independent experiments. The reduced association of PI3K with IRS-1 in the absence of Nrf2 was also verified for the in vivo situation. Thus, association of PI3K with IRS-1 was strongly reduced in injured liver of Nrf2 knockout mice (3 h after hepatectomy) compared to wild-type mice (Figure 7D) in three independent experiments. Taken together, these results provide the link among Nrf2 deficiency, oxidative stress and insulin resistance, and demonstrate that the oxidative stress-induced insulin/IGF resistance is mediated at the level of the IRS in vivo and in cultured primary hepatocytes.
Figure 7.
Nrf2 deficiency in hepatocytes causes insulin resistance. (A) Primary hepatocytes from Nrf2 knockout or wild-type mice were serum-starved, and treated for the indicated time with 100 nM insulin. Protein lysates (40 μg) were analyzed by western blotting using phosphospecific antibodies to IGF-1R/IR and Akt, or antibodies to total IR, Akt and GAPDH. Representative blots from four independent experiments are shown. (B) Primary hepatocytes from Nrf2 knockout or wild-type mice were serum-starved, and treated for 15 min with insulin. Lysates were subjected to immunoprecipitation with an IR antibody, and precipitates were analyzed by western blotting using antibodies against IR or pIR/IGF-1R. (C) Alternatively, lysates were subjected to immunoprecipitation with an IRS-1 antibody, and association of IRS-1 with PI3K-p85α was monitored by immunoblotting (upper panel). To ensure equal loading, 30 μg of the lysates used for immunoprecipitation was analyzed by western blotting for the levels of total IRS-1, PI3K-p85α and GAPDH (lower panel). Representative blots from two independent experiments are shown. (D) Liver samples from Nrf2 knockout mice and wild-type control animals were harvested 3 h after hepatectomy. Lysates were immunoprecipitated with an antibody to IRS-1. Precipitates were analyzed by western blotting for the presence of PI3K (p85α subunit). Analysis of IRS-1 levels in the precipitate served as a control. In addition, levels of IRS-1, PI3K (p85α subunit), p-Akt and GAPDH were analyzed in the lysate before immunoprecipitation (input: western blots shown in the lower panel). Representative blots from three experiments are shown.
Discussion
Nrf2: an important redox regulator in hepatocytes
In this study, we demonstrate a novel role of Nrf2 in liver regeneration. Deficiency in this transcription factor resulted in enhanced oxidative stress in the normal and particularly in the injured liver. Thus, in addition to NF-κB (Schwabe and Brenner, 2006), Nrf2 is another master regulator of the intracellular redox balance in hepatocytes. Nrf1 is also involved in ROS detoxification in the liver. It is required for hepatocyte survival during liver development (Chen et al, 2003). Further, mice lacking Nrf1 in the adult liver revealed enhanced levels of ROS in hepatocytes. They also developed severe steatosis and spontaneous liver cancer (Xu et al, 2005), a phenotype not observed in Nrf2-deficient mice. Therefore, both Nrf1 and Nrf2 are apparently important redox regulators in hepatocytes with partially overlapping, but also individually different functions. In the future, it will be interesting to determine the consequences of a loss of both transcription factors in the liver.
Oxidative stress causes insulin/IGF-1 resistance in the injured liver
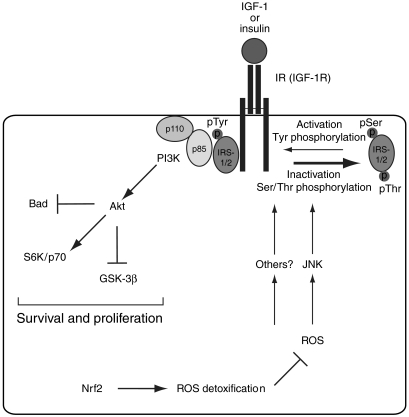
The enhanced oxidative stress in the liver of Nrf2 knockout mice was accompanied by a severe defect in liver regeneration. In view of the findings described in this study and on previously published data, we propose a model for the role and mechanism of action of Nrf2 in liver regeneration, which is shown schematically in Figure 8.
Figure 8.
Schematic representation of Nrf2 action in the injured liver. Nrf2 induces the expression of ROS-detoxifying enzymes, which prevents ROS accumulation. Its deficiency causes oxidative stress, resulting in reduced tyrosine phosphorylation of IRS-1 and -2, most likely by ROS-activated JNK and possibly other Ser/Thr IRS kinases. Since tyrosine phosphorylation of IRS-1 is required for PI3K activation, phosphorylation of Akt and downstream targets is reduced.
Nrf2 deficiency reduces the expression of ROS-detoxifying enzymes in normal liver and after hepatectomy, resulting in oxidative stress in hepatocytes (Figure 2). Our experiments performed with cultured primary hepatocytes revealed that ROS are directly responsible for the impairment of insulin/IGF signaling and subsequent inhibition of the PI3K-Akt pathway (Figures 6 and 7). This result is consistent with recent data obtained with adipocytes, which demonstrated a causal role of ROS in insulin resistance (Houstis et al, 2006). Notably, the chronic oxidative stress, which is already present in the non-injured liver of Nrf2-deficient mice, seems to be predominantly responsible for the phenotypic abnormalities. Thus, the deficiency in insulin/IGF signaling was seen within a few hours after injury, before the upregulation of Nrf2 target genes occurred (Figure 2A). This assumption is further strengthened by the impaired insulin-mediated Akt activation in primary hepatocytes of Nrf2 knockout mice, which had not been pretreated with ROS. These results provide the link among Nrf2 deficiency, chronic oxidative stress and insulin/IGF-1 resistance.
The inhibitory effect of ROS on IGF-1R/IR signaling was shown to be mediated through activation of kinases by ROS, which in turn phosphorylate IRS-1 and -2 at serine/threonine residues. This results in insulin/IGF resistance due to dissociation of IRS from the receptor and from PI3K (reviewed by Zick, 2005; Morino et al, 2006). This mechanism is most likely also responsible for the insulin/IGF-1 resistance in Nrf2-deficient hepatocytes, since in the cultured primary cells of Nrf2 knockout mice association between IRS-1 and PI3K as well as subsequent Akt activation in response to insulin was inhibited, whereas IR phosphorylation occurred normally (Figure 7A and B). Thus, the inhibitory effect of ROS apparently occurs downstream of the receptor, most likely at IRS-1 and/or IRS-2. One of the IRS kinases responsible could be JNK, which is activated in response to ROS (Ventura et al, 2004; Kamata et al, 2005). Consistent with this assumption, we found enhanced activation of JNK in the injured liver (Figure 3A). JNK can phosphorylate IRS-1 at Ser307 (Ser312 in human IRS-1) and thereby inhibit its phosphorylation at Tyr608 (Tyr612 in human IRS-1) by the insulin or IGF-1 receptor (Aguirre et al, 2002). Through this mechanism, JNK is a crucial mediator of insulin resistance in vivo (Hirosumi et al, 2002). Although we were not able to detect Ser307 phosphorylation of IRS-1 in the liver under our experimental conditions using commercially available antibodies, phosphorylation of Tyr608 of IRS-1 and the corresponding site in IRS-2 was strongly reduced in hepatectomized liver of Nrf2 knockout mice (Figure 5A), and GO also prevented the insulin-mediated phosphorylation of this site in the human HepG2 hepatoma cell line (data not shown).
In addition to JNK, other IRS kinases may be involved in the inhibition of insulin signaling in the Nrf2-deficient liver. Our in vivo results (Supplementary Figure 3B) argue against a role of another major IRS kinase—IKK-β (Cai et al, 2005)—, but the possible involvement of additional kinases, for example, protein kinase Cζ and others (Zick, 2005) remains to be determined. Independent of the kinase(s) responsible, it seems likely that serine/threonine phosphorylation of IRS is responsible for the transient insulin/IGF resistance in injured liver of Nrf2 knockout mice.
Tyrosine phosphorylation, in particular of the IR, was also severely reduced in injured liver of Nrf2 knockout mice compared to controls (Figure 5A). This difference is unlikely to result from reduced availability of ligands in Nrf2 knockout mice, since mRNA levels of IGF-1 in the liver were similar in mice of both genotypes (Supplementary Table 1). Serum levels of IGF-1 protein were slightly reduced in Nrf2-deficient animals, but high concentrations were present in animals of both genotypes (Supplementary Figure 2C). Insulin serum levels strongly decline after hepatectomy (Michalopoulos and DeFrances, 1997), suggesting that insulin is not responsible for the increased IGF-1R/IR receptor phosphorylation seen in the injured liver. Rather, it is possible that IGF-1 is released from binding proteins upon hepatectomy and activates in particular the IR in mice of both genotypes. However, alterations in the phosphorylation status of IRS-1 and -2 in Nrf2 knockout mice (enhanced serine/threonine and reduced tyrosine phosphorylation) reduce their association with the receptor (Bloch-Damti et al, 2006). This could render the receptor more accessible to inhibitory phosphatases, in particular in vivo.
Insulin/IGF-1 resistance impairs the activation of anti-apoptotic and pro-mitogenic signaling pathways in the injured liver
We propose that the reduction in insulin/IGF-1 signaling is at least partially responsible for the regeneration defect. This assumption is consistent with the delayed liver regeneration seen in IGF-1R-deficient animals (Desbois-Mouthon et al, 2006). The impaired activation of the IR and the reduced tyrosine phosphorylation of IRS-1 are likely to be responsible for the reduced phosphorylation of Akt and p38, since Tyr608 phosphorylation of IRS-1 is crucial for the activation of both signaling pathways (Cheng and Feldman, 1998; Bloch-Damti et al, 2006).
A role of reduced p38 activation (Figure 3A) in the regeneration defect is consistent with findings from a recent study, where systemic treatment of rats with a p38 inhibitor suppressed cell cycle entry of hepatocytes after partial hepatectomy (Hsu et al, 2006). On the other hand, enhanced and prolonged p38 phosphorylation as seen in c-Jun knockout mice resulted in reduced proliferation and enhanced mortality (Stepniak et al, 2006), demonstrating that the levels/activity of this signaling protein need to be tightly controlled in the injured liver.
Most importantly, Nrf2 knockout mice showed strongly reduced activation of Akt early after injury (Figures 4, 5A and 7D). Consequently, phosphorylation of the Akt targets GSK-3β, p70/S6 kinase, and Bad was also reduced (Figure 4). Since the latter are crucial for cell proliferation and survival (Lawlor and Alessi, 2001), the observed abnormalities in their activation are likely to further contribute to the delayed liver regeneration in Nrf2 knockout mice.
Nrf2: a novel target for the improvement of liver regeneration and for treatment of insulin resistance
Taken together, our results demonstrate for the first time a crucial role of Nrf2 in tissue repair. They also reveal that oxidative stress associated with Nrf2 deficiency in the liver causes a transient insulin/IGF-1 resistance after hepatectomy. Although the increased expression of Nrf2 target genes that occurs within a few days after hepatectomy suggests that Nrf2 is at least partially activated, preliminary studies with ARE reporter mice (Johnson et al, 2002) revealed that Nrf2 is only activated in a small percentage of hepatocytes in the injured liver. Furthermore, activation of Nrf2 may be insufficient in diseased liver. Therefore, pharmacological activation of Nrf2, for example, by synthetic triterpenoids or avicins (Hanausek et al, 2001; Hyer et al, 2005; Liby et al, 2005), could be a promising new strategy to improve regeneration in patients with acute or chronic liver damage. Finally, it will be worthwhile to explore if Nrf2 activation can be used for the therapy of insulin resistance.
Materials and methods
Animals
Nrf2 knockout mice (Chan and Kan, 1999) and wild-type littermates were housed and fed according to federal guidelines. Animal experiments had been approved by the local veterinary authorities of Zurich, Switzerland.
Two-third hepatectomy
Male mice (8- to 10-week old) were anesthetized by intraperitoneal (i.p.) injection of ketamine (100 μg/g body weight)/xylazine (5 μg/g body weight) and subjected to two-third hepatectomy (Steiling et al, 2003). After surgery, mice were injected with buprenorphine (Temgesic; Essex Chemie AG, Bern, Switzerland; 0.1 μg/g body weight). At different stages after injury, they were euthanized by CO2 inhalation, and remaining livers were harvested. Resting liver from non-injured mice and the liver removed during surgery served as a control. For sham operation, mice were anesthetized as described above, the abdomen was opened and the liver lobes were briefly removed from the abdominal cavity, but subsequently returned to their original site.
Hepatocyte isolation, cell culture and treatment with GO and insulin
Male mice (8- to 10-week old) were killed by i.p. injection of pentobarbital (Eutha 77; Essex Chemie AG; 2.5 mg/10 g) dissolved in isotonic saline containing 1000 IE heparin/ml. Primary hepatocytes were isolated by the two-step collagenase (Sigma; 0.5 mg/ml) perfusion method (Huh et al, 2004) and subsequently purified by centrifugation (200 g) and isodensity purification in Percoll (Amersham, Braunschweig, Germany). Cells were plated on collagen R (Serva, Heidelberg, Germany; 0.2 mg/ml)-coated dishes in Dulbecco's modified Eagle's medium (DMEM; 4500 mg/l glucose; Sigma), supplemented with 10% fetal calf serum (BioConcept, Allschwil, Switzerland), 100 μg/ml kanamycin at a density of 4 × 104/cm2, and left to adhere for 4 h. The medium was then changed to serum-free DMEM containing kanamycin. After 16 h incubation in serum-free medium, they were analyzed for GST activity or used for DCF measurements. For signaling experiments, they were serum-starved for 16 h, subsequently treated with 100 nM insulin for 15–30 min and analyzed by western blotting or immunoprecipitation (see Supplementary data for description of the procedure and information about antibodies).
To determine the effect of oxidative stress, serum-starved primary murine hepatocytes were pretreated with GO (Sigma; 50 or 100 mU/ml) for 2 h, washed twice with pre-warmed PBS, subsequently treated with 100 nM insulin (Sigma) in DMEM for 15 or 30 min and harvested for western blot analysis.
Flow cytometry
Cultured hepatocytes were loaded with 2,7-dichlorofluoresceine diacetate (H2DCF-DA) (100 μmol/l; Molecular Probes) for 30 min. This cell-permeable compound is converted into a non-fluorescent product (H2DCF) after deacetylation by intracellular esterases and oxidized to the highly fluorescent dichlorofluoresceine (DCF). Cells were trypsinized, washed with PBS and analyzed on a FACSCalibur flow cytometer (BD Bioscience, San Jose, CA).
Dihydroethidine hydrochloride staining
Freshly cut frozen liver sections (7 μm) were stained with 2 μM dihydroethidine hydrochloride (DEH) (Molecular Probes) for 30 min at 37°C and analyzed by fluorescence microscopy.
Analysis of GST activity
Cultured primary hepatocytes or liver tissues were lysed in 100 mM potassium phosphate pH 7, containing 2 mM EDTA, and protein content was determined. Enzymatic activity toward 1-chloro-2,4-dinitrobenzene (CNDB) (Sigma) was assayed in a buffer containing 100 mM potassium phosphate pH 6.5, 0.1% Triton X-100 (v/v), 1 mM glutathione and 1 mM CNDB. Formation of glutathione/CNDB conjugate was measured in a spectrophotometer at 340 nm.
Statistical analysis
Statistical analysis was performed using the Prism4 statistical program. P-values are two-tailed and were calculated using the Mann–Whitney U-test.
Supplementary Material
Supplementary Information
Acknowledgments
We are grateful to Dr Claes Wollheim, Geneva, Switzerland, and Dr Alfred Nordheim, Tübingen, Germany, for very helpful suggestions. We thank Drs Hubert Rehrauer and Marzanna Künzli from the Functional Genomics Center Zurich for invaluable help with the microarray experiments; Christiane Born and Nicole Hallschmid, ETH Zurich, for excellent technical assistance; Claudia Defila, ETH Zurich, for performing RPAs; Dr Markus Weiller, Konstanz, Germany, for help with the culture of primary hepatocytes and Dr Anke Klippel, New York, USA, for the PI3K-p85α antibody and for helpful suggestions with the PI3K signaling experiments. This study was supported by a grant from the Swiss National Science Foundation (3100A9-109340/1 to SW). UadK was a recipient of a Boehringer-Ingelheim predoctoral fellowship. WX was supported by the China Scholarship Council, Jinan, China.
References
- Aguirre V, Werner ED, Giraud J, Lee YH, Shoelson SE, White MF (2002) Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J Biol Chem 277: 1531–1537 [DOI] [PubMed] [Google Scholar]
- auf dem Keller U, Huber M, Beyer T, Kumin A, Siemes C, Braun S, Bugnon P, Mitropoulos V, Johnson D, Johnson J, Hohl D, Werner S (2006) Nrf transcription factors in keratinocytes are essential for skin tumor prevention but not for wound healing. Mol Cell Biol 26: 3773–3784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch-Damti A, Potashnik R, Gual P, Le Marchand-Brustel Y, Tanti JF, Rudich A, Bashan N (2006) Differential effects of IRS1 phosphorylated on Ser307 or Ser632 in the induction of insulin resistance by oxidative stress. Diabetologia 49: 2463–2473 [DOI] [PubMed] [Google Scholar]
- Borowiak M, Garratt A, Wustefeld T, Strehle M, Trautwein C, Birchmeier C (2004) Met provides essential signals for liver regeneration. Proc Natl Acad Sci USA 101: 10608–10613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun S, Hanselmann C, Gassmann M, auf dem Keller U, Born-Berclaz C, Chan K, Kan Y, Werner S (2002) Nrf2 transcription factor, a novel target of keratinocyte growth factor action which regulates gene expression and inflammation in the healing skin wound. Mol Cell Biol 22: 5492–5505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE (2005) Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med 11: 183–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K, Han X, Kan Y (2001) An important function of Nrf2 in combating oxidative stress: detoxification of acetaminophen. Proc Natl Acad Sci USA 98: 4611–4616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K, Kan Y (1999) Nrf2 is essential for protection against acute pulmonary injury in mice. Proc Natl Acad Sci USA 96: 12731–12736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Kwong M, Lu R, Ginzinger D, Lee C, Leung L, Chan J (2003) Nrf1 is critical for redox balance and survival of liver cells during development. Mol Cell Biol 23: 4673–4686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng HL, Feldman EL (1998) Bidirectional regulation of p38 kinase and c-Jun N-terminal protein kinase by insulin-like growth factor-I. J Biol Chem 273: 14560–14565 [DOI] [PubMed] [Google Scholar]
- Cressman D, Diamond R, Taub R (1995) Rapid activation of the Stat3 transcription complex in liver regeneration. Hepatology 21: 1443–1449 [PubMed] [Google Scholar]
- Desbois-Mouthon C, Wendum D, Cadoret A, Rey C, Leneuve P, Blaise A, Housset C, Tronche F, Le Bouc Y, Holzenberger M (2006) Hepatocyte proliferation during liver regeneration is impaired in mice with liver-specific IGF-1R knockout. FASEB J 20: 773–775 [DOI] [PubMed] [Google Scholar]
- Diehl A (2002) Liver regeneration. Front Biosci 7: e301–e314 [DOI] [PubMed] [Google Scholar]
- Fausto N (2000) Liver regeneration. J Hepatol 32: 19–31 [DOI] [PubMed] [Google Scholar]
- FitzGerald M, Webber E, Donovan J, Fausto N (1995) Rapid DNA binding by nuclear factor kappa B in hepatocytes at the start of liver regeneration. Cell Growth Differ 6: 417–427 [PubMed] [Google Scholar]
- Hanausek M, Ganesh P, Walaszek Z, Arntzen C, Slaga T, Gutterman J (2001) Avicins, a family of triterpenoid saponins from Acacia victoriae (Bentham), suppress H-ras mutations and aneuploidy in a murine skin carcinogenesis model. Proc Natl Acad Sci USA 98: 11551–11556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim M, Gamboni G, Beglinger C, Gyr K (1997) Specific activation of AP-1 but not Stat3 in regenerating liver in mice. Eur J Clin Invest 27: 948–955 [DOI] [PubMed] [Google Scholar]
- Hirosumi J, Tuncman G, Chang L, Gorgun C, Uysal K, Maeda K, Karin M, Hotamisligil G (2002) A central role for JNK in obesity and insulin resistance. Nature 420: 333–336 [DOI] [PubMed] [Google Scholar]
- Houstis N, Rosen ED, Lander ES (2006) Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 440: 944–948 [DOI] [PubMed] [Google Scholar]
- Hsu M, Qiao L, Ho V, Zhang B, Zhang H, Teoh N, Dent P, Farrell G (2006) Ethanol reduces p38 kinase activation and cyclin D1 protein expression after partial hepatectomy in rats. J Hepatol 44: 375–382 [DOI] [PubMed] [Google Scholar]
- Huh C, Factor V, Sanchez A, Uchida K, Conner E, Thorgeirsson S (2004) Hepatocyte growth factor/c-met signaling pathway is required for efficient liver regeneration and repair. Proc Natl Acad Sci USA 101: 4477–4482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyer M, Croxton R, Krajewska M, Krajewski S, Kress C, Lu M, Suh N, Sporn M, Cryns V, Zapata J, Reed J (2005) Synthetic triterpenoids cooperate with tumor necrosis factor-related apoptosis-inducing ligand to induce apoptosis of breast cancer cells. Cancer Res 65: 4799–4808 [DOI] [PubMed] [Google Scholar]
- Jaiswal A (2004) Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med 36: 1199–1207 [DOI] [PubMed] [Google Scholar]
- Johnson DA, Andrews GK, Xu W, Johnson JA (2002) Activation of the antioxidant response element in primary cortical neuronal cultures derived from transgenic reporter mice. J Neurochem 81: 1233–1241 [DOI] [PubMed] [Google Scholar]
- Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M (2005) Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell 120: 649–661 [DOI] [PubMed] [Google Scholar]
- Lawlor M, Alessi D (2001) PKB/Akt: a key mediator of cell proliferation, survival and insulin responses? J Cell Sci 114: 2903–2910 [DOI] [PubMed] [Google Scholar]
- Li J, Stein T, Johnson J (2004) Genetic dissection of systemic autoimmune disease in Nrf2-deficient mice. Physiol Genomics 18: 261–272 [DOI] [PubMed] [Google Scholar]
- Liby K, Hock T, Yore M, Suh N, Place A, Risingsong R, Williams C, Royce D, Honda T, Honda Y, Gribble G, Hill-Kapturczak N, Agarwal A, Sporn M (2005) The synthetic triterpenoids, CDDO and CDDO-imidazolide, are potent inducers of heme oxygenase-1 and Nrf2/ARE signaling. Cancer Res 65: 4789–4798 [DOI] [PubMed] [Google Scholar]
- Luedde T, Beraza N, Trautwein C (2006) Evaluation of the role of nuclear factor-kappaB signaling in liver injury using genetic animal models. J Gastroenterol Hepatol 21 (Suppl 3): S43–S46 [DOI] [PubMed] [Google Scholar]
- Malhi H, Gores G, Lemasters J (2006) Apoptosis and necrosis in the liver: a tale of two deaths? Hepatology 43: S31–S44 [DOI] [PubMed] [Google Scholar]
- Mead J, Fausto N (1989) Transforming growth factor alpha may be a physiological regulator of liver regeneration by means of an autocrine mechanism. Proc Natl Acad Sci USA 86: 1558–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalopoulos GK, DeFrances MC (1997) Liver regeneration. Science 276: 60–66 [DOI] [PubMed] [Google Scholar]
- Mitchell C, Nivison M, Jackson L, Fox R, Lee D, Campbell J, Fausto N (2005) Heparin-binding epidermal growth factor-like growth factor links hepatocyte priming with cell cycle progression during liver regeneration. J Biol Chem 280: 2562–2568 [DOI] [PubMed] [Google Scholar]
- Morino K, Petersen KF, Shulman GI (2006) Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes 55 (Suppl 2): S9–S15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motohashi H, O'Connor T, Katsuoka F, Engel J, Yamamoto M (2002) Integration and diversity of the regulatory network composed of Maf and CNC families of transcription factors. Gene 294: 1–12 [DOI] [PubMed] [Google Scholar]
- Nguyen T, Sherratt P, Pickett C (2003) Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu Rev Pharmacol Toxicol 43: 233–260 [DOI] [PubMed] [Google Scholar]
- Oe S, Lemmer E, Conner E, Factor V, Leveen P, Larsson J, Karlsson S, Thorgeirsson S (2004) Intact signaling by transforming growth factor beta is not required for termination of liver regeneration in mice. Hepatology 40: 1098–1105 [DOI] [PubMed] [Google Scholar]
- Pennisi PA, Kopchick JJ, Thorgeirsson S, LeRoith D, Yakar S (2004) Role of growth hormone (GH) in liver regeneration. Endocrinology 145: 4748–4755 [DOI] [PubMed] [Google Scholar]
- Ramos-Gomez M, Kwak M, Dolan P, Itoh K, Yamamoto M, Talalay P, Kensler T (2001) Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc Natl Acad Sci USA 98: 3410–3415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreck R, Rieber P, Baeuerle PA (1991) Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J 10: 2247–2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe R, Brenner D (2006) Mechanisms of liver injury. I. TNF-alpha-induced liver injury: role of IKK, JNK, and ROS pathways. Am J Physiol Gastrointest Liver Physiol 290: G583–G589 [DOI] [PubMed] [Google Scholar]
- Shteyer E, Liao Y, Muglia LJ, Hruz PW, Rudnick DA (2004) Disruption of hepatic adipogenesis is associated with impaired liver regeneration in mice. Hepatology 40: 1322–1332 [DOI] [PubMed] [Google Scholar]
- Song G, Ouyang G, Bao S (2005) The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med 9: 59–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiling H, Wustefeld T, Bugnon P, Brauchle M, Fassler R, Teupser D, Thiery J, Gordon J, Trautwein C, Werner S (2003) Fibroblast growth factor receptor signalling is crucial for liver homeostasis and regeneration. Oncogene 22: 4380–4388 [DOI] [PubMed] [Google Scholar]
- Stepniak E, Ricci R, Eferl R, Sumara G, Sumara I, Rath M, Hui L, Wagner E (2006) c-Jun/AP-1 controls liver regeneration by repressing p53/p21 and p38 MAPK activity. Genes Dev 20: 2306–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub R (2004) Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol 5: 836–847 [DOI] [PubMed] [Google Scholar]
- Ventura J, Cogswell P, Flavell R, Baldwin AJ, Davis R (2004) JNK potentiates TNF-stimulated necrosis by increasing the production of cytotoxic reactive oxygen species. Genes Dev 18: 2905–2915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JJ, Roth RJ, Anderson EJ, Hong EG, Lee MK, Choi CS, Neufer PD, Shulman GI, Kim JK, Bennett AM (2006) Mice lacking MAP kinase phosphatase-1 have enhanced MAP kinase activity and resistance to diet-induced obesity. Cell Metab 4: 61–73 [DOI] [PubMed] [Google Scholar]
- Xu Z, Chen L, Leung L, Yen T, Lee C, Chan J (2005) Liver-specific inactivation of the Nrf1 gene in adult mouse leads to nonalcoholic steatohepatitis and hepatic neoplasia. Proc Natl Acad Sci USA 102: 4120–4125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zick Y (2005) Ser/Thr phosphorylation of IRS proteins: a molecular basis for insulin resistance. Sci STKE 2005: pe4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information