Abstract
The term Pulmonary–renal syndrome refers to the combination of diffuse alveolar haemorrhage and rapidly progressive glomerulonephritis. A variety of mechanisms such as those involving antiglomerular basement membrane antibodies, antineutrophil cytoplasm antibodies or immunocomplexes and thrombotic microangiopathy are implicated in the pathogenesis of this syndrome. The underlying pulmonary pathology is small-vessel vasculitis involving arterioles, venules and, frequently, alveolar capillaries. The underlying renal pathology is a form of focal proliferative glomerulonephritis. Immunofluorescence helps to distinguish between antiglomerular basement membrane disease (linear deposition of IgG), lupus and postinfectious glomerulonephritis (granular deposition of immunoglobulin and complement) and necrotizing vasculitis (pauci-immune glomerulonephritis). Patients may present with severe respiratory and/or renal failure and require admission to the intensive care unit. Since the syndrome is characterized by a fulminant course if left untreated, early diagnosis, exclusion of infection, close monitoring of the patient and timely initiation of treatment are crucial for the patient's outcome. Treatment consists of corticosteroids in high doses, and cytotoxic agents coupled with plasma exchange in certain cases. Renal transplantation is the only alternative in end-stage renal disease. Newer immunomodulatory agents such as those causing TNF blockade, B-cell depletion and mycophenolate mofetil could be used in patients with refractory disease.
Introduction
Pulmonary–renal syndrome is defined as the combination of diffuse alveolar haemorrhage (DAH) and glomerulonephritis [1-3]. Several types of immunologic injury as well as other nonimmunologic mechanisms such as antiglomerular basement membrane (anti-GBM) antibodies, antineutrophil cytoplasm antibodies (ANCA), immunocomplexes and thrombotic microangiopathy are involved in the syndrome's pathogenesis [4-8] (Table 1).
Table 1.
Pulmonary–renal syndromes
| Clinical entities classified according to the pathogenetic mechanism involved |
| Pulmonary–renal syndrome associated with anti-GBM antibodies: Goodpasture's syndrome |
| Pulmonary–renal syndrome in ANCA-positive systemic vasculitis |
| Wegener's granulomatosis |
| Microscopic polyangiitis |
| Churg–Strauss syndrome |
| Other vasculitis |
| Pulmonary–renal syndrome in ANCA-negative systemic vasculitis |
| Henoch–Schönlein purpura |
| Mixed cryoglobulinaemia |
| Behçet's disease |
| IgA nephropathy |
| ANCA-positive Pulmonary–renal syndrome without systemic vasculitis: idiopathic Pulmonary–renal syndrome |
| Pauci-immune necrotic glomerulonephritis and pulmonary capillaritis |
| Pulmonary–renal syndrome in drug-associated ANCA-positive vasculitis |
| Propylthiouracil |
| D-Penicillamine |
| Hydralazine |
| Allopurinol |
| Sulfasalazine |
| Pulmonary–renal syndrome in anti-GBM-postive and ANCA-positive patients |
| Pulmonary–renal syndrome in autoimmune rheumatic diseases (immune complexes and/or ANCA mediated) |
| Systemic lupus erythematosus |
| Scleroderma (ANCA?) |
| Polymyositis |
| Rheumatoid arthritis |
| Mixed collagen vascular disease |
| Pulmonary–renal syndrome in thrombotic microangiopathy |
| Antiphospholipid syndrome |
| Thrombotic thrombocytopenic purpura |
| Infections |
| Neoplasms |
| Diffuse alveolar haemorrhage complicating idiopathic pauci-immune glomerulonephritis |
anti-GBM, antiglomerular basement membrane; ANCA, antineutrophil cytoplasm antibodies.
A significant number of patients will present with rapid clinical deterioration and require admission to the intensive care unit (ICU) [9-12]. This is attributed either to exacerbation of the disease activity itself, or to infectious complications secondary to severe immunosuppressive treatment [10,12]. Pulmonary–renal syndromes represent a major challenge in the ICU since the outcome is based on early and accurate diagnosis and aggressive treatment [13]. Nevertheless, mortality can reach 25–50% [14].
The aim of the present article is to provide the intensivist with an overview of Pulmonary–renal syndrome, focusing on new concepts of its pathogenesis and treatment innovations.
Pathology of Pulmonary–renal syndrome
The underlying pulmonary lesion in the majority of cases of Pulmonary–renal syndrome is small-vessel vasculitis, characterized by a destructive inflammatory process that involves arterioles, venules and alveolar capillaries (necrotic pulmonary capillaritis). These lesions disrupt perfusion and the continuity of the pulmonary capillary wall, allowing blood to extravasate in the alveolar space. This is clinically expressed with DAH [15].
The underlying renal pathology in the majority of cases of Pulmonary–renal syndrome is a form of focal proliferative glomerulonephritis [16]. Fibrinoid necrosis is frequently seen, as well as microvascular thrombi. Extensive crescent formation regularly accompanies glomerular tuft disease. Interstitial infiltration, fibrosis and tubular atrophy are poor prognostic factors. Necrotizing granulomas and small-vessel vasculitis are rare findings. Immunofluorescence helps to distinguish among anti-GBM disease (linear deposition of IgG), lupus and postinfectious glomerulonephritis (granular deposition of immunoglobulin and complement), and necrotizing vasculitis (pauci-immune glomerulonephritis) [17,18].
Epidemiology and pathogenesis of Pulmonary–renal syndrome
Pulmonary–renal syndrome associated with anti-GBM antibodies: Goodpasture's syndrome
The term 'Goodpasture's syndrome' is used for the clinical entity of DAH and rapidly progressive glomerulonephritis associated with anti-GBM antibodies [19,20].
Goodpasture's syndrome is extremely rare (one case per 1,000,000 population per year). The disease predominantly affects Caucasians of every age but mostly those in the second to third decades and the fifth to sixth decades of life, with a slight predominance of males. Although rare, this syndrome is responsible for about 20% of acute renal failure cases due to rapidly progressive glomerulonephritis [19]. Both genetic and environmental factors have been implicated in the pathogenesis of Goodpasture's syndrome. The disease has been described in brothers and in identical twins. More than 80% of patients carry the HLA alleles DR15 or DR4 whereas the alleles DR7 and DR1 are rarely found, suggesting that the latter may play a protective role [21]. The fact that most cases present sporadically implies an additional aetiology beyond hereditary predisposition. Environmental factors, such as smoking, infections and previous hydrocarbon exposure, have been implicated in triggering the disease [22].
Human anti-GBM antibodies belong mostly to the IgG class and reactwith a limited number of epitopes (EA and EB) on the noncollageneous domain of the α3 chain of type IV collagen (NC1 α3 IV), a molecule expressed in the basement membranes of renal glomerulus, renal tubule, alveoli, chorioid plexus, retinal capillaries and Bruchs's membrane [16,20]. Anti-GBM antibodies bind the glomerular basement membrane, activating compliment and proteases, resulting in the disruption of the filtration barrier and Bowman's capsule and causing proteinuria and crescent formation [23,24]. The pathogenetic role of anti-GBM has been proved in multiple studies [20]. As an example, in genetically engineered mice that produce human IgG antibodies, immunization with the NC1 α3 IV domains leads to the production of human anti-GBM antibodies and proliferative glomerulonephritis [25].
Pulmonary–renal syndrome in ANCA-positive systemic vasculitis
Circulating ANCA autoantibodies are detected in the majority of patients presenting with Pulmonary–renal syndrome [26,27]. ANCA do not confirm a specific entity but practically lead the differential diagnosis to three major systemic vasculitides syndromes: Wegener's granulomatosis, microscopic polyangiitis and Churg–Strauss syndrome [26].
Wegener's disease or Wegener's granulomatosis is characterized by the triad of systemic necrotizing vasculitis, necrotizing granulomatous inflammation of the upper and lower respiratory tract, and necrotizing glomerulonephritis [28]. The incidence of the disease is estimated up to 8.5/million (range 5.2–12.9/million) with a male-to-female ratio of 1:1. The disease usually involves Caucasians (80–97%) with a mean age at the time of diagnosis of 40–55 years, although persons of every age may be affected [29]. The lungs are involved in 90% of cases. In a small percentage of patients, a limited form of the disease that spares the kidney has been described [29,30].
Microscopic polyangiitis is a systemic small-vessel vasculitis manifested by pauci-immune necrotic glomerulonephritis (80–100% of patients), pulmonary capillaritis (10–30%), skin lesions and arthralgias [31].
Churg–Strauss syndrome is a systemic disease, typically presenting with an initial asthma/sinusitis phase, followed by eosinophilia and vasculitis [9]. In Churg–Strauss syndrome, renal involvement is milder compared with Wegener's disease, Goodpasture's syndrome and microscopic polyangitis [32].
ANCA include three categories of antibodies based on their pattern of indirect immunofluorescence on ethanol-fixed neutrophils: a diffuse cytoplasmic granular pattern, a perinuclear pattern, and an atypical pattern [33-35]. The antigenic target for cytoplasmic ANCA is proteinase 3 (Pr3), and that for perinuclear ANCA is myeloperoxidase (MPO).
ANCA are detected both through indirect immunofluorescence and ELISA [36,37].
Several lines of evidence suggest that ANCA are involved in the pathogenesis of ANCA-associated diseases. Xiao and colleagues demonstrated that anti-MPO IgG administration in mice causes focal necrotizing and crescentic glomerulonephritis [38]. In humans, a newborn developed glomerulonephritis and pulmonary haemorrhage after intrauterine transplacental transfer of ANCA IgG against MPO [39,40]. On the other hand, administration of anti-Pr3 antibodies in mice alone does not induce glomerulonephritis. This administration does, however, aggravate TNF-α-elicited inflammation, suggesting that Pr3 ANCA have a proinflammatory activity in conjunction with a primary inflammatory stimulus [41]. In addition, ANCA were shown to enhance interactions between leukocytes and endothelial cells and to cause microvascular haemorrhage [42,43]. More precisely, the majority of target antigens of ANCA such as Pr3 and MPO are proteolytic enzymes of the azurophilic granules of neutrophils [44]. Fixation of ANCA with Pr3 on the endothelial surface induces expression of adhesion molecules and release of IL-8 that causes recruitment and attachment of neutrophils on the endothelial cell surface, leading to vessel wall inflammation, obliteration and damage [45].
Pulmonary–renal syndrome in ANCA-negative systemic vasculitis
Pulmonary–renal syndrome in ANCA-negative systemic vasculitis is very rare and has been described only occasionally in Behçet's disease, in Henoch–Schönlein purpura, in IgA nephropathy and in mixed cryoglobulinaemia [46]. In Henoch–Schönlein purpura, acute capillaritis and DAH involve deposition of IgA immuno-complexes along the pulmonary alveoli [47].
ANCA-positive Pulmonary–renal syndrome without systemic vasculitis: idiopathic Pulmonary–renal syndrome
This entity includes the patients presenting with DAH, rapidly progressive glomerulonephritis and positive ANCA (either Pr3 or MPO), but with no other manifestation of systemic vasculitis. Fever, malaise, weight loss, myalgias and arthralgias may coexist. Mortality during the first episode of the syndrome exceeds 50%. It is argued that the syndrome represents either a limited type of microscopic polyangiitis or a variant of Wegener's syndrome [5].
Pulmonary–renal syndrome in drug-associated ANCA-positive vasculitis
Drugs provide one of the potentially reversible causes of ANCA-positive vasculitis. Most frequently they cause perinuclear ANCA/MPO ANCA vasculitis, although cytoplasmic ANCA/Pr3 ANCA vasculitis has also been described [48]. The drugs most frequently implicated in the pathogenesis of the syndrome are propylthiouracil and hydralazine. ANCA are detected in 20% of patients receiving propylthiouracil, but only a minority of these patients develop clinical manifestations of systemic vasculitis including Pulmonary–renal syndrome [49]. D-Penicillamine, allopurinol and sulfasalazine have also been associated with Pulmonary–renal syndrome.
Discontinuation of the causative drug most frequently leads to regression of the disease; however, some patients continue to present positive ANCA or even recurrent disease, requiring long-term immunosuppressive treatment. In general, drug-induced disease has a more benign course than ANCA-positive Pulmonary–renal syndrome of other aetiology [50]. Drugs should therefore be considered a potential cause of MPO vasculitis, particularly among patients with high titres of these antibodies [48].
Pulmonary–renal syndrome in both anti-GBM-positive and ANCA-positive patients
In patients with Pulmonary–renal syndrome, anti-GBM antibodies are occasionally detected simultaneously with ANCA, most frequently MPO ANCA [51,52]. The significance of this finding is unknown. No cross-reactivity between the targets of ANCA and anti-GBM antibodies has been found. It has been speculated that ANCA-associated damage of the glomerular membrane uncovers 'hidden antigen' inducing the formation of anti-GBM antibodies [53].
Pulmonary–renal syndrome in autoimmune rheumatic diseases (immune complexes and/or ANCA mediated)
Pulmonary–renal syndrome has been reported more often in systemic lupus erythematosus and systemic sclerosis, and rarely in rheumatoid arthritis and mixed connective tissue disease. DAH ± glomerulonephritis occurs in 2% of systemic lupus erythematosus patients and rarely is the first manifestation of the disease [54,55]. Immune complex deposition is frequently detected in both the pulmonary and renal vessels with mortality rates between 70% and 90%, among the highest of all causes of Pulmonary–renal syndrome [54,55]. Pulmonary–renal syndrome is a rare but potentially lethal complication of systemic sclerosis, and often coexists with pulmonary fibrotic disease [56]. In this case, renal failure is normotensive, in contrast to the hypertensive nephropathy characterizing systemic sclerosis. ANCA, more often the perinuclear ANCA or MPO ANCA, have been detected in some systemic sclerosis patients [57].
Pulmonary–renal syndrome in thrombotic microangiopathy
Pulmonary–renal syndrome has been described in the context of diseases characterized by thrombotic microangiopathy, such as antiphospholipid syndrome (APS), thrombotic thrombocytopenic purpura, malignancies and infections.
Antiphospholipid syndrome
The term APS was used initially to characterize patients presenting with the combination of antiphospholipid antibodies and hypercoagulation syndrome. The diagnosis of the disease actually requires the criteria defined in the very informative paper of Levine and colleagues [58]. Antiphospholipid antibodies are heterogeneous, and they target negatively charged phospholipids and serum phospholipid-binding proteins. The antibodies are frequently associated with thrombosis, foetal loss and other clinical manifestations of APS, and are thought to play an important role in the pathogenesis of the syndrome. Antiphospholipid antibodies inhibit activated protein C, antithrombin III and fibrinolysis and upregulate tissue factor activity, thus leading to a procoagulant state [59].
Pulmonary–renal syndrome has been described in the context of acute catastrophic APS, defined as the APS that develops over days or weeks characterized by multiple thromboses in small and large vessels of at least three different organ systems [60]. The kidney is the organ most commonly involved (78%), followed by the lungs (66%), the central nervous system (56%), the heart (50%), and the skin (50%). Acute catastrophic APS results in adult respiratory distress syndrome and in renal failure, leading up to 25% of patients to haemodialysis [60,61].
Thrombotic thrombocytopenic purpura
Pulmonary–renal syndrome has also been described in patients with thrombotic thrombocytopenic purpura [62]. Thrombotic thrombocytopenic purpura is an often-fatal multisystem disease characterized by thrombocytopenia, microangiopathic haemolytic anaemia and ischemic manifestations due to aggregation of platelets in the arterial microcirculation [63].
Recent studies suggest that the insufficiency of a specific plasma metalloprotease responsible for the degradation of von Willebrand factor cleavage protein (ADAMTS-13) is involved in the pathogenesis of many familial and idiopathic cases [64]. In some patients, inhibitory anti-von Willebrand factor cleavage protein antibodies have been detected in serum. Pregnancy, disseminated neoplasms and chemotherapy are considered predisposing factors. The detection of hyaline thrombi in arterioles, venules and capillaries without evidence of vascular inflammation is diagnostic [65-67].
Diffuse alveolar haemorrhage complicating idiopathic pauci-immune glomerulonephritis
The term 'pauci-immune' glomerulonephritis has mainly been used to indicate that no immunoglobulins, immune complexes or complement can be detected in renal biopsy, either by immunofluorescence or by electronic microscopy. Rarely, the course of patients with idiopathic pauci-immune glomerulonephritis may be complicated by DAH [5,6].
Clinical manifestation of Pulmonary–renal syndrome and evaluation of the critically ill patient
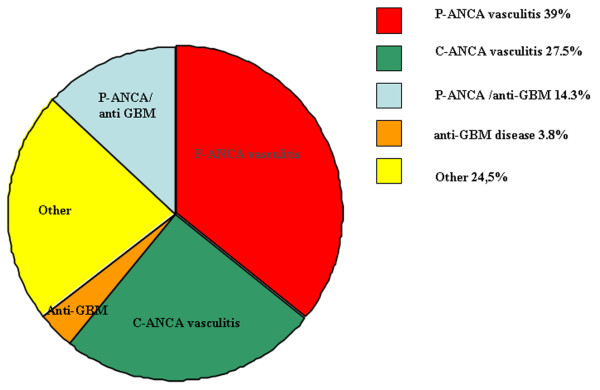
Patients with Pulmonary–renal syndrome may require admission to the ICU either because of the disease itself or because of a complication of the treatment [11]. The most frequent diagnoses in patients with Pulmonary–renal syndrome admitted to the ICU are perinuclear ANCA vasculitis, followed by cytoplasmic ANCA vasculitis, Goodpasture's syndrome, systemic lupus erythematosus and catastrophic APS [68-71] (Table 2 and Figure 1). The diagnosis is already known in the majority of those patients admitted to the ICU; the main cause of admission in these patients is infection or adverse drug effects, including severe infectious complications related to the immunosuppressive treatment. More than one-third of the patients treated in ICU settings, however, present with serious renal impairment and adult respiratory distress syndrome of unknown aetiology [70,72].
Table 2.
Relative frequencies of conditions contributing to Pulmonary–renal syndrome in the intensive care unit [68-71]
| Pourrat et al. (2000) [68] | Gallagher et al. (2002) [70] | Cruz et al. (2003) [69] | Bucciarrelli et al. (2006) [71] | |
| Number of patients | 33a | 14b | 26c | 220d |
| Perinuclear ANCA vasculitis | - | 5 | 11 | - |
| Cytoplasmic ANCA vasculitis | 2 | 5 | 5 | - |
| Goodpasture's syndrome | 3 | - | 1 | - |
| Antiglomerular basement membrane/perinuclear ANCA positive | - | 2 | - | - |
| Catastrophic antiphospholipid syndrome with adult respiratory distress syndrome | - | - | 56 | |
| Other | 28 | 2 | 10 | 164 |
aThirty-three patients admitted to the intensive care unit with 'systemic disease'. 'Other' includes eight cases of systemic lupus erythematosus. bFourteen patients with Pulmonary–renal syndrome. 'Other' included one case of systemic lupus erythematosus. cTwenty-six patients with systemic necrotizing vasculitis. 'Perinuclear antineutrophil cytoplasm antibodies (ANCA) vasculitis' included Churg–Strauss syndrome and microscopic polyangiitis. 'Other' included polyarteritis nodosa, HIV-related vasculitis, cryoglobulinaemic vasculitis and Henoch–Schönlein purpura. dTwo hundred and twenty patients with catastrophic antiphospholipid syndrome included in the catastrophic antiphospholipid syndrome registry. 'Other' included catastrophic antiphospholipid syndrome without adult respiratory distress syndrome.
Figure 1.
Relative frequencies of conditions contributing to Pulmonary–renal syndrome in the intensive care unit. Relative frequencies of conditions contributing to Pulmonary–renal syndrome in the intensive care unit based on mean values from data on patients' characteristics provided by [69,70] (shown in detail in Table 2). Perinuclear antineutrophil cytoplasmic antibodies (P-ANCA) vasculitis is the most frequent cause of Pulmonary–renal syndrome for patients admitted to the intensive care unit. 'Other' includes systemic lupus erythematosus, catastrophic antiphospholipid syndrome, polyarteritis nodosa, HIV-related vasculitis, cryoglobulinaemic vasculitis and Henoch–Schönlein purpura. C-ANCA, cytoplasmic antineutrophil cytoplasmic antibodies; anti-GBM, antiglomerular basement membrane.
Establishing the diagnosis is a particularly difficult task in patients presenting with pulmonary infiltrates and fever, having no prior disease label and without haemoptysis – a clinical scenario resembling 'pneumonia'. Even though the lack of large prospective trials does not permit strict recommendations, we propose that the possibility of a Pulmonary–renal syndrome should be considered in those patients with bilateral pulmonary infiltrates in the face of the following: falling haemoglobin levels, renal failure necessitating haemodialysis, sinusitis, mononeuritis multiplex, polyarthalgia, severe asthma attack, pericarditis, cerebral ischaemia, purpura or congestive heart failure [69,73]. Furthermore, the treating physician should always bear in mind that Pulmonary–renal syndrome at first presentation may not only mimic pneumonia, but in certain cases could be triggered by pneumonia. Treatment of all these patients should therefore include broad antibiotic cover until further workup is performed [74].
Haemoptysis is the most common clinical manifestation of DAH [5,6]. However, 30–35% of patients may have DAH without evidence of haemoptysis. Breathlessness, cough and low-grade fever may also be present. In about 50% of cases of DAH, patients suffer acute respiratory failure requiring mechanical ventilation [6]. The most common renal manifestation of Pulmonary–renal syndrome is haematuria, proteinuria and active urinary sediment. If left untreated, patients can progress to end-stage renal failure, requiring haemodialysis [17].
Chest roentgenograms and computerized tomography scanning are used to depict DAH. The former may be normal in up to 22% of cases [75]. Common findings include coalescent alveolar infiltrates or consolidations with air bronchogram, and rarely ground glass opacities. The distribution of the infiltrates is mainly perihilar or predominates in the middle and lower pulmonary fields. Complete roentgenographic resolution usually takes 3–4 days (or occasionally even 1 day) provided the haemorrhage has ceased. A persistence of the interstitial pattern may be related to underlying disease or may indicate the presence of primary pulmonary haemosiderosis, a result of indolent chronic or recurrent DAH [76,77]. The presence of a diffuse alveolar pattern with Kerley A, B, C linear shadows denotes other causes such as veno-occlusive disease of the lung, mitral valve stenosis or cardiogenic pulmonary oedema [78]. Urinalysis reveals dysmorphic red cells of glomerular origin, red-cell casts and other cellular and granular casts. Proteinuria is always present, but rarely in the range of nephrotic syndrome [17,18]. In the vast majority of patients, bound urea nitrogen and creatinine levels are elevated, associated with oliguria, hypertension and oedema. A normochromic normocytic anaemia is frequently observed and is more profound than expected from the degree of renal failure [16]. Laboratory findings of Coomb's negative haemolytic anaemia with schistocytes or fragmented red cells on peripheral blood examination in combination with thrombocytopenia and minimal activation of coagulation mechanisms are suggestive of thrombotic thrombocytopenic purpura [63].
All necessary samples such as sputum and blood cultures, as well as serology tests, should be obtained to rule out bacterial infection or viral infection. When Pulmonary–renal syndrome is clinically suspected, the detection in serum of antibodies such as anti-GBM and/or ANCA is of major importance. The use of serology to direct therapeutic decisions may be extremely complicated and should be based on the performance characteristics of the test (sensitivity and specificity) as well as on the pretest probability of the disease. In this regard, ANCA testing can be safely interpreted as 'documentation of the diagnosis' in patients with strong clinical suspicion for pauci-immune crescentic glomerulonephritis; on the contrary, in patients with weak clinical evidence of the disease, a positive result requires further testing while a negative result can be used to exclude such a diagnosis [79].
Anti-GBM antibodies detected using different immunoassays have a sensitivity of 95–100% and a specificity of 90–100% for Goodpasture's syndrome [80-82]. Cytoplasmic ANCA are found in more than 85% of patients with generalized Wegener's granulomatosis and in 60% of patients with the limited form of the disease [83]. Approximately 40–80% of patients with microscopic polyangiitis have ANCA, mainly perinuclear ANCA/MPO ANCA. Positive perinuclear ANCA/MPO ANCA and a negative serological test for hepatitis B are, in general, suggestive of microscopic polyangiitis [84]. Of the patients with Churg–Strauss syndrome, 35–70% have positive perinuclear ANCA/MPO ANCA, while only 10% have positive Pr3 ANCA [85,86].
According to the International Consensus Statement on Testing and Reporting of antineutrophil cytoplasmic antibodies, combining indirect immunofluorescence essays and enzyme immunoassays (ELISAs) for Pr3 and MPO is more accurate than either assay alone [87]. It is important to note, however, that not all patients with ANCA-associated vasculitis will test positive for ANCA, and therefore ANCA are not considered a diagnostic criterion [88]. On the other hand, ANCA have also been detected in several other autoimmune nonvasculitic disorders, such as inflammatory bowel disease, rheumatoid arthritis and autoimmune hepatitis as well as in infectious and neoplastic diseases [89,90].
Bronchoscopy should be performed to rule out infection and to evaluate the presence of DAH. Recovery of haemorrhagic fluid on bronchoalveolar lavage, especially if the sample becomes bloodier from the first to the last suctioned syringe, and acute decrease of the haematocrit coupled with a chest roentgenogram showing multiple coalescent alveolar shadows strongly suggest the diagnosis of DAH [6].
The gold standard for diagnosis of Pulmonary–renal syndrome is pulmonary and/or renal biopsy. Percutaneous renal biopsy is often performed and specimens undergo conventional histopathology and immunofluorescence study [91,92]. When the lung is involved, a surgical or a thoracoscopic lung biopsy may be performed. Tissue should always be processed for additional immunofluorescence and microbiology studies [81].
In the case of Goodpasture's syndrome, anti-GBM antibody deposition on the glomerular and alveolar basement membrane can be detected in renal and/or lung tissue by immunofluorescence as a linear staining along the glomerular and/or the alveolar basement membrane, respectively. In Wegener's granulomatosis, the three major pathologic features on lung biopsy include granuloma, inflammation of the vascular wall (arteriolar, venular or capillary) and areas of geographic necrosis [83,91]. The histologic criteria of Churg–Strauss syndrome include necrotizing vasculitis in affected tissues, eosinophilic tissue infiltration and extravascular granulomas [84].
Critically ill patients are unfortunately high-risk operative candidates for lung or renal biopsy [93]. Although biopsies of other organs (skin, sinus, nerves) can be used, appropriate treatment should be promptly initiated even in the absence of histopathological confirmation to minimize morbidity and mortality in patients with high clinical suspicion of ANCA-associated or anti GBM-associated vasculitis and with a positive ANCA or anti-GBM antibodies result, respectively [14,34]. When initial treatment is initiated (see below), patients should be closely monitored for response to therapy. Improvement of a chest X-ray, of arterial blood gases, of renal function, of neurologic signs and of other signs (such as purpura) is expected to start during the first few days of the initiation of treatment in those patients responding to therapy. Recovery is less common for patients on dialysis, but dialysis can be discontinued in more than one-half of them. When patients deteriorate, the differential diagnosis includes refractory Pulmonary–renal syndrome, drug-adverse effects, infection with sepsis and another underlying disease. In these cases, invasive diagnostic efforts should be performed without further delay and empirical treatment should be reevaluated with a highly expert team of physicians along with the treating doctors [9,10].
Treatment of Pulmonary–renal syndrome in the critically ill patient
Therapy is subdivided into the induction-remission phase and the maintenance phase [94,95]. In the following, treatment for ANCA-associated vasculitides, for Goodpasture's syndrome and for Pulmonary–renal syndrome of variant aetiology will be discussed. It is uncommon that the intensivist treats patients with Pulmonary–renal syndrome in remission, unless drug toxicity and infectious immunosuppressive treatment complications ensue.
ANCA-associated Pulmonary–renal syndrome
Immunosuppression is the cornerstone of treatment in ANCA-associated Pulmonary–renal syndrome. Standard induction-remission regimens include pulse intravenous methylprednisolone (500–1,000 mg) for 3–5 days. As the life-threatening features subside, the dose can then be reduced to 1 mg/kg prednisone (or equivalent) daily for the first month, tapered over the next 3–4 months. Glucocorticoid therapy is combined with cytotoxic agents. Cyclophosphamide is the treatment of choice in critically ill patients with generalized disease, at a dose of 0.5–1 g/m2 administered intravenously as a pulse per month or orally (1–2 mg/kg/day) [87,88]. Severe disease defined by major renal impairment (serum creatinine > 5.7 mg/dl) was recently suggested to be treated with corticosteroids and cyclophosphamide coupled with plasma exchange at least for the first week to increase the likelihood for renal function restoration [96,97]. There are reports suggesting that extracorporeal membrane oxygenation and activated human factor VII may be beneficial in some critically ill patients with DAH [98-100].
With this treatment, approximately 85% of patients achieve remission [94,95]. Transition to maintenance therapy may occur 6–12 months after the initiation of induction therapy or after clinical remission [101]. The maintenance therapy includes low-dose corticosteroids coupled with cytotoxic agents
Relapse will occur in 11–57% of patients in remission. Some relapses are severe, resulting in end-organ damage. Female or black patients and those patients with severe kidney disease, lung disease or upper airway disease and anti-Pr3 serum antibodies are shown to be more resistant to initial treatment [95]. In these cases, the use of alternative agents must be considered. Recent investigation has focused on TNF-α inhibitors, B-cell depletion agents, mycophenolate mofetil, leflunomide and antithymocyte globulin [102-113]. As indicated in Table 3, new agents are shown to be effective in certain cases but are followed by high relapse and complication rates. Most data are preliminary and further studies are needed for definite conclusions.
Table 3.
Novel agents for the treatment of Pulmonary–renal syndrome [102-113]
| Biological agent | Mechanism of action | Indication-study population | Comments |
| Etanercept | TNFα inhibitor | Maintenance therapy in Wegener's granulomatosis | Not effective, high rate of treatment-related complications |
| Infliximab | TNFα inhibitor | ANCA-associated vasculitis | Effective, severe infection rate, severe relapse rate |
| Rituximab | Anti-CD20 antibody for B lymphocytes | ANCA-associated vasculitis, refractory to or contraindication to treatment | Effective, preliminary data |
| Mycofenolate mofetil | Suppressor of B lymphocytes and T lymphocytes | ANCA-associated vasculitis, remission maintenance | Well tolerated, high relapse rate |
| Leflunomide | Suppressor of T cells | Wegener's granulomatosis, remission maintenance | Well tolerated, high relapse rate |
| Antithymocyte globulin | Suppressor of T cells | Severe refractory Wegener's granulomatosis | Partial or complete remission, high complication rate |
ANCA, antineutrophil cytoplasmic antibodies.
Goodpasture's syndrome
Immunosuppressive treatment should also be urgently initiated in the case of Goodpasture's syndrome. Daily plasma exchange should be started; if tests for anti-GBM antibodies are found to be negative, plasmapheresis is then discontinued. A mean of 14 courses of treatment is usually needed until the anti-GBM antibody titre is normalized. Prompt and aggressive plasmapheresis for ANCA-positive, anti-GBM-positive patients may portend a greater likelihood of renal recovery [11,114].
Systemic lupus erythematosus
DAH due to systemic lupus erythematosus carries a grave prognosis, and lupus nephritis needs immediate immunosuppressive treatment with cyclophosphamide to prevent end-stage renal disease [115]. To avoid the severe side effects of the treatment of systemic lupus erythematosus, including bone marrow suppression, haemorrhagic cystitis, opportunistic infections, malignant diseases and premature gonadal failure, new agents such as mycophenolate mofetil and rituximab are under investigation. Both drugs have led to effective disease remission with low toxicity but with a high relapse rate [116,117].
Acute catastrophic antiphospholipid syndrome
In Pulmonary–renal syndrome related to acute catastrophic APS, the mainstay of therapy is anticoagulation [59].
Thrombotic thrombocytopenic purpura
In cases of Pulmonary–renal syndrome and thrombotic thrombocytopenic purpura, mortality exceeded 90% before the application of plasmapheresis. Today's response to treatment with plasmapheresis reaches 80%. While waiting for plasmapheresis treatment, plasma transfusions are indicated to make up for the inadequate von Willebrand factor cleavage protein [67].
Despite rigorous treatment, almost 66% of patients with small-vessel vasculitis and Pulmonary–renal syndrome will need renal transplantation within less than 4 years of initial presentation. The ICU physician will have to care for patients with end-stage renal disease due to Pulmonary–renal syndrome because of an increased rate of fluid and electrolyte abnormalities, cardiovascular disease, haematological and neurological abnormalities, and bacterial infections. In the post-transplant period, the ICU admission rates for these patients are high and their prognosis remains poor [118].
Conclusion
Pulmonary renal syndrome in the ICU is a life-threatening entity with an acute onset and with a fulminant course if left untreated. Appropriate management of such patients includes early and accurate diagnosis, exclusion of infection, close monitoring and specialized immunosuppressive treatment coupled with plasma exchange in certain cases. Newer immunomodulatory agents could confer life-saving options for refractory disease in the future. Renal transplantation remains the only alternative for patients with Pulmonary–renal syndrome who develop end-stage renal disease.
Abbreviations
anti-GBM = antiglomerular basement membrane; ANCA = antineutrophil cytoplasm antibodies; APS = antiphospholipid syndrome; DAH = diffuse alveolar haemorrhage; ELISA = enzyme-linked immunosorbent assay; ICU = intensive care unit; IL = interleukin; MPO = myeloperoxidase; Pr3 = proteinase 3; TNF = tumour necrosis factor.
Conflicts of interest
The authors declare that they have no competing interests.
Authors' contributions
SAP contributed to the concept, design and drafting of the manuscript. EDM and IK contributed to the drafting of the manuscript. GEK contributed to critically revising the manuscript. AK and ChR contributed to the final approval of the version to be published.
Acknowledgments
Acknowledgements
The authors would like to express the deepest gratitude to Professor Haralampos M Moutsopoulos, MD, FACP, FRCP(Edin), for his continuous support and invaluable inspiration, as well as for his critical review of the manuscript. This work was supported by the 'Thorax' Foundation
Contributor Information
Spyros A Papiris, Email: papiris@otenet.gr.
Effrosyni D Manali, Email: fmanali@otenet.gr.
Ioannis Kalomenidis, Email: ikalom@med.uoa.gr.
Giorgios E Kapotsis, Email: gkapotsis@hotmail.com.
Anna Karakatsani, Email: annakara@otenet.gr.
Charis Roussos, Email: croussos@cc.uoa.gr.
References
- Gallagher H, Kwan J, Jayne RW. Pulmonary renal syndrome: a 4-year, single center experience. Am J Kidney Dis. 2002;38:42–47. doi: 10.1053/ajkd.2002.29876. [DOI] [PubMed] [Google Scholar]
- Goodpasture EW. The significance of certain pulmonary lesions in relation to the aetiology of pneumonia. Am J Med Sci. 1919;158:863–870. doi: 10.1097/00000441-191911000-00012. [DOI] [PubMed] [Google Scholar]
- Stanton MC, Tange JD. Goodpasture's syndrome (pulmonary haemorrhage associated with glomerulonephritis) Australas Ann Med. 1958;7:132–144. doi: 10.1111/imj.1958.7.2.132. [DOI] [PubMed] [Google Scholar]
- Lerner RA, Glassock KJ, Dixon FJ. The role of antiglomerular basement membrane antibody in the pathogenesis of human glomerulonephritis. J Exp Med. 1967;126:989–1004. doi: 10.1084/jem.126.6.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specks U. Diffuse alveolar hemorrhage syndromes. Curr Opin Rheumatol. 2001;13:12–17. doi: 10.1097/00002281-200101000-00003. [DOI] [PubMed] [Google Scholar]
- Collard HR, Schwarz MI. Diffuse alveolar hemorrhage. Clin Chest Med. 2004;25:583–592. doi: 10.1016/j.ccm.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Wiik A. Autoantibodies in vasculitis. Arthritis Res Ther. 2003;5:147–152. doi: 10.1186/ar758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford C, Balow JE. New insights into the immunopathogenesis and treatment of small vessel vasculitis of the kidney. Curr Opin Nephrol Hypertens. 2003;12:267–272. doi: 10.1097/00041552-200305000-00007. [DOI] [PubMed] [Google Scholar]
- Brown KK. Pulmonary vasculitis. Proc Am Thorac Soc. 2006;3:48–57. doi: 10.1513/pats.200511-120JH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez W, Hanania N, Guy E, Guntupalli J. Pulmonary–renal syndromes in the intensive care unit. Crit Care Clin. 2002;18:881–895. doi: 10.1016/S0749-0704(02)00029-5. [DOI] [PubMed] [Google Scholar]
- Merkel PA, Choi H, Niles JL. Evaluation and treatment of vasculitis in the critically ill patient. Crit Care Clin. 2002;18:321–344. doi: 10.1016/S0749-0704(01)00006-9. [DOI] [PubMed] [Google Scholar]
- Camargo JF, Tobon GJ, Fonseca N, Diaz JL, Uribe M, Molina F, Anaya J-M. Autoimmune rheumatic diseases in the intensive care unit: experience from a tertiary referral hospital and review of the literature. Lupus. 2005;14:315–320. doi: 10.1191/0961203305lu2082oa. [DOI] [PubMed] [Google Scholar]
- Semple D, Keogh J, Forni L, Venn R. Clinical review: vasculitis on the intensive care unit-part 1: diagnosis. Crit Care. 2005;9:92–97. doi: 10.1186/cc2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith M, Brett S. The pulmonary physician in critical care illustrative case 3: pulmonary vasculitis. Thorax. 2003;58:543–546. doi: 10.1136/thorax.58.6.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies DJ. Small vessel vasculitis. Cardiovasc Pathol. 2005;14:335–346. doi: 10.1016/j.carpath.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Erlich JH, Sevastos J, Pussell B. Goodpasture's disease: antiglomerular basement membrane disease. Nephrology. 2004;9:49–51. doi: 10.1111/j.1440-1797.2004.00244.x. [DOI] [PubMed] [Google Scholar]
- Lau K, Wyatt R. Glomerulonephritis. Adolesc Med. 2005;16:67–85. doi: 10.1016/j.admecli.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Contreras G, Pardo V, Leclercq B, Lenz O, Tozman E, O'Nan P, Roth Sequential therapies for proliferative lupus nephritis. N Engl J Med. 2004;350:971–980. doi: 10.1056/NEJMoa031855. [DOI] [PubMed] [Google Scholar]
- Salama AD, Levy J, Lightstone L, Pusey CD. Goodpasture's disease. Lancet. 2001;358:917–920. doi: 10.1016/S0140-6736(01)06077-9. [DOI] [PubMed] [Google Scholar]
- Hudson BG, Tryggvason K, Sundaramoorthy M, Neilson EG. Alport's syndrome, Goodpasture's syndrome, and type IV collagen. N Engl J Med. 2003;348:2543–2556. doi: 10.1056/NEJMra022296. [DOI] [PubMed] [Google Scholar]
- Bombassei GJ, Kaplan AA. The association between hydrocarbon exposure and anti-glomerular basement membrane antibody-mediated disease (Goodpasture's syndrome) Am J Ind Med. 1992;21:141–153. doi: 10.1002/ajim.4700210204. [DOI] [PubMed] [Google Scholar]
- Phelps RG, Jones V, Turner AN, Rees AJ. Properties of HLA class II molecules divergently associated with Goodpasture's disease. Int Immunol. 2000;12:1135–1143. doi: 10.1093/intimm/12.8.1135. [DOI] [PubMed] [Google Scholar]
- Lou YH. Anti-GBM glomerulonephritis: a T cell-mediated autoimmune disease. Arch Immunol Ther Exp. 2004;52:96–103. [PubMed] [Google Scholar]
- Sheerin NS, Springall T, Carroll MC, Sacks SH. Protection against antiglomerular basement membrane (GBM)-mediated nephritis in C3 and C4 deficient mice. Clin Exp Immunol. 1997;110:403–409. doi: 10.1046/j.1365-2249.1997.4261438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers KE, Allen J, Gehret J, Jacobovits A, Gallo M, Neilson EG, Hopfer H, Kalluri R, Madaio MP. Human antiglomerular basement membrane autoantibody disease in Xenomouse II. Kidney Int. 2002;61:1666–1673. doi: 10.1046/j.1523-1755.2002.00312.x. [DOI] [PubMed] [Google Scholar]
- Csernok E. Anti-neutrophil cytoplasmic antibodies and pathogenesis of small vessel vasculitides. Autoimmun Rev. 2003;2:158–164. doi: 10.1016/S1568-9972(03)00010-7. [DOI] [PubMed] [Google Scholar]
- Frankel S, Cosgrove G, Fischer A, Meehan RT, Brown KK. Update in the diagnosis and management of pulmonary vasculitis. Chest. 2006;129:452–465. doi: 10.1378/chest.129.2.452. [DOI] [PubMed] [Google Scholar]
- Bacon PA. The spectrum of Wegener's granulomatosis and disease relapse. N Engl J Med. 2005;352:330–332. doi: 10.1056/NEJMp048338. [DOI] [PubMed] [Google Scholar]
- Langford C, Hoffman G. Wegener's granulomatosis. Thorax. 1999;54:629–637. doi: 10.1136/thx.54.7.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savige J, Davies D, Falk R, Jennette JC, Wiik A. Antineutrophil cytoplasmic antibodies and associated diseases: a review of the clinical and laboratory features. Kidney Int. 2000;57:846–862. doi: 10.1046/j.1523-1755.2000.057003846.x. [DOI] [PubMed] [Google Scholar]
- Schwarz M, Brown K. Small vessel vasculitis of the lung. Thorax. 2000;55:502–510. doi: 10.1136/thorax.55.6.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillevin L, Cohen P, Gayraud M, Lhote F, Jarrousse B, Casassus P. Churg Strauss syndrome: clinical study and long-term follow-up of 96 patients. Medicine. 1999;78:26–37. doi: 10.1097/00005792-199901000-00003. [DOI] [PubMed] [Google Scholar]
- Davies DJ, Moran JE, Niall JF, Ryan GB. Segmental necrotizing glomerulonephritis with antineutrophil antibody: possible arbovirus aetiology? [Short report] BMJ. 1982;285:606. doi: 10.1136/bmj.285.6342.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Woude FJ, Rasmussen N, Lobatto S, Wiik A, Permin H, van ES LA, van der Giessen M, van der Hem GK, The TH. Autoantibodies against neutrophils and monocytes: tool for diagnosis and marker of disease activity in Wegener's granulomatosis. Lancet. 1985;23:425–429. doi: 10.1016/S0140-6736(85)91147-X. [DOI] [PubMed] [Google Scholar]
- Bosch X, Guilabert A, Font J. Antineutrophil cytoplasmic antibodies. Lancet. 2006;368:404–418. doi: 10.1016/S0140-6736(06)69114-9. [DOI] [PubMed] [Google Scholar]
- Falk RJ, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N Engl J Med. 1988;318:1651–1657. doi: 10.1056/NEJM198806233182504. [DOI] [PubMed] [Google Scholar]
- Niles JL, McCluskey RT, Ahmad MF, Arnaout MA. Wegener's granulomatosis autoantigen is a novel neutrophil serine proteinase. Blood. 1989;74:1888–1893. [PubMed] [Google Scholar]
- Xiao H, Heeringa P, Hu P, Liu Z, Zhao M, Aratani Y, Maeda N, Falk RJ, Jennette JC. Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest. 2002;110:955–963. doi: 10.1172/JCI200215918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlieben DJ, Korbet SM, Kimura RE, Schwartz MM, Lewis EJ. Pulmonary–renal syndrome in a newborn with placental transmission of ANCAs. Am J Kidney Dis. 2005;45:758–761. doi: 10.1053/j.ajkd.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Jennette JC, Xiao H, Falk RJ. Pathogenesis of vascular inflammation by antineutrophil cytoplasmic antibodies. J Am Soc Nephrol. 2006;17:1235–1242. doi: 10.1681/ASN.2005101048. [DOI] [PubMed] [Google Scholar]
- Pfister H, Ollert M, Frolich LF, Quintanilla-Martinez L, Colby TV, Specks U, Jenne DE. Antineutrophil cytoplasmic autoantibodies against the murine homolog of proteinase 3 (Wegener autoantigen) are pathogenic in vivo. Blood. 2004;104:1411–1418. doi: 10.1182/blood-2004-01-0267. [DOI] [PubMed] [Google Scholar]
- Little MA, Smyth CL, Yadav R, Ambrose L, Cook HT, Nourshargh S, Pusey CD. Antineutrophil cytoplasm antibodies directed against myeloperoxidase augment leukocyte microvascular interactions in vivo. Blood. 2005;106:2050–2058. doi: 10.1182/blood-2005-03-0921. [DOI] [PubMed] [Google Scholar]
- Xiao H, Heeringa P, Liu Z, Huugen D, Hu P, Maeda N, Falk PJ, Jennette JC. The role of neutrophils in the induction of glomerulonephritis by anti-myeloperoxidase antibodies. Am J Pathol. 2005;167:39–45. doi: 10.1016/S0002-9440(10)62951-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo P, Stone J. The antineutrophil cytoplasmic antibody-associated vasculitides. Am J Med. 2004;117:39–50. doi: 10.1016/j.amjmed.2004.02.030. [DOI] [PubMed] [Google Scholar]
- Booth A, Pusey C, Jayne D. Renal vasculitis – an update in 2004. Nephrol Dial Transplant. 2004;19:1964–1968. doi: 10.1093/ndt/gfh318. [DOI] [PubMed] [Google Scholar]
- Niles JL, Böttinger EP, Saurina GR, Kelly KJ, Pan G, Collins AB, McCluskey RT. The syndrome of lung hemorrhage and nephritis is usually an ANCA-associated condition. Arch Intern Med. 1996;156:440–445. doi: 10.1001/archinte.156.4.440. [DOI] [PubMed] [Google Scholar]
- Green R, Ruoss S, Kraft S, Dunkan SR, Berry GJ, Raffin TA. Pulmonary capillaritis and alveolar hemorrhage. Chest. 1996;110:1305–1316. doi: 10.1378/chest.110.5.1305. [DOI] [PubMed] [Google Scholar]
- Choi HK, Merkel PA, Walker AM, Niles JL. Drug associated anti-neutrophil cytoplasmic antibody positive vasculitis. Arthritis Rheum. 2000;43:405–413. doi: 10.1002/1529-0131(200002)43:2<405::AID-ANR22>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Schwarz MI, Fontenot AP. Drug-induced diffuse alveolar hemorrhage syndromes and vasculitis. Clin Chest Med. 2004;25:133–140. doi: 10.1016/S0272-5231(03)00139-4. [DOI] [PubMed] [Google Scholar]
- Bonaci-Nikolic B, Nikolic MM, Andrejevic S, Zoric S, Bukilica M. Antineutrophil cytoplasmic antibody (ANCA)-associated autoimmune diseases induced by antithyroid drugs: comparison with idiopathic ANCA vasculitides. Arthritis Res Ther. 2005;7:R1072–R1081. doi: 10.1186/ar1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayne DR, Marshall PD, Jones SJ, Lockwood CM. Autoantibodies to GBM and neutrophil cytoplasm in rapidly progressive glomerulonephritis. Kidney Int. 1990;37:965–970. doi: 10.1038/ki.1990.72. [DOI] [PubMed] [Google Scholar]
- Bosch X, Mirapeix E, Font J, Lopez-Soto A, Rodriguez R, Vivancos J, Revert L, Ingelmo M, Urbano-Marquez A. Prognostic implication of anti-neutrophil cytoplasmic autoantibodies with myeloperoxidase specificity in antiglomerular basement disease. Clin Nephrol. 1991;36:107–113. [PubMed] [Google Scholar]
- Serratrice J, Chiche L, Dussol B, Granel B, Daniel L, Jego-Desplat S, Disdier P, Swiader L, Berland Y, Weiller PJ. Sequential development of perinuclear ANCA-associated vasculitis and anti-glomerular basement membrane glomerulonephritis. Am J Kidney Dis. 2004;43:e26–e30. doi: 10.1053/j.ajkd.2003.11.019. [DOI] [PubMed] [Google Scholar]
- Hughson M, Zhi H, Henegar J, McMurray R. Alveolar hemorrhage and renal microangiopathy in systemic lupus erythematosus. Arch Pathol Lab Med. 2001;125:475–483. doi: 10.5858/2001-125-0475-AHARMI. [DOI] [PubMed] [Google Scholar]
- Keane M, Lynch J. Pleuropulmonary manifestations of systemic lupus erythematosus. Thorax. 2000;55:159–166. doi: 10.1136/thorax.55.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar J, Ehrenfeld M, Rozenman J, Perelman M, Sidi Y, Gur H. Pulmonary renal syndrome in systemic sclerosis. Semin Arthritis Rheum. 2001;30:403–410. doi: 10.1053/sarh.2001.21904. [DOI] [PubMed] [Google Scholar]
- Wutzl A, Foley R, O'Driscoll B, Reeve RS, Chisholm R, Herrick AL. Microscopic polyangiitis presenting as pulmonary renal syndrome in a patient with long-standing diffuse cutaneous systemic sclerosis and antibodies to myeloperoxidase. Arthritis Care Res. 2001;45:533–536. doi: 10.1002/1529-0131(200112)45:6<533::AID-ART379>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Levine JS, Branch DW, Rauch J. The antiphospholipid syndrome. N Engl J Med. 2002;346:752–763. doi: 10.1056/NEJMra002974. [DOI] [PubMed] [Google Scholar]
- Hanly JG. Antiphospholipid syndrome: an overview. CMAJ. 2003;168:1675–1682. [PMC free article] [PubMed] [Google Scholar]
- Gezer S. Antiphospholipid syndrome. Dis Mon. 2003;49:696–741. doi: 10.1016/S0011-5029(03)00184-6. [DOI] [PubMed] [Google Scholar]
- Deane KD, West SG. Antiphospholipid antibodies as a cause of pulmonary capillaritis and diffuse alveolar hemorrhage: a case series and literature review. Semin Arthritis Rheum. 2005;35:154–165. doi: 10.1016/j.semarthrit.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Panoskaltsis N, Derman MP, Perillo I, Brennan JK. Thrombotic thrombocytopenic purpura in Pulmonary–renal syndromes. Am J Hematol. 2000;65:50–55. doi: 10.1002/1096-8652(200009)65:1<50::AID-AJH9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- George JN. Thrombotic thrombocytopenic purpura. N Engl J Med. 2006;354:1927–1935. doi: 10.1056/NEJMcp053024. [DOI] [PubMed] [Google Scholar]
- Levy GG, Nichols WC, Lian EC, Foroud T, McClintick JN, McGee B, Yang AY, Siemieniak DR, Stark KR, Gruppo R, et al. Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature. 2001;413:488–494. doi: 10.1038/35097008. [DOI] [PubMed] [Google Scholar]
- Soejima K, Nakagaki T. Interplay between ADAMTS 13 and von Willebrand factor in inherited and acquired thrombotic microangiopathies. Semin Hematol. 2005;42:56–62. doi: 10.1053/j.seminhematol.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Tsai HM, Rice L, Sarode R, Chow TW, Moake JL. Antibody inhibitors to von Willebrand factor metalloproteinase and increased binding of von Willebrand factor to platelets in ticlopidine-associated thrombotic thrombocytopenic purpura. Ann Intern Med. 2000;132:794–799. doi: 10.7326/0003-4819-132-10-200005160-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana S, Kremer Hovinga JA, Lammle B, Mansouri Taleghani B. Treatment of thrombotic thrombocytopenic purpura. Vox Sang. 2006;90:245–254. doi: 10.1111/j.1423-0410.2006.00747.x. [DOI] [PubMed] [Google Scholar]
- Pourrat O, Bureau JM, Hira M, Martin-Barbaz F, Descamps JM, Robert R. Pronostic des maladies systémiques admises en réanimation: étude rétrospective de 39 séjours. Rev Méd Intern. 2000;21:147–151. doi: 10.1016/S0248-8663(00)88243-0. [DOI] [PubMed] [Google Scholar]
- Cruz BA, Ramanoelina J, Mahr A, Cohen P, Mouthon L, Cohen Y, Hoang P, Guillevin L. Prognosis and outcome of 26 patients with systemic necrotizing vasculitis admitted to the intensive care unit. Rheumatology. 2003;42:1183–1188. doi: 10.1093/rheumatology/keg322. [DOI] [PubMed] [Google Scholar]
- Gallagher H, Kwan JT, Jayne DR. Pulmonary–renal syndrome: a 4-year, single-center experience. Am J Kidney Dis. 2002;39:42–47. doi: 10.1053/ajkd.2002.29876. [DOI] [PubMed] [Google Scholar]
- Bucciarelli S, Espinosa G, Asherson RA, Cervera R, Claver G, Gomez-Puerta JA, Ramos-Casals M, Ingelmo M, Catastrophic Antiphospholipid Syndrome Registry Project Group The acute respiratoty distress syndrome in catastrophic antiphospholipid syndrome: analysis of a series of 47 patients. Ann Rheum Dis. 2006;65:81–86. doi: 10.1136/ard.2005.037671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manganelli P, Fietta P, Carotti M, Pesci A, Salaffi F. Respiratory system involvement in systemic vasculitides. Clin Exp Rheumatol. 2006;24:S48–S59. [PubMed] [Google Scholar]
- Bouachour G, Roy PM, Tirot P, Quérin O, Gouello JR, Alquier P. Prognosis of systemic diseases diagnosed in intensive care units [in French] Presse Méd. 1996;25:837–841. [PubMed] [Google Scholar]
- Cervera R, Asherson RA, Acevedo ML, Gomez-Puerta JA, Espinosa G, de la Red G, Gil V, Ramos-Casals M, Garcia-Carrasco M, Ingelmo M, Font J. Antiphospholipid syndrome associated with infections: clinical and microbiological characteristics of 100 patients. Ann Rheum Dis. 2004;63:1312–1317. doi: 10.1136/ard.2003.014175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowley NB, Steiner RE, Chin WS. The chest X-ray in antiglomerular basement membrane antibody disease (Goodpasture's syndrome) Clin Radiol. 1979;30:419–429. doi: 10.1016/S0009-9260(79)80223-8. [DOI] [PubMed] [Google Scholar]
- Papiris SA, Manoussakis MN, Drosos A, Kontogiannis D, Constantopoulos SH, Moutsopoulos HM. Imaging of thoracic Wegener's granulomatosis: the computed tomographic appearance. Am J Med. 1992;93:529–536. doi: 10.1016/0002-9343(92)90581-U. [DOI] [PubMed] [Google Scholar]
- Ando Y, Okada F, Matsumoto S, Mori H. Thoracic manifestation of myeloperoxidase-antineutrophil cytoplasmic antibody (MPO-ANCA) related disease. J Comput Assist Tomogr. 2004;28:710–716. doi: 10.1097/01.rct.0000135280.79012.c7. [DOI] [PubMed] [Google Scholar]
- Jennings C, King T, Tuder R, Cherniack RM, Schwarz MI. Diffuse alveolar hemorrhage with underlying isolated, pauciimmune pulmonary capillaritis. Am J Respir Crit Care Med. 1997;155:1101–1109. doi: 10.1164/ajrccm.155.3.9116994. [DOI] [PubMed] [Google Scholar]
- Jennette JC, Wilkman AS, Falk RJ. Diagnostic predictive value of ANCA serology [editorial] Kidney Int. 1998;53:796–798. doi: 10.1038/ki.1998.36. [DOI] [PubMed] [Google Scholar]
- Sinico RA, Radice A, Corace C, Sabadini E, Bollini B. Antiglomerular basement membrane antibodies in the diagnosis of Goodpasture syndrome: a comparison of different assays. Nephrol Dial Transplant. 2006;21:397–401. doi: 10.1093/ndt/gfi230. [DOI] [PubMed] [Google Scholar]
- Salama A, Dougan T, Levy J, Cook HT, Morgan SH, Naudeer S, Maidment G, George AJ, Evans D, Lightstone L, Pussey CD. Goodpasture's disease in the absence of circulating anti-glomerular membrane antibodies as detected by standard techniques. Am J Kidney Dis. 2002;39:1162–1167. doi: 10.1053/ajkd.2002.33385. [DOI] [PubMed] [Google Scholar]
- Hellmann M, Gerhardt T, Rabe C, Haas S, Sauerbruch T, Woitas RP. Goodpasture's syndrome with massive pulmonary haemorrhage in the absence of circulating anti-GBM antibodies? Nephrol Dial Transplant. 2006;21:526–529. doi: 10.1093/ndt/gfi279. [DOI] [PubMed] [Google Scholar]
- Travis WD, Hoffman GS, Leavitt RY, Pass HI, Fauci AS. Surgical pathology of the lung in Wegener's granulomatosis. Review of 87 open lung biopsies from 67 patients. Am J Surg Pathol. 1991;15:315–333. doi: 10.1097/00000478-199104000-00001. [DOI] [PubMed] [Google Scholar]
- Frankel SK, Sullivan EJ, Brown KK. Vasculitis: Wegener granulomatosis, Churg–Strauss syndrome, microscopic polyangiitis, polyarteritis nodosa, and Takayasu arteritis. Crit Care Clin. 2002;18:855–879. doi: 10.1016/S0749-0704(02)00031-3. [DOI] [PubMed] [Google Scholar]
- Sano K, Sakaguchi N, Ito M, Koyama M, Kobayashi M, Hotchi M. Histological diversity of vasculitic lesions in MPO-ANCA positive autopsy cases. Pathol Intern. 2001;51:460–466. doi: 10.1046/j.1440-1827.2001.01225.x. [DOI] [PubMed] [Google Scholar]
- Katzenstein AL. Diagnostic features and differential diagnosis of Churg–Strauss syndrome in the lung. A review. Am J Clin Pathol. 2000;114:767–772. doi: 10.1309/F3FW-J8EB-X913-G1RJ. [DOI] [PubMed] [Google Scholar]
- Savige J, Dimech W, Fritzler M, Goeken J, Hagen EC, Jennette JC, McEvoy R, Pucey C, Pollack W, Trevisin M, et al. Addentum to the International Consencus Statement on testing and reporting antineutrophil cytoplasmic antibodies. Quality control guidelines, comments, and recommendations for testing in other autoimmune diseases. Am J Clin Pathol. 2003;120:312–318. doi: 10.1309/WAEP-ADW0-K4LP-UHFN. [DOI] [PubMed] [Google Scholar]
- Sable-Fourtassou R, Cohen P, Mahr A, Pagnoux C, Mouthon L, Jayne D, Blockmans D, Cordier JF, Delaval P, Puechal X, et al. Antineutrophil cytoplasmic antibodies and the Churg–Strauss syndrome. Ann Intern Med. 2005;143:632–638. doi: 10.7326/0003-4819-143-9-200511010-00006. [DOI] [PubMed] [Google Scholar]
- Falk RJ, Jennette JC. Thoughts about the classification of small vessel vasculitis. J Nephrol. 2004;17(Suppl 8):S3–S9. [PubMed] [Google Scholar]
- Vassilopoulos D, Hoffman G. Clinical utility of testing for anti-neutrophil cytoplasmic antibodies. Clin Diagn Lab Immunol. 1999;6:645–651. doi: 10.1128/cdli.6.5.645-651.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennette JC, Falk RJ. The pathology of vasculitis involving the kidney. Am J Kidney Dis. 1994;24:130–141. doi: 10.1016/s0272-6386(12)80171-5. [DOI] [PubMed] [Google Scholar]
- Agard C, Mouthon L, Mahr A, Guillevin L. Microscopic polyangiitis and polyarteritis nodosa: how and when do they start? Arthritis Rheum. 2003;49:709–715. doi: 10.1002/art.11387. [DOI] [PubMed] [Google Scholar]
- Niles JA. A renal biopsy is essential for the management of ANCA-positive patients with glomerulonephritis, the contra view. Sarcoidosis Vasc Diffuse Lung Dis. 1996;13:232–234. [PubMed] [Google Scholar]
- Tesar V, Rihova Z, Jancova E, Rysova R, Merta M. European randomized trials: current treatment strategies in ANCA-positive renal vasculitis-lessons from European randomized trials. Nephrol Dial Transplant. 2003;18:v2–v4. doi: 10.1093/ndt/gfg1032. [DOI] [PubMed] [Google Scholar]
- Hogan S, Falk RJ, Chin H, Cai J, Jennette CE, Jennette JC, Nachman PH. Predictors of relapse and treatment resistance in antineutrophil cytoplasmic antibody-associated small vessel vasculitis. Ann Intern Med. 2005;143:621–631. doi: 10.7326/0003-4819-143-9-200511010-00005. [DOI] [PubMed] [Google Scholar]
- Rihova Z, Jancova E, Merta M, Tesar V. Daily oral versus pulse intravenous cyclophosphamide in the therapy of ANCA-associated vasculitis – preliminary single center experience. Prague Med Rep. 2004;105:64–68. [PubMed] [Google Scholar]
- Gaskin G, Pusey C. Plasmapheresis in antineutrophil cytoplasmic antibody-associated systemic vasculitis. Ther Apher. 2001;5:176–181. doi: 10.1046/j.1526-0968.2001.00300.x. [DOI] [PubMed] [Google Scholar]
- Klemmer P, Chalermskulrat W, Reif M, Hogan SL, Henke DC, Falk RJ. Plasmapheresis therapy for diffuse alveolar hemorrhage in patients with small vessel vasculitis. Am J Kidney Dis. 2003;42:1149–1153. doi: 10.1053/j.ajkd.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Aziz T, Cochran J, Highland K. Use of extracorporeal membrane oxygenation in a patient with diffuse alveolar hemorrhage. Chest. 2004;126:305–309. doi: 10.1378/chest.126.1.305. [DOI] [PubMed] [Google Scholar]
- Henke DC, Falk RJ, Gabriel DA. Successful treatment of diffuse alveolar hemorrhage with activated factor VII. Ann Intern Med. 2004;140:493–494. doi: 10.7326/0003-4819-140-6-200403160-00033. [DOI] [PubMed] [Google Scholar]
- Jayne D, Rasmussen N, Andrassy K, Bacon P, Tervaert JW, Dadoniene J, Ekstrand A, Gaskin G, Gregorini G, de Groot K, et al. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med. 2003;349:36–44. doi: 10.1056/NEJMoa020286. [DOI] [PubMed] [Google Scholar]
- White ES, Lynch JP. Pharmacological therapy for Wegener's granulomatosis. Drugs. 2006;66:1209–1228. doi: 10.2165/00003495-200666090-00004. [DOI] [PubMed] [Google Scholar]
- Booth A, Harper L, Hammad T, Bacon P, Griffith M, Levy J, Savage C, Pusey C, Jayne D. Prospective study of TNF alpha blockade with infliximab in antineutrophil cytoplasmic antibody-assocaited systemic vasculitis. J Am Soc Nephrol. 2004;15:717–721. doi: 10.1097/01.ASN.0000114554.67106.28. [DOI] [PubMed] [Google Scholar]
- Lamprecht P, Voswinkel J, Lilienthal T, Nolle B, Heller M, Gross WL, Gause A. Effectiveness of TNF-alpha blockade with infliximab in refractory Wegener's granulomatosis. Rheumatology (Oxford) 2002;41:1303–1307. doi: 10.1093/rheumatology/41.11.1303. [DOI] [PubMed] [Google Scholar]
- Wegener's Granulomatosis Etanercept Trial (WGET) Research Group Etanercept plus standard therapy for Wegener's granulomatosis. N Engl J Med. 2005;352:351–361. doi: 10.1056/NEJMoa041884. [DOI] [PubMed] [Google Scholar]
- Feldman M, Pusey CD. Is there a role for TNF-alpha in anti-neutrophil cytoplasmic antibody-associated vasculitis? Lessons fron other chronic inflammatory diseases. J Am Soc Nephrol. 2006;17:1243–1252. doi: 10.1681/ASN.2005121359. [DOI] [PubMed] [Google Scholar]
- Stasi R, Stipa E, del Poeta GD, Amadori S, Newland AC, Provan D. Long term observation of patients with anti-neutrophil cytoplasmic antibody-associated vasculitis treated with rituximab. Rheumatology (Oxford) 2006;45:1432–1436. doi: 10.1093/rheumatology/kel098. [DOI] [PubMed] [Google Scholar]
- Antoniu SA. Rituximab for refractory Wegener's granulomatosis. Exp Opin Invest Drugs. 2006;15:1115–1117. doi: 10.1517/13543784.15.9.1115. [DOI] [PubMed] [Google Scholar]
- Nowack R, Gobel U, Klooker P, Hergesell O, Andrassy K, van der Woude FJ. Mycophenolate mofetil for maintenance therapy of Wegener's granulomatosis and microscopic polyangiitis: a pilot study in 11 patients with renal involvement. J Am Soc Nephrol. 1999;10:1965–1971. doi: 10.1681/ASN.V1091965. [DOI] [PubMed] [Google Scholar]
- Langford CA, Talar-Williams C, Sneller MC. Mycophenolate mofetil for remission maintenance in the treatment of Wegener's granulomatosis. Arthritis Rheum. 2004;51:278–283. doi: 10.1002/art.20240. [DOI] [PubMed] [Google Scholar]
- Koukoulaki M, Jayne DR. Mycophenolate mofetil in anti-neutrophil cytoplasm antibodies-associated systemic vasculitis. Nephron Clin Pract. 2006;102:c100–c107. doi: 10.1159/000089667. [DOI] [PubMed] [Google Scholar]
- Metzler C, Fink C, Lamprecht P, Gross WL, Reinhold-Keller E. Maintenance of remission with leflunomide in Wegener's granulomatosis. Rheumatology (Oxford) 2004;43:315–320. doi: 10.1093/rheumatology/keh009. [DOI] [PubMed] [Google Scholar]
- Schmitt WH, Hagen EC, Neumann I, Nowack R, Flores-Suarez LF, van der Woude FJ, European Vasculitis Study Group Treatment of refractory Wegener's granulomatosis with antithymocyte globulin (ATG): an open study in 15 patients. Kidney Int. 2004;65:1440–1448. doi: 10.1111/j.1523-1755.2004.00534.x. [DOI] [PubMed] [Google Scholar]
- Levy JB, Turner AN, Rees AJ, Pusey CD. Long term outcome of antiglomerular basement membrane antibody disease treated with plasma exchange and immunosuppression. Ann Intern Med. 2001;134:1033–1042. doi: 10.7326/0003-4819-134-11-200106050-00009. [DOI] [PubMed] [Google Scholar]
- Austin HA, 3rd, Klippel JH, Balow JE, le Riche NG, Steinberg AD, Plotz PH, Decker JL. Therapy of lupus nephritis. Controlled trial of prednisone and cytotoxic drugs. N Engl J Med. 1986;314:614–619. doi: 10.1056/NEJM198603063141004. [DOI] [PubMed] [Google Scholar]
- Ginzler E, Dooley MA, Aranow C, Kim MY, Buyon J, Merrill JT, Petri M, Gilkeson GS, Wallace DJ, Weisman MH, et al. Mycophenolate mofetil or intravenous cyclophosphamide for lupus nephritis. N Engl J Med. 2005;353:2219–2228. doi: 10.1056/NEJMoa043731. [DOI] [PubMed] [Google Scholar]
- Smith KG, Jones RB, Burns SM, Jayne DR. Long term comparison of rituximab treatment for refractory systemic lupus erythematosus and vasculitis: remission, relapse, and re-treatment. Arthritis Rheum. 2006;54:2970–2982. doi: 10.1002/art.22046. [DOI] [PubMed] [Google Scholar]
- Candan S, Pirat A, Varol A, Torgay A, Zeyneloglou P, Arslan G. Respiratory problems in renal transplant recipients admitted to intensive care during long-term follow-up. Transplant Proc. 2006;38:1354–1356. doi: 10.1016/j.transproceed.2006.02.083. [DOI] [PubMed] [Google Scholar]