Abstract
The increase of herbal medicine use led many scientists to contribute to the research in this field. Also a few pharmacologists, after an initial phase of correct criticisms, today recognize the possibility of investigating the scientific value of medicinal products composed essentially of vegetable extracts. However, it is logical to pose the questions: (i) is there a role for the pharmacologist in herbal medicine (or phytotherapy)? (ii) can we do without pharmacologists’? First, two worlds—drug researchers (pharmacologists) and herbal medicines—yesterday appearing in opposition, are today closer and it is not unusual to read scientific works describing herbal extracts in journals traditionally dedicated to the study of synthetic drugs. Second, clinical application of herbal medicines is evaluable through the methods of modern clinical pharmacology. Efficacy and safety of medicinal plants represent naturally the object of interest for the pharmacologist and it is surely this aspect which gives the most important information on herbal medicine use. Many plants have been studied and results published showing, one time good or another poor, efficacy. Safety aspects of some of the most frequently used plants are now well known. For example, today we learn to use hypericum and we do not give it to patients taking other drugs because the interactions of hypericum with them. Contraindications of other plants, often represented by interactions with drugs, are finally known (Ginkgo biloba and drugs acting on blood coagulation). In conclusion, antagonistic behavior of pharmacologists versus herbal medicines is not useful. On the contrary, modern phytotherapy needs the contribution of researchers usually trained to evaluate efficacy and safety of medicinals.
Keywords: herbal medicines, herbal medicine, herbs, medicinal plants, phytotherapy
Herbal Medicine and Pharmacologists: Debating is Possible
In the last decade there has been a rapidly growing use of herbal medicines. This phenomenon which captured the attention of many scientists led them to play a role in this research. Modern pharmacology is for the most part directly derived from plants, but it is also well known that the use of active principles (drugs) contained in them has been considered an important progress in the care of diseases. However, a growing group of pharmacologists today, after an initial phase of pertinent criticisms of some aspects of herbal medicine still extremely based on experience, recognize the possibility of analyzing the scientific basis of biological effects produced by medicinal products composed essentially of vegetable extracts (1).
The times have changed and we think that to debate on herbal medicines without a strong influence of cultural barriers with respect to the recent past is possible. It is reasonable to pose some questions: (i) is there a role for the pharmacologist in herbal medicine (or phytotherapy)? can herbal medicine context do without pharmacologist?
Two considerations are interesting. First, two worlds—drug researchers (pharmacologists) and herbal medicines—yesterday appearing in opposition, are today closer. During the last decade reading scientific works on pharmacological effects and mechanisms of action of herbal extracts described by pharmacologists (including the authors of this article) and published in journals traditionally dedicated to synthetic drugs became more frequent (2,3).
Second, also clinical application of herbal medicines is evaluable through the methods of modern clinical pharmacology. It is difficult to challenge the assumption that clinical trial methodology is not applicable to vegetable products. Actually the production of extracts, tinctures and other herbal preparations has reached acceptable levels allowing them to be standardized and giving to these medicinals characteristics adequate to the application of rules regulating clinical research of synthetic drugs (4).
Furthermore, efficacy and safety of products based on medicinal plants represent a natural object of interest for the pharmacologist. The evaluation of clinical efficacy and safety of herbal medicines with the goal to know if they are efficient to treat diseases and if their use is free of damage to the health of consumers is surely the most important aspect either for medical community or public opinion (5). In this way, different plants have been studied and results published showing, one time good or another poor, efficacy. Thanks to this work, actually in progress, safety aspects of certain most frequently used plants are now well known (6).
Safety from Long Standing Use
Can a medicinal product be considered not safe, if it has been used for a long time in folk medicine? This is one question frequently posed by those who do not consider legislative rules necessary for the use of herbal medicines.
Safety of herbal medicines is guaranteed in some cases by long-standing use, but it is not always this case. Modern phytotherapy includes sometimes the intake of new plants or new uses of old plants. A lesson for pharmacologists and physicians on safety aspects of supposed known plants is deriving from the recent history of Hypericum perforatum.
A Lesson from Hypericum perforatum
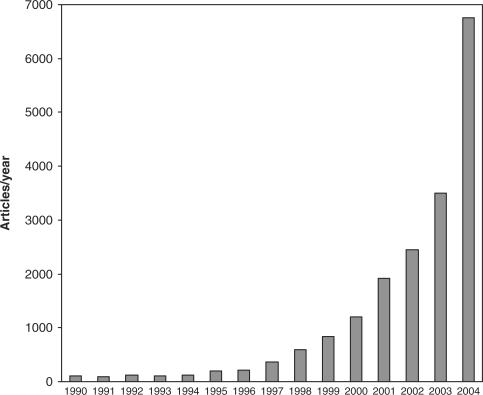
Interest of the scientific community in this plant changed the approach to medicinal plants and it is possible to compare this interest with the birth of modern phytotherapy and the progressive shift from folk medicine in the western world. Publication of meta-analysis of Linde et al. (7) in 1996 marked this important step. Work of Linde et al. (7) entitled ‘St John's wort for depression-an overview and meta-analysis of randomised clinical trials’ examined clinical works published on the effects of Hypericum perforatum on mild-to-moderate depression. We think that it is not a pure casuality if the number of scientific works reporting experiments or clinical studies on phytotherapy is progressively and rapidly increased from this point (Fig. 1). Even though Fig. 1 is not the fruit of a fully scientific methodology, it is surprising to observe, simply typing the word ‘phytotherapy’ in one of the most used scientific data banks in the world, the growing use of this term by scientists.
Figure 1.
Number of scientific articles listed each year on the data banks Pubmed reporting the word ‘Phytotherapy’ in the period 1990–2004.
The authors of this meta-analysis reported two innovative conclusions: (i) the efficacy of extracts of the flowers of the plant Hyperium perforatum, standardized to hypericin (considered for many years the only active chemical responsible for the effects) at the daily dosage of 300 mg subdivided in three times, was similar to that of tricyclic anti-depressants and (ii) side effects of Hypericum perforatum were not relevant and any way better tolerated than those of tricyclic anti-depressants. We know that the first part of these conclusions have been largely confirmed. On the contrary the conclusions related to safety have been necessarily revised.
What is the reason? What has happened? The facts linked to the revision of safety of Hypericum perforatum are easily explainable comparing recent history of the plant to those of synthetic drugs when they are recently released in the market. It is well known that a reduced number of persons have experienced the effects of new drugs before their commercialization. This underexposition produces limits in the knowledge of aspects regarding safety. Some kinds of adverse reactions require a number of patients larger than included inside the clinical research occurring before commercialization (generally about 2000–3000 for drugs). Entry in the market creates a new situation in which a larger number of people can potentially intake the new drug. In this new condition the possibility exists that adverse reactions not appeared during clinical trials can be observed. In other words the ratio risk/benefit of a drug still not frequently used is automatically not well defined. This aspect becomes clearer every day and with every patient. This is well known and leads to monitoring with particular attention to recently marketed drugs through pharmacovigilance programs in every country.
The same happened with Hypericum perforatum. Differently used in folk medicine and whatever poorly used as antidepressant, rapidly this plant has been used largely by many people for depressive symptoms. Use of effective dosage, without contraindications, without medical supervision and generally for self-medication, revealed what are the real problems related to its safety.
Today we have learnt to use Hypericum and we do not give it to patients taking other antidepressants (in particular serotoninergic drugs) or certain drugs because of the possible interactions of Hypericum with them. For its effects on metabolism of other substances, commercialization of Hypericum perforatum in Europe requires a registration like that of synthetic drugs (8).
The story of Hypericum perforatum has been observed also for other medicinal herbs. Contraindications for plants, often represented by interactions with drugs, are now known also for other plants such as Ginkgo biloba, Allium sativum and Panax ginseng (9). It is fitting to remember that medicinal plants still sell as dietary supplements in most countries. This state of things worsens the safety use of herbal medicines and it will be this way until new rules are in effect. Moreover, the need to better understand the potential toxicity of some toxic compounds contained in some popular plants (such as estragole in fennel and anise) and the medicinal plants that could be used (and at what dosage) in children and/or adolescents is emerging (10). In conclusion, antagonistic behavior of pharmacologists versus herbal medicines is not useful. On the contrary, modern phytotherapy needs the contribution of researchers without preconceptions trained to evaluate efficacy and safety of medicinals.
References
- 1.Miyata T. Pharmacological basis of traditional medicines and health supplements as curatives. J Pharmacol Sci. 2007;103:127–31. doi: 10.1254/jphs.cpj06016x. [DOI] [PubMed] [Google Scholar]
- 2.Calapai G, Crupi A, Firenzuoli F, Marciano MC, Squadrito F, Inferrera G, et al. Neuroprotective effects of Ginkgo biloba extract in brain ischemia are mediated by inhibition of nitric oxide synthesis. Life Sci. 2000;67:2673–83. doi: 10.1016/s0024-3205(00)00858-4. [DOI] [PubMed] [Google Scholar]
- 3.Calapai G, Crupi A, Firenzuoli F, Costantino G, Inferrera G, Campo GM, et al. Effects of Hypericum perforatum on levels of 5-hydroxytryptamine, noradrenaline and dopamine in the cortex, diencephalon and brainstem of the rat. J Pharm Pharmacol. 1999;51:723–8. doi: 10.1211/0022357991772862. [DOI] [PubMed] [Google Scholar]
- 4.Gagnier JJ, Boon H, Rochon P, Moher D, Barnes J, Bombardier C, et al. Reporting randomized, controlled trials of herbal interventions: an elaborated CONSORT statement. Ann Intern Med. 2006;144:364–7. doi: 10.7326/0003-4819-144-5-200603070-00013. [DOI] [PubMed] [Google Scholar]
- 5.Fong HH. Integration of herbal medicine into modern medical practices: issues and prospects. Integr Cancer Ther. 2002;1:287–93. doi: 10.1177/153473540200100313. [DOI] [PubMed] [Google Scholar]
- 6.Ernst E. Herbal medicines – they are popular, but are they also safe? Eur J Clin Pharmacol. 2006;62:1–2. doi: 10.1007/s00228-005-0070-2. [DOI] [PubMed] [Google Scholar]
- 7.Linde K, Ramirez G, Mulrow CD, Pauls A, Weidenhammer W, Melchart D. St John's wort for depression – an overview and meta-analysis of randomised clinical trials. Br Med J. 1996;313:253–8. doi: 10.1136/bmj.313.7052.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linde K, Berner M, Egger M, Mulrow C. St John's wort for depression: meta-analysis of randomised controlled trials. Br J Psychiatry. 2005;186:99–107. doi: 10.1192/bjp.186.2.99. [DOI] [PubMed] [Google Scholar]
- 9.De Smet PA. Clinical risk management of herb-drug interactions. Br J Clin Pharmacol. 2007;63:258–67. doi: 10.1111/j.1365-2125.2006.02797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rietjens IM, Martena MJ, Boersma MG, Spiegelenberg W, Alink GM. Molecular mechanisms of toxicity of important food-borne phytotoxins. Mol Nutr Food Res. 2005;49:131–58. doi: 10.1002/mnfr.200400078. [DOI] [PubMed] [Google Scholar]