Abstract
Introduction
There is limited information on whether the incidence of acute kidney injury (AKI) in critically ill patients has changed over time and there is controversy on whether its outcome has improved.
Methods
We interrogated the Australian New Zealand Intensive Care Society Adult Patient Database to obtain data on all adult admissions to 20 Australian intensive care units (ICUs) for ≥ 24 hours from 1 January 1996 to 31 December 2005. Trends in incidence and mortality for ICU admissions associated with early AKI were assessed.
Results
There were 91,254 patient admissions to the 20 study ICUs, with 4,754 cases of AKI, for an estimated crude cumulative incidence of 5.2% (95% confidence interval, 5.1 to 5.4). The incidence of AKI increased during the study period, with an estimated annual increment of 2.8% (95% confidence interval, 1.0 to 5.6, P = 0.04). The crude hospital mortality was significantly higher for patients with AKI than those without (42.7% versus 13.4%; odds ratio, 4.8; 95% confidence interval, 4.5 to 5.1; P < 0.0001). There was also a decrease in AKI crude mortality (annual percentage change, -3.4%; 95% confidence interval, -4.7 to -2.12; P < 0.001), however, which was not seen in patients without AKI. After covariate adjustment, AKI remained associated with a higher mortality (odds ratio, 1.23; 95% confidence interval, 1.14 to 1.32; P < 0.001) and there was a declining trend in the odds ratio for hospital mortality.
Conclusion
Over the past decade, in a large cohort of critically ill patients admitted to 20 Australian ICUs, there has been a significant rise in the incidence of early AKI while the mortality associated with AKI has declined.
Introduction
Acute kidney injury (AKI) is a common clinical problem in critically ill patients and typically portends an increase in morbidity and mortality [1]. Multiple epidemiologic investigations have provided a broad range of estimates of the incidence of AKI in critically ill patients [2-9]. Likewise, numerous studies have shown that AKI in the intensive care unit (ICU) is associated with high short-term and long-term case fatality rates, with dialysis dependence, with reduced quality of life and with excess utilization of health resources [2-6,9-20].
Regrettably, many of these studies suffer from limited generalizability as a result of disparities in the study methodology, the study population and the definitions of AKI. Moreover, no study has purposely evaluated or been capable of assessing trends in the incidence and outcome of AKI in critically ill patients over time, once changes in illness severity have been taken into account [21]. Accordingly, there is limited information on whether the incidence of AKI in the ICU has changed significantly over time and there is considerable controversy on whether its outcome has improved [22,23]. On the other hand, the Australian New Zealand Intensive Care Society (ANZICS) Adult Patient Database (APD) is a high-quality clinical database containing data from > 600,000 individual adult admissions to 135 ICUs from 1987 to the present that now captures approximately > 80% of all admissions to ICUs in Australia and New Zealand [24]. Twenty of these units have contributed data for a decade, making it possible to assess changes in incidence and outcome over a significant timespan.
We therefore interrogated the ANZICS APD to obtain information on the incidence and outcome of AKI in a cohort of critically ill patients from 20 Australian hospitals over a decade. We sought to describe the 10-year trend in the incidence of AKI at the time of or within 24 hours of admission to ICU, and the 10-year trend in the crude and adjusted hospital mortality rates associated with AKI.
Methods
We conducted an observational surveillance cohort study to determine the incidence of and outcomes associated with AKI. We interrogated the ANZICS APD for all adult (age ≥ 18 years) ICU admissions for ≥ 24 hours with a diagnosis of AKI during the period from 1 January 1996 to 31 December 2005. In the event of multiple admissions for a particular patient, only the initial ICU admission was considered. Those patients readmitted to the ICU within 72 hours after their initial discharge were considered part of the index admission. We selected only those Australian ICUs that had continuously contributed data to the APD during this 10-year period. This cohort included 20 ICUs (nine tertiary referral centres, six metropolitan hospitals, four peripheral regional/rural hospitals and one private hospital).
Identification of cases
We used two strategies to identify all ICU admissions associated with AKI. First, the APD has a prespecified data element for the presence of AKI [25]. For this data element, AKI was defined as an acute serum creatinine level ≥ 133 μmol/l or a 24-hour urine output < 410 ml and not having received prior renal replacement therapy. In addition, the APD verifies and validates any patient designated with AKI and a serum creatinine level < 200 μmol/l. Second, we evaluated the Acute Physiology and Chronic Health Evaluation (APACHE) III diagnostic codes for AKI in order to identify any additional patients. To further corroborate admissions with AKI, all identified patients were then referenced with APACHE II and APACHE III diagnostic codes for chronic renal replacement therapy and/or kidney transplant.
Data collection
Standard demographic, clinical and physiologic data were retrieved. Demographic information included age, sex, dates of admission to the hospital and the ICU, and source of admission. Clinical data encompassed the primary diagnosis, surgical status, the presence of selected comorbid illnesses and a need for mechanical ventilation. Data on kidney function extracted included the peak serum creatinine and urea, and the total 24-hour urine output within the first 24 hours of ICU admission [25]. Severity of illness during the first 24 hours of ICU admission was assessed using the APACHE II, APACHE III and Simplified Acute Physiology Score II scoring systems [26,27].
Pre-existing comorbid illnesses were defined using the chronic health evaluation for the APACHE II, APACHE III and Simplified Acute Physiology Score II scoring systems, as outlined in the ANZICS APD data dictionary [25].
Several primary admission diagnostic categories were created [25]. Sepsis/septic shock encompassed admissions for primarily sepsis-related diagnoses, and included sepsis associated with pneumonia, gastrointestinal disease, urinary tract infections, central nervous system infections, soft tissue infections, and the ANZICS APD-specific diagnostic code additions for sepsis with shock of undetermined source. A primary cardiac diagnosis encompassed nonsurgical admissions with cardiogenic shock, cardiac arrest, congestive heart failure and acute myocardial infarction. A primary hepatic diagnosis included admission with hepatic failure or liver transplant. A diagnosis of gastrointestinal haemorrhage included bleeding due to peptic ulcers, diverticulosis and varices. A metabolic/poisoning diagnosis incorporated nonoperative causes of metabolic coma, diabetic ketoacidosis, drug overdoses or other endocrinopathies. A primary respiratory diagnosis encompassed primary respiratory arrests, aspiration syndrome, noncardiogenic pulmonary oedema, exacerbations of chronic obstructive pulmonary disease or asthma, and pulmonary embolism. A primary neurologic diagnosis incorporated stroke, intracerebral haemorrhage, subarachnoid haemorrhage, epidural haematoma or other neurologic cause for coma.
Clinical outcomes
Outcomes extracted from the APD included an incidence of early AKI at or within 24 hours of ICU admission (as a proportion of all ICU admissions) and the hospital mortality rate. If patients were readmitted to the ICU prior to hospital discharge, subsequent ICU admissions were not included in the analysis of mortality. The ICU and hospital lengths of stay and the hospital discharge location were also evaluated.
Statistical analysis
Analysis was performed using Stata version 8.2 (Stata Corp, College Station, TX, USA). In the event of missing data values, data were not replaced or estimated. Normally or near-normally distributed variables are reported as means with standard deviations and were compared by Student's t test. Non-normally distributed continuous data are reported as medians with interquartile ranges and were compared by the Mann–Whitney U test. Categorical data are reported as proportions and were compared using Fisher's exact test.
Incidence estimates for early AKI on admission to the ICU were calculated as a proportion of all admissions to the ICU with 95% confidence intervals (CIs). Incidence estimates are presented as cumulative over 10 years, as time-stratified by 2-year intervals and as stratified by demographics, baseline characteristics and primary diagnosis. To determine changes over time, parametric and nonparametric tests for trend were performed as appropriate.
The estimated annual percentage changes in the incidence of AKI were determined by fitting a straight-line regression of the natural logarithm of the rates, with the calendar year used as an independent variable. The estimated annual percentage change was equal to [100 × (exp(b) - 1)], where b represents the slope of the regression. If the estimated annual percentage change is statistically greater than zero, then the incidence rate has an increasing trend over the study period [28].
Multivariable logistic regression was used to calculate the adjusted odds ratios (ORs) with 95% CIs for the association of AKI at ICU admission with hospital mortality. The variables age, sex, comorbidity, surgical/medical admission, primary diagnosis, severity of illness (APACHE II score), mechanical ventilation and hospital site were included. Model fit was assessed by the goodness-of-fit test and discrimination was assessed by the area under the receiver operator characteristic curve. P < 0.05 was considered statistically significant for all comparisons.
Results
During the 10-year study period, 91,254 patients were admitted to the 20 study ICUs. Overall, these patients had a median (interquartile range) age of 64.1 (49 to 74.1) years, 60.6% were male, 21.5% had evidence of comorbid disease, 50.4% were medical admissions and the initial mean (± standard deviation) APACHE II score was 16.4 (± 7.8).
Incidence
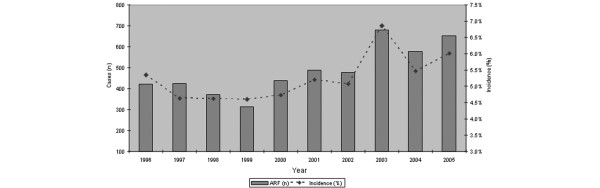
In total, 4,754 patients had a diagnosis of AKI at the time of or during the first 24 hours after ICU admission. This translated into an estimated crude cumulative incidence of 5.2% (95% CI, 5.1 to 5.4). The range in incidence was 4.6 to 6.9%. There was a significant increasing trend in incidence over the study period, with an estimated annual percentage increment of 2.8% (95% CI, 1.0 to 5.6; P = 0.04) (Figure 1). The incidence was significantly greater for admissions in 2001–2005 compared with admissions during 1996–2000 (5.6% versus 4.8%; OR, 1.16; 95% CI, 1.10 to 1.23; P < 0.0001); this difference persisted after taking into account the apparent high 6.9% incidence in 2003 (5.2% versus 4.8%; OR, 1.10; 95% CI, 1.03 to 1.16; P = 003).
Figure 1.
Summary of cases of acute kidney injury and incidence from the Australia New Zealand Intensive Care Society Adult Patient Database, 1996–2005. ARF, acute renal failure.
Demographics
Older patient age was associated with a higher incidence of AKI (Table 1). There were no significant changes in incidence of AKI stratified by age. There was, however, a nonsignificant increase in incidence for patients aged ≥ 75 years (annual percentage change, 2.0%; 95% CI, -0.5 to 4.6; P = 0.1). There was no significant difference in the cumulative incidence stratified by sex (5.1% for males versus 5.4% for females; OR, 0.96; 95% CI, 0.90 to 1.01; P = 0.12) or evidence for a change over the study period (Table 1).
Table 1.
Incidence rates (95% confidence intervals) of acute kidney injury stratified by two-year intervals, age, sex and comorbid illness from the Australia New Zealand Intensive Care Society Adult Patient Database 1996–2005
| Covariate | Cumulative | Incidence rates per two-year period | |||||
| Cases (n = 4,754) |
Incidence (5.2 (5.1–5.4)) |
1996/1997 (n = 849) |
1998/1999 (n = 684) |
2000/2001 (n = 926) |
2002/2003 (n = 1,158) |
2004/2005 (n = 1,137) |
|
| Age | |||||||
| 18–49 years | 838 | 3.6 (3.3–3.8) | 3.4 (2.8–3.9) | 3.3 (2.8–3.9) | 3.7 (3.2–4.3) | 3.9 (3.4–4.4) | 3.5 (3.0–3.9) |
| 50–64 years | 1,026 | 4.5 (4.3–4.8) | 4.3 (3.7–4.9) | 4.3 (3.6–4.9) | 4.0 (3.5–4.6) | 5.1 (4.4–5.7) | 4.9 (4.3–5.4) |
| 65–74 years | 1,304 | 5.7 (5.4–6.0) | 5.7 (5.0–6.3) | 4.8 (4.2–5.5) | 5.4 (4.7–6.0) | 6.6 (5.9–7.3) | 5.9 (5.2–6.5) |
| ≥ 75 years | 1,545 | 7.5 (7.2–7.9) | 7.0 (6.1–7.9) | 6.8 (5.9–7.7) | 7.2 (6.4–8.0) | 8.8 (8.0–9.6) | 7.4 (6.7–8.1) |
| Sex | |||||||
| Male | 2,831 | 5.1 (4.9–5.3) | 4.9 (4.5–5.4) | 4.5 (4.0–4.9) | 4.8 (4.4–5.2) | 5.7 (5.3–6.1) | 5.5 (5.1–5.9) |
| Female | 1,923 | 5.4 (5.1–5.6) | 5.0 (4.5–5.5) | 4.9 (4.3–5.4) | 5.2 (4.7–5.8) | 6.4 (5.8–6.9) | 5.1 (4.7–5.6) |
| Comorbid illness | |||||||
| None | 3,335 | 4.7 (4.5–4.8) | 4.2 (3.9–4.6) | 4.1 (3.7–4.4) | 4.3 (4.0–4.7) | 5.5 (5.1–5.9) | 4.9 (4.5–5.1) |
| 1 comorbidity | 1,067 | 6.8 (6.4–7.2) | 6.6 (5.7–7.4) | 6.1(5.1–7.0) | 6.8 (5.9–7.6) | 7.6 (6.7–8.5) | 6.9 (6.1–7.7) |
| 2 comorbidities | 312 | 8.7 (7.7–9.6) | 12.2 (9.6–14.8) | 7.6 (5.3–9.9) | 8.6 (6.7–10.6) | 7.5 (5.8–9.3) | 7.9 (6.1–9.8) |
| ≥ 3 comorbidities | 40 | 12.4 (8.8–16.0) | 12.5 (4.1–20.8) | 16.4 (6.8–26.0) | 15.0 (7.0–23.0) | 9.2 (2.6–15.9) | 7.3 (0–15.6) |
| Any comorbidity | 1,419 | 7.2 (6.9–7.6) | 7.6 (6.7–8.4) | 6.5 (5.6–7.4) | 7.3 (6.5–8.1) | 7.6 (6.8–8.4) | 7.1 (6.3–7.8) |
| Comorbid conditions | |||||||
| Cardiovascular | 553 | 6.3 (5.8–6.8) | 7.7 (6.4–9.0) | 5.0 (3.9–6.0) | 6.3 (5.2–7.3) | 5.7 (4.7–6.8) | 6.7 (5.5–7.8) |
| Respiratory | 404 | 6.9 (6.3–7.6) | 6.2 (4.9–7.6) | 5.4 (3.9–6.9) | 8.0 (6.4–9.6) | 8.3 (6.8–9.9) | 6.2 (4.9–7.5) |
| Liver | 230 | 12.1 (10.6–13.6) | 12.1 (8.8–15.6) | 12.8 (8.5–17.1) | 12.1 (8.7–15.5) | 13.2 (9.9–16.5) | 10.9 (8.3–13.6) |
| Metastatic cancer | 143 | 8.2 (6.9–9.5) | 8.4 (5.3–11.6) | 9.4 (5.8–13.1) | 7.7 (4.6–10.9) | 8.6 (5.9–11.3) | 7.3 (4.9–9.6) |
| Haematologic malignancy | 134 | 10.5 (8.8–12.2) | 15.6 (10.5–20.7) | 16.2 (10.3–22.1) | 9.9 (6.3–13.6) | 6.9 (3.9–9.9) | 8.7 (5.9–11.6) |
| Immunocompromised | 351 | 8.2 (7.4–9.0) | 8.9 (7.0–10.8) | 8.7 (6.6–10.8) | 8.3 (6.5–10.1) | 7.9 (6.2–9.6) | 7.1 (5.4–8.9) |
Patient characteristics
The incidence of AKI was considerably higher when stratified by both the presence of pre-existing comorbid illness and by specific comorbid illnesses (Table 1). There was a nonsignificant trend for an increase in the incidence of AKI for patients with no comorbid illness (annual percentage change, 2.9%; 95% CI, -0.4 to 6.2; P = 0.08). There were no significant changes, however, in incidence stratified by the number of comorbid diseases. For the specific comorbid diseases evaluated, all were associated with a significantly higher incidence AKI. In particular, comorbid liver disease (OR, 2.58; 95% CI, 2.24 to 2.98; P < 0.0001) and haematologic malignancy (OR, 2.18; 95% CI, 1.82 to 2.61; P < 0.0001) showed the highest risk. During the study period, only haematologic malignancy showed a significant change in incidence of AKI, characterized by a decreasing trend (annual percentage change, -65%; 95% CI, -86 to -12; P = 0.03).
Nonelective admissions compared with elective admissions were associated with a higher incidence of AKI (7.2% versus 1.7%; OR, 4.6; 95% CI, 4.20 to 5.04; P < 0.0001) (Table 2). Over the study period, there was a nonsignificant but increasing trend in the incidence of AKI for elective ICU admissions (annual percentage change, 6.4%; 95% CI, -1.2 to 14.6; P = 0.09). There was no change for nonelective admissions, however.
Table 2.
Incidence rates (95% confidence intervals) of acute kidney injury stratified by two-year intervals, and admission characteristics from the Australia New Zealand Intensive Care Society Adult Patient Database 1996–2005
| Covariate | Cumulative | Incidence rates per two-year period | |||||
| Cases (n = 4,754) |
Incidence (5.2 (5.1–5.4)) |
1996/1997 (n = 849) |
1998/1999 (n = 684) |
2000/2001 (n = 926) |
2002/2003 (n = 1,158) |
2004/2005 (n = 1,137) |
|
| Admission category | |||||||
| Elective | 544 | 1.7 (1.5–1.8) | 1.4 (1.1–1.7) | 1.0 (0.7–1.3) | 1.5 (1.3–1.8) | 2.4 (2.1–2.8) | 1.8 (1.4–2.1) |
| Nonelective | 4,209 | 7.2 (7.0–7.4) | 7.0 (6.5–7.4) | 6.8 (6.3–7.3) | 7.2 (6.7–7.7) | 7.9 (7.5–8.4) | 7.0 (6.6–7.5) |
| Admission type | |||||||
| Surgical | 957 | 2.1 (2.0–2.2) | 2.5 (2.2–2.8) | 2.0 (1.7–2.3) | 1.7 (1.4–2.0) | 2.6 (2.3–2.9) | 1.9 (1.7–2.2) |
| Medical | 3,752 | 8.3 (8.0–8.5) | 7.5 (7.0–8.1) | 7.6 (7.0–8.2) | 8.5 (7.9–9.1) | 9.3 (8.7–9.9) | 8.1 (7.6–8.6) |
| Surgical subcategory | |||||||
| Cardiovascular | 376 | 1.6 (1.4–1.8) | 1.9 (1.5–2.2) | 1.8 (1.4–2.3) | 1.3 (1.0–1.6) | 1.7 (1.4–2.1) | 1.3 (1.0–1.6) |
| Trauma | 72 | 1.7 (1.3–2.1) | 2.9 (1.6–4.2) | 1.6 (0.7–2.4) | 1.4 (0.7–2.1) | 1.5 (0.7–2.2) | 1.7 (0.8–2.6) |
| Diagnostic category | |||||||
| Sepsis/septic shock | 1,109 | 19.5 (18.5–20.5) | 23.0 (20.0–26.0) | 19.1 (16.0–22.2) | 20.6 (18.1–23.1) | 22.9 (20.6–25.2) | 15.4 (13.8–17.0) |
| Cardiac | 658 | 11.2 (10.4–12.0) | 9.6 (8.0–11.3) | 12.5 (10.3–14.6) | 10.9 (9.0–12.7) | 11.8 (10.0–13.6) | 11.6 (9.9–13.3) |
| Hepatic | 362 | 8.0 (7.2–8.8) | 7.4 (5.6–9.2) | 7.5 (5.5–9.5) | 9.4 (7.3–11.4) | 7.9 (6.1–9.6) | 7.9 (6.5–9.4) |
| Gastrointestinal bleeding | 108 | 6.1 (5.0–7.3) | 4.7 (2.3–7.1) | 5.3 (2.2–8.4) | 5.7 (3.0–8.4) | 8.9 (6.1–11.7) | 5.5 (3.6–7.4) |
| Metabolic/poisoning | 191 | 3.9 (3.4–4.4) | 3.1 (1.9–4.2) | 3.3 (2.1–4.6) | 4.2 (2.9–5.5) | 3.9 (2.7–5.1) | 4.5 (3.4–5.7) |
| Respiratory | 383 | 3.4 (3.0–3.7) | 2.7 (2.1–3.4) | 3.0 (2.3–3.8) | 3.7 (3.0–4.5) | 4.2 (3.4–5.0) | 3.1 (2.4–3.8) |
| Neurologic | 101 | 2.1 (1.7–2.5) | 1.9 (1.0–2.8) | 2.0 (1.0–3.1) | 2.1 (1.2–3.0) | 2.0 (1.2–2.9) | 2.3 (1.4–3.1) |
Medical admissions compared with primarily surgical admissions were associated with a higher incidence of AKI (8.3% versus 2.1%; OR, 4.11; 95% CI, 3.82 to 4.42; P < 0.0001) (Table 2). There was a nonsignificant decreasing trend in the incidence of AKI associated with cardiovascular surgery (annual percentage change, -4%; 95% CI, -8.9 to 12; P = 0.1) and a significant decrease in the incidence of AKI associated with trauma (annual percentage change, -8%; 95% CI, -13 to -2.3; P = 0.009) over the study period.
Several admission diagnoses were associated with an increased incidence of AKI (Table 2). There were no significant changes in incidence by diagnostic category over the study period, with the exception of an increasing trend in incidence of AKI associated with metabolic/poisoning diagnoses (annual percentage change, 5.5%; 95% CI, 0.6–10.7; P = 0.03).
Details of kidney function and severity of illness scores for the first 24 hours after ICU admission for patients with AKI are presented in Table 3.
Table 3.
Summary of kidney function for patients admitted to the intensive care unit with acute kidney injury from the Australia New Zealand Intensive Care Society Adult Patient Database 1996–2005
| Kidney function parameter | Overall | 1996/1997 | 1998/1999 | 2000/2001 | 2002/2003 | 2004/2005 |
| Incidence of acute kidney injury (%) (95% confidence interval) | 5.2 (5.1–5.4) | 5.0 (4.6–5.3) | 4.6 (4.3–4.9) | 5.0 (4.7–5.3) | 6.0 (5.7–6.3) | 5.3 (5.0–5.6) |
| APACHE II score (mean (standard deviation)) | 27 (8.4) | 27.8 (8.5) | 27.1 (8.4) | 27.1 (8.4) | 26.6 (8.6) | 26.7 (8.0) |
| Simplified Acute Physiology Score II score (mean (standard deviation)) | 52.3 (18.6) | 56 (18.8) | 55.4 (18.7) | 50.9 (18.1) | 50.4 (18.9) | 50.9 (17.8) |
| Serum creatinine (μmol/l) (median (interquartile range)) | 245 (170–362) | 261 (200–390) | 255 (190–365) | 240 (148–356) | 230 (157–353) | 243 (170–360) |
| Serum creatinine ≥ 133 μmol/l (%) | 86.8 | 93.3 | 91 | 78.4 | 84 | 89.1 |
| Serum urea (mmol/l) (mean (standard deviation)) | 20.4 (12.5) | 21.7 (13.1) | 20.4 (11.6) | 20.4 (12.3) | 19.6 (12.5) | 20.1 (12.5) |
| Urine output (ml/hour) (median (interquartile range)) | 40 (11.6–95) | 23.6 (9.6–90) | 22.9 (9–82) | 50.6 (14.1–105) | 51 (15–105) | 36.8 (11.6–89) |
| Oliguria (< 410 ml/24 hour) (%) | 38.7 | 48.7 | 47.4 | 32.4 | 32.3 | 37.1 |
APACHE, Acute Physiology and Chronic Health Evaluation.
Mortality
The crude hospital mortality was significantly higher for patients with AKI than those without (42.7% versus 13.4%; OR, 4.8; 95% CI, 4.5 to 5.1; P < 0.0001) (Table 4 and Figure 2). There was, however, a significant decrease over time in the crude mortality rate associated with AKI (annual percentage change, -3.4%; 95% CI, -4.7 to -2.12; P < 0.001). There was no change for those without AKI over the study period. The presence of AKI remained associated with higher mortality after adjustment for age, sex, comorbidity, surgical/medical admission, primary diagnosis, severity of illness (APACHE II score), mechanical ventilation and hospital site (OR, 1.39; 95% CI, 1.3 to 1.5; P < 0.001). Over the study period, there was a trend for decreasing ORs for death associated with AKI.
Table 4.
Crude and age, sex, comorbidity and severity of illness-adjusted odds ratios (95% confidence intervals) for the association of acute kidney injury and hospital mortality stratified by two-year intervals from the Australia New Zealand Intensive Care Society Adult Patient Database 1996–2005
| Mortality outcome | Overall | 1996/1997 | 1998/1999 | 2000/2001 | 2002/2003 | 2004/2005 |
| Crude | 4.80 (4.5–5.1) | 6.03 (5.2–7.0) | 6.47 (5.5–7.6) | 4.74 (4.1–5.4) | 3.94 (3.5–4.5) | 4.11 (3.6–4.7) |
| Age and sex adjusted | 4.41 (4.1–4.7) | 5.63 (4.9–6.5) | 6.05 (5.2–7.1) | 4.40 (3.8–5.1) | 3.56 (3.1–4.0) | 3.73 (3.3–4.2) |
| Age, sex and comorbidity adjusted | 4.28 (4.2–4.6) | 5.29 (4.6–6.1) | 5.90 (5.0–6.9) | 4.21 (3.7–4.8) | 3.54 (3.1–4.0) | 3.66 (3.2–4.2) |
| Age, sex, comorbidity and severity adjusted | 1.42 (1.3–1.5) | 1.47 (1.2–1.7) | 1.48 (1.2–1.8) | 1.59 (1.3–1.9) | 1.25 (1.1–1.5) | 1.39 (1.2–1.6) |
| Adjusted odds ratioa | 1.39 (1.3–1.5) | 1.54 (1.3–1.9) | 1.64 (1.3–2.0) | 1.38 (1.2–1.6) | 1.20 (1.02–1.4) | 1.33 (1.1–1.6) |
aAdjustment for age, sex, comorbidity, surgical/medical admission, primary diagnosis, severity of illness (Acute Physiology and Chronic Health Evaluation II score), mechanical ventilation and hospital site. Goodness-of-fit test, P = 1.0; area under the receiver operator characteristic curve, 0.84.
Figure 2.
Summary of crude mortality for patients with and without acute kidney injury from the Australia New Zealand Intensive Care Society Adult Patient Database, 1996–2005. ARF, acute renal failure.
Additional clinical outcomes
Those patients with AKI had a longer median (interquartile range) stay in both the ICU and the hospital than those without AKI (Table 5). Specifically, AKI increased both the duration of the ICU stay (4.4 (2.1–9.5) days for AKI versus 2.6 (1.7 to 4.9) days for no AKI, P < 0.0001) and of the hospital stay (14.2 (6.5 to 28.9) days for AKI versus 11.7 (7.0 to 21.9) days for no AKI, P < 0.0001). The total duration of stay was also significantly longer in survivors to hospital discharge stratified by AKI than in nonsurvivors (19.8 (10.8 to 37.2) days versus 11.9 (7.2 to 21.9) days, P < 0.0001). There were no significant changes in ICU or hospital lengths of stay over the study period.
Table 5.
Clinical outcomes in critically ill patients admitted with acute kidney injury from the Australia New Zealand Intensive Care Society Adult Patient Database 1996–2005
| Overall | 1996/1997 | 1998/1999 | 2000/2001 | 2002/2003 | 2004/2005 | |
| Intensive care unit stay (days) (median (interquartile range)) |
||||||
| Dead | 3.4 (1.8–8.5) | 3.0 (1.6–8.2) | 3.2 (1.6–8.6) | 3.1 (1.8–7.9) | 3.5 (1.8–8.4) | 4.3 (1.9–9.8) |
| Alive | 5.0 (2.7–10.0) | 5.6 (2.9–11.8) | 6.1 (2.8–11.7) | 5.0 (2.6–10.1) | 4.7 (2.4–9.0) | 4.8 (2.8–9.5) |
| Hospital stay (days) (median (interquartile range)) | ||||||
| Dead | 7.5 (2.9–17.7) | 6.5 (2.5–15.7) | 7.1 (2.9–16.5) | 7.8 (3.0–18.8) | 7.8 (2.9–19.7) | 8.0 (3.2–18.6) |
| Alive | 19.8 (10.8–37.2) | 21.9 (11.8–40.1) | 20.5 (11.1–35.4) | 19.7 (11.0–37.6) | 18.7 (10.0–35.2) | 18.8 (9.9–37.6) |
| Discharge location of survivors (%) | ||||||
| Home | 74.9 | 75.5 | 70.9 | 74.9 | 76.1 | 75.1 |
| Transfer to another acute care hospital | 16.6 | 14.7 | 19.8 | 17.2 | 14.8 | 17.6 |
| Rehabilitation/long-term care facility | 8.6 | 9.9 | 9.3 | 7.8 | 9.1 | 7.3 |
The hospital discharge location was significantly different for patients with AKI compared with those patients with no AKI (Table 5). Fewer patients with AKI were discharged home than patients without AKI (74.8% versus 84.8%, P < 0.001); instead, AKI patients were more likely to have been transferred to another acute care hospital (16.6% versus 9.6%, P < 0.001) or a rehabilitation facility (8.6% versus 5.5%, P < 0.001). There were no significant changes in hospital discharge location over the study period.
Discussion
We conducted a 10-year observational study of > 90,000 ICU admissions to 20 ICUs in Australia, using a high-quality clinical database, to evaluate trends in the incidence and mortality associated with AKI. We found that approximately 5.2% of critically ill patients are diagnosed with AKI at the time of ICU admission and that the incidence of AKI has increased significantly over the past decade. We also found that the incidence of AKI associated with admission for metabolic diagnoses and/or poisonings has increased but that the incidence has declined in those patients admitted with trauma or haematologic malignancies. We confirmed that the mortality rate associated with AKI remains high and that the increased risk of death associated with AKI persisted after adjustment for several relevant covariates. Finally, we found that, despite an increasing incidence, the multivariate adjusted odds of death associated with AKI have shown a declining trend over the 10-year study period.
Numerous epidemiologic investigations have estimated the occurrence and associated burden of AKI on clinical outcomes and health resources in critically ill patients [1-5,7,8,11,12,15,19,29]. Very few studies, however, have assessed whether the incidence or outcomes associated with AKI have changed over time [21,30,31]. Moreover, these studies are often limited to a single centre and compare two discrete periods in time separated by several years [21]. Two recent large epidemiologic investigations using administrative databases showed similar patterns of increasing incidence and decreasing mortality with AKI; however, these studies are limited by focusing on all hospitalized patients rather than on only those admitted to ICU. Overall, this paucity of data examining for trends in incidence over time is unfortunate when taking into account the poor clinical outcome and high cost of care for critically ill patients with AKI [10,13,32].
The key findings from our study, specifically that AKI is common and its occurrence is on the rise, may have important health resource and economic implications. For instance, our data support the findings of prior investigations showing that AKI may play a role in prolonging the duration of stay in the ICU and the hospital and may lead to higher rates of hospital discharge to long-term care or rehabilitation facilities [2,11]. One consequence of these differences in clinical outcomes would undoubtedly be the consumption of considerable health resources [10,13,33,34].
Additionally, there has been considerable controversy as to whether the clinical outcomes – in particular, mortality associated with AKI – have improved [22,23]. For example, Ympa and colleagues reported in a systematic review that mortality associated with AKI has shown no consistent change over several decades [23]. Regrettably, their study was highly prone to bias and was limited by only reporting crude mortality rates across those studies included and by the inability to show equivalent illness severity. On the contrary, we have found over the past decade that the mortality associated with AKI, when adjusted for covariates, has shown a declining trend. Whether this decline can be attributed to an improvement in the overall care of critically ill patients or by specific interventions or therapies aimed at those with AKI remains unknown [35-38]. This decline in mortality has, however, occurred despite reported changes to the clinical profile and characteristics of critically ill patients with AKI [8,22]. Observational studies suggest that critically ill patients with AKI are increasingly older, have more comorbid disease, are more probably septic, and have greater severity of illness and organ failure [2,6].
In our study, we evaluated for changes in the profile and characteristics of patients that might have also corresponded to changes in the incidence of AKI. We found no notable trends when stratified by age or the presence of comorbid illness, with the exception of a decline in AKI associated with haematologic malignancy. Similarly, while ICU admissions for sepsis, acute cardiac conditions and hepatic failure were all associated with a higher risk for AKI, there were no significant trends in incidence for these conditions over the study period, with the exception of a rise in AKI associated with admissions for acute metabolic/poisoning conditions. Interestingly, however, we found a declining trend in the incidence of AKI associated with trauma. While the number of cases of AKI associated with trauma in our study was relatively small, there are plausible explanations for this finding – such as an increase in regionalized trauma systems [39,40], advancements in prehospital care [41] and earlier identification of patients at high risk for AKI, due to conditions such as rhabdomyolysis, with initiation of timely prophylactic interventions [42,43].
There are both limitations and strengths to our study. First, the definition of AKI used in our study, as mandated by the APD, will invariably influence the overall incidence estimates. We have, however, used several measures to capture patients designated with acute reductions in kidney function consistent with the syndrome of AKI. Second, we were unable to determine the precise prevalence of chronic kidney disease with the exception of those patients requiring chronic renal replacement therapy. This may also influence the overall incidence estimates. To minimize misclassification, we have attempted to exclude all patients with known end-stage renal disease or all admissions to the ICU that were related to kidney transplantation. Reassuringly, our incidence estimates appear largely consistent with the current literature [1]. Third, we were unable to provide estimates of the proportion of patients requiring acute renal replacement therapy. Fourth, we were only able to collect data on patients within the first 24 hours of admission to the ICU. The incidence estimates of AKI therefore probably underestimate the true burden of AKI as some patients would have developed delayed AKI several days after admission [44]. Moreover, we are unable to assess long-term outcome or renal recovery. On the other hand, this is by far the largest study of AKI ever conducted in terms of the overall screened population and target cohort, and the only study where outcomes and illness severity could be studied in the same units over an entire decade.
Conclusion
To our knowledge, we conducted the first large multicentre study of AKI in critically ill patients to evaluate long-term trends in incidence and mortality. In this heterogeneous cohort of critically ill patients, we found a significant rise in the incidence of AKI. Moreover, despite modest changes in the profile of patients with AKI, the associated mortality has declined.
Key messages
• The incidence of AKI has increased over the past decade.
• AKI associated with ICU admissions for metabolic diagnoses and/or poisonings appears to have increased.
• AKI associated with ICU admissions for trauma has decreased.
• AKI carries an independent increased risk of death.
• The associated mortality for patients with AKI remains high but has declined over the past decade.
Abbreviations
AKI = acute kidney injury; ANZICS = Australia New Zealand Intensive Care Society; APACHE = Acute Physiology and Chronic Health Evaluation; APD = Adult Patient Database; CI = confidence interval; ICU = intensive care unit; OR = odds ratio.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
SMB developed the study protocol, analysed data, and wrote and revised the manuscript. CG extracted the data from the ANZICS APD. RB conceived the study, assisted in developing the study protocol and provided critiques of successive drafts of the manuscript. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
SMB is supported by Clinical Fellowships from the Alberta Heritage Foundation for Medical Research and by the Canadian Institutes for Health Research. This study was supported in part by the Austin Hospital Anaesthesia and by the Intensive Care Trust Fund.
See related commentary by Cruz and Ronco, http://ccforum.com/content/11/4/149
Contributor Information
Sean M Bagshaw, Email: bagshaw@ualberta.ca.
Carol George, Email: adult.data@anzics.com.au.
Rinaldo Bellomo, Email: rinaldo.bellomo@austin.org.au.
References
- Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- Bagshaw SM, Laupland KB, Doig CJ, Mortis G, Fick GH, Mucenski M, Godinez-Luna T, Svenson LW, Rosenal T. Prognosis for long-term survival and renal recovery in critically ill patients with severe acute renal failure: a population-based study. Crit Care. 2005;9:R700–R709. doi: 10.1186/cc3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metnitz PG, Krenn CG, Steltzer H, Lang T, Ploder J, Lenz K, Le Gall JR, Druml W. Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med. 2002;30:2051–2058. doi: 10.1097/00003246-200209000-00016. [DOI] [PubMed] [Google Scholar]
- Metcalfe W, Simpson M, Khan IH, Prescott GJ, Simpson K, Smith WC, MacLeod AM. Acute renal failure requiring renal replacement therapy: incidence and outcome. QJM. 2002;95:579–583. doi: 10.1093/qjmed/95.9.579. [DOI] [PubMed] [Google Scholar]
- Silvester W, Bellomo R, Cole L. Epidemiology, management, and outcome of severe acute renal failure of critical illness in Australia. Crit Care Med. 2001;29:1910–1915. doi: 10.1097/00003246-200110000-00010. [DOI] [PubMed] [Google Scholar]
- de Mendonca A, Vincent JL, Suter PM, Moreno R, Dearden NM, Antonelli M, Takala J, Sprung C, Cantraine F. Acute renal failure in the ICU: risk factors and outcome evaluated by the SOFA score. Intensive Care Med. 2000;26:915–921. doi: 10.1007/s001340051281. [DOI] [PubMed] [Google Scholar]
- Cole L, Bellomo R, Silvester W, Reeves JH. A prospective, multicenter study of the epidemiology, management, and outcome of severe acute renal failure in a 'closed' ICU system. Am J Respir Crit Care Med. 2000;162:191–196. doi: 10.1164/ajrccm.162.1.9907016. [DOI] [PubMed] [Google Scholar]
- Liano F, Junco E, Pascual J, Madero R, Verde E. The spectrum of acute renal failure in the intensive care unit compared with that seen in other settings. The Madrid Acute Renal Failure Study Group. Kidney Int Suppl. 1998;66:S16–S24. [PubMed] [Google Scholar]
- Brivet FG, Kleinknecht DJ, Loirat P, Landais PJ. Acute renal failure in intensive care units – causes, outcome, and prognostic factors of hospital mortality; a prospective, multicenter study. French Study Group on Acute Renal Failure. Crit Care Med. 1996;24:192–198. doi: 10.1097/00003246-199602000-00003. [DOI] [PubMed] [Google Scholar]
- Ahlstrom A, Tallgren M, Peltonen S, Rasanen P, Pettila V. Survival and quality of life of patients requiring acute renal replacement therapy. Intensive Care Med. 2005;31:1222–1228. doi: 10.1007/s00134-005-2681-6. [DOI] [PubMed] [Google Scholar]
- Chertow GM, Christiansen CL, Cleary PD, Munro C, Lazarus JM. Prognostic stratification in critically ill patients with acute renal failure requiring dialysis. Arch Intern Med. 1995;155:1505–1511. doi: 10.1001/archinte.155.14.1505. [DOI] [PubMed] [Google Scholar]
- Groeneveld A, Tran D, van der Meulen J, Nauta J, Thijs L. Acute renal failure in the medical intensive care unit: predisposing, complicating factors and outcome. Nephron. 1991;59:602–610. doi: 10.1159/000186651. [DOI] [PubMed] [Google Scholar]
- Korkeila M, Ruokonen E, Takala J. Costs of care, long-term prognosis and quality of life in patients requiring renal replacement therapy during intensive care. Intensive Care Med. 2000;26:1824–1831. doi: 10.1007/s001340000726. [DOI] [PubMed] [Google Scholar]
- Maynard SE, Whittle J, Chelluri L, Arnold R. Quality of life and dialysis decisions in critically ill patients with acute renal failure. Intensive Care Med. 2003;29:1589–1593. doi: 10.1007/s00134-003-1837-5. [DOI] [PubMed] [Google Scholar]
- Mehta RL, Pascual MT, Soroko S, Savage BR, Himmelfarb J, Ikizler TA, Paganini EP, Chertow GM. Spectrum of acute renal failure in the intensive care unit: the PICARD experience. Kidney Int. 2004;66:1613–1621. doi: 10.1111/j.1523-1755.2004.00927.x. [DOI] [PubMed] [Google Scholar]
- Morgera S, Kraft AK, Siebert G, Luft FC, Neumayer HH. Long-term outcomes in acute renal failure patients treated with continuous renal replacement therapies. Am J Kidney Dis. 2002;40:275–279. doi: 10.1053/ajkd.2002.34505. [DOI] [PubMed] [Google Scholar]
- Schaefer JH, Jochimsen F, Keller F, Wegscheider K, Distler A. Outcome prediction of acute renal failure in medical intensive care. Intensive Care Med. 1991;17:19–24. doi: 10.1007/BF01708404. [DOI] [PubMed] [Google Scholar]
- Chertow GM, Lazarus JM, Paganini EP, Allgren RL, Lafayette RA, Sayegh MH. Predictors of mortality and the provision of dialysis in patients with acute tubular necrosis. The Auriculin Anaritide Acute Renal Failure Study Group. J Am Soc Nephrol. 1998;9:692–698. doi: 10.1681/ASN.V94692. [DOI] [PubMed] [Google Scholar]
- Spiegel DM, Ullian ME, Zerbe GO, Berl T. Determinants of survival and recovery in acute renal failure patients dialyzed in intensive-care units. Am J Nephrol. 1991;11:44–47. doi: 10.1159/000168271. [DOI] [PubMed] [Google Scholar]
- Spurney RF, Fulkerson WJ, Schwab SJ. Acute renal failure in critically ill patients: prognosis for recovery of kidney function after prolonged dialysis support. Crit Care Med. 1991;19:8–11. doi: 10.1097/00003246-199101000-00007. [DOI] [PubMed] [Google Scholar]
- Jayakumar M, Prabahar MR, Fernando EM, Manorajan R, Venkatraman R, Balaraman V. Epidemiologic trend changes in acute renal failure – a tertiary center experience from South India. Ren Fail. 2006;28:405–410. doi: 10.1080/08860220600689034. [DOI] [PubMed] [Google Scholar]
- Bellomo R. The epidemiology of acute renal failure: 1975 versus 2005. Curr Opin Crit Care. 2006;12:557–560. doi: 10.1097/01.ccx.0000247443.86628.68. [DOI] [PubMed] [Google Scholar]
- Ympa YP, Sakr Y, Reinhart K, Vincent JL. Has mortality from acute renal failure decreased? A systematic review of the literature. Am J Med. 2005;118:827–832. doi: 10.1016/j.amjmed.2005.01.069. [DOI] [PubMed] [Google Scholar]
- Stow PJ, Hart GK, Higlett T, George C, Herkes R, McWilliam D, Bellomo R. Development and implementation of a high-quality clinical database: the Australian and New Zealand Intensive Care Society Adult Patient Database. J Crit Care. 2006;21:133–141. doi: 10.1016/j.jcrc.2005.11.010. [DOI] [PubMed] [Google Scholar]
- ANZICS APD Data Dictionary http://www.anzics.com.au/section.asp?Section=adult
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- Le Gall J, Lemeshow S, Saulnier F. A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957–2963. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- Ries LA, Wingo PA, Miller DS, Howe HL, Weir HK, Rosenberg HM, Vernon SW, Cronin K, Edwards BK. The annual report to the nation on the status of cancer, 1973–1997, with a special section on colorectal cancer. Cancer. 2000;88:2398–2424. doi: 10.1002/(SICI)1097-0142(20000515)88:10<2398::AID-CNCR26>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Cosentino F, Chaff C, Piedmonte M. Risk factors influencing survival in ICU acute renal failure. Nephrol Dial Transplant. 1994;9(Suppl 4):179–182. [PubMed] [Google Scholar]
- Waikar SS, Curhan GC, Wald R, McCarthy EP, Chertow GM. Declining mortality in patients with acute renal failure, 1988 to 2002. J Am Soc Nephrol. 2006;17:1143–1150. doi: 10.1681/ASN.2005091017. [DOI] [PubMed] [Google Scholar]
- Xue JL, Daniels F, Star RA, Kimmel PL, Eggers PW, Molitoris BA, Himmelfarb J, Collins AJ. Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol. 2006;17:1135–1142. doi: 10.1681/ASN.2005060668. [DOI] [PubMed] [Google Scholar]
- Manns B, Doig CJ, Lee H, Dean S, Tonelli M, Johnson D, Donaldson C. Cost of acute renal failure requiring dialysis in the intensive care unit: clinical and resource implications of renal recovery. Crit Care Med. 2003;31:449–455. doi: 10.1097/01.CCM.0000045182.90302.B3. [DOI] [PubMed] [Google Scholar]
- Gopal I, Bhonagiri S, Ronco C, Bellomo R. Out of hospital outcome and quality of life in survivors of combined acute multiple organ and renal failure treated with continuous venovenous hemofiltration/hemodiafiltration. Intensive Care Med. 1997;23:766–772. doi: 10.1007/s001340050407. [DOI] [PubMed] [Google Scholar]
- Hamel MB, Phillips RS, Davis RB, Desbiens N, Connors AF, Jr, Teno JM, Wenger N, Lynn J, Wu AW, Fulkerson W, Tsevat J. Outcomes and cost-effectiveness of initiating dialysis and continuing aggressive care in seriously ill hospitalized adults. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. Ann Intern Med. 1997;127:195–202. doi: 10.7326/0003-4819-127-3-199708010-00003. [DOI] [PubMed] [Google Scholar]
- Jorres A, Gahl GM, Dobis C, Polenakovic MH, Cakalaroski K, Rutkowski B, Kisielnicka E, Krieter DH, Rumpf KW, Guenther C, et al. Haemodialysis-membrane biocompatibility and mortality of patients with dialysis-dependent acute renal failure: a prospective randomised multicentre trial. International Multicentre Study Group. Lancet. 1999;354:1337–1341. doi: 10.1016/S0140-6736(99)01213-1. [DOI] [PubMed] [Google Scholar]
- Ronco C, Bellomo R, Homel P, Brendolan A, Dan M, Piccinni P, La Greca G. Effects of different doses in continuous veno-venous haemofiltration on outcomes of acute renal failure: a prospective randomised trial. Lancet. 2000;356:26–30. doi: 10.1016/S0140-6736(00)02430-2. [DOI] [PubMed] [Google Scholar]
- Gettings LG, Reynolds HN, Scalea T. Outcome in post-traumatic acute renal failure when continuous renal replacement therapy is applied early vs. late. Intensive Care Med. 1999;25:805–813. doi: 10.1007/s001340050956. [DOI] [PubMed] [Google Scholar]
- Schiffl H, Lang S, Fischer R. Daily hemodialysis and the outcome of acute renal failure. N Engl J Med. 2002;346:305–310. doi: 10.1056/NEJMoa010877. [DOI] [PubMed] [Google Scholar]
- Sampalis JS, Denis R, Frechette P, Brown R, Fleiszer D, Mulder D. Direct transport to tertiary trauma centers versus transfer from lower level facilities: impact on mortality and morbidity among patients with major trauma. J Trauma. 1997;43:288–295. doi: 10.1097/00005373-199708000-00014. [DOI] [PubMed] [Google Scholar]
- Sampalis JS, Denis R, Lavoie A, Frechette P, Boukas S, Nikolis A, Benoit D, Fleiszer D, Brown R, Churchill-Smith M, Mulder D. Trauma care regionalization: a process–outcome evaluation. J Trauma. 1999;46:565–579. doi: 10.1097/00005373-199904000-00004. discussion 579–581. [DOI] [PubMed] [Google Scholar]
- Turner J, Nicholl J, Webber L, Cox H, Dixon S, Yates D. A randomised controlled trial of prehospital intravenous fluid replacement therapy in serious trauma. Health Technol Assess. 2000;4:1–57. [PubMed] [Google Scholar]
- Sharp LS, Rozycki GS, Feliciano DV. Rhabdomyolysis and secondary renal failure in critically ill surgical patients. Am J Surg. 2004;188:801–806. doi: 10.1016/j.amjsurg.2004.08.050. [DOI] [PubMed] [Google Scholar]
- Rosen CL, Adler JN, Rabban JT, Sethi RK, Arkoff L, Blair JA, Sheridan R. Early predictors of myoglobinuria and acute renal failure following electrical injury. J Emerg Med. 1999;17:783–789. doi: 10.1016/S0736-4679(99)00084-0. [DOI] [PubMed] [Google Scholar]
- Guerin C, Girard R, Selli JM, Perdrix JP, Ayzac L. Initial versus delayed acute renal failure in the intensive care unit. A multicenter prospective epidemiological study. Rhone-Alpes Area Study Group on Acute Renal Failure. Am J Respir Crit Care Med. 2000;161:872–879. doi: 10.1164/ajrccm.161.3.9809066. [DOI] [PubMed] [Google Scholar]