Abstract
The body growth of animals is regulated by growth hormone and IGF-I. The classical theory of this regulation is that most IGF-I in the blood originates in the liver and that body growth is controlled by the concentration of IGF-I in the blood. We have abolished IGF-I production in the livers of mice by using the Cre/loxP recombination system. These mice demonstrated complete inactivation of the IGF-I gene in the hepatocytes. Although the liver accounts for less than 5% of body mass, the concentration of IGF-I in the serum was reduced by 75%. This finding confirms that the liver is the principal source of IGF-I in the blood. However, the reduction in serum IGF-I concentration had no discernible effect on postnatal body growth. We conclude that postnatal body growth is preserved despite complete absence of IGF-I production by the hepatocytes.
Growth hormone (GH) is the major regulator of postnatal body growth (1). The anabolic and growth-promoting effects of GH are to a large extent mediated by a stimulation of expression of insulin-like growth factor I (IGF-I) in the liver and in peripheral tissues. The relative importance of liver-derived IGF-I (endocrine-acting) versus locally produced IGF-I (autocrine/paracrine-acting) has been a matter of debate (2, 3). During the last 10 years it has become evident that systemic administration of IGF-I to GH-deficient/GH receptor-mutated animals or humans stimulates body growth. These findings suggest that both IGF-I and GH have the capacity to stimulate body growth (4–8). Elimination of IGF-I and the IGF-I receptor by homologous gene recombination in mice results in severe retardation of growth in stature that first becomes apparent at day 12 of gestation and continues postnatally (9–12). Furthermore, a patient with a deletion of the IGF-I gene demonstrated intrauterine growth retardation and postnatal growth failure (13). These experimental and clinical studies clearly demonstrate that IGF-I, as well as its receptor, plays a critical role for normal body growth. However, these experiments did not clarify the relative importance of liver-derived circulating IGF-I and locally produced IGF-I for body growth.
The aim of the present study was to investigate the role of liver-derived IGF-I for postnatal body growth. To achieve a liver-specific inducible inactivation of IGF-I in mice (LI-IGF-I −/− mice) we used the Cre/loxP recombination system. Cre-mediated gene targeting can be used to inactivate genes in vivo in a tissue-specific and inducible manner (14–16).
MATERIALS AND METHODS
Animal and Experimental Protocol.
The Mx-Cre 31 strain was generated by injection into (C57BL/6 × CBA)F2 eggs as previously described (15). The offspring were then backcrossed with C57BL/6 mice. Mice with exon 4 of the IGF-I gene flanked with loxP sites were, as earlier described, generated in embryonic stem (ES) cells derived from 129sv mice (12). The ES cells were injected into C57BL/6 blastocysts. Male chimeric offspring were backcrossed with C57BL/6 female mice. Mx-Cre 31 mice were intercrossed with mice with exon 4 of the IGF-I gene flanked with loxP sites. Mice homozygous for loxP and heterozygous for Mx-Cre were given interferon (IFN-α2/α1, 4 × 108 units/kg of body weight) in three i.p. injections on days 24, 26, and 28 after birth. The animal care was in accordance with institutional guidelines. The animal procedure was approved by the ethical committee at the University of Gothenburg.
Southern Blotting and RNase-Protection Assay.
Southern blotting and the probe used for it have been described by Liu et al. (12). The RNase-protection assay was done with a kit according to the manufacturer’s instructions (RPA kit; Ambion, Austin, TX). The 178-bp probe used in the RNase-protection assay was generated with PCR and corresponds to exon 4 and part of exon 3 of the IGF-I gene. cDNA was synthesized from total mouse liver RNA and then PCR was performed with the following primers: 5′-GGTGGATGCTCTTCAGTT-3′ and 5′-TGCTTTTGTAGGCTTCAG-3′. The PCR fragment was isolated and T/A cloned into the PCR-II vector (Invitrogen, Leek, the Netherlands), and the insert was verified by sequencing. The vector was linearized with XhoI prior to in vitro transcription with SP6 RNA polymerase in the presence of [α-32P]UTP. Bands of correct size in both Southern blotting and RNase-protection assay were visualized and quantified with a PhosphorImager (Molecular Dynamics). Recombination, as determined by Southern blotting, was calculated by dividing the density of the exon 4 deleted band (Δ) with the sum of the density of the exon 4 deleted (Δ) band and the band corresponding to intact exon 4 of the IGF-I gene flanked with loxP sites (Flox) and is expressed in percent. IGF-I mRNA levels determined by RNase-protection assay were expressed as a ratio between the density of the band corresponding to the IGF-I transcript and the density of the band corresponding to the internal standard, 18S rRNA.
Preparation of Purified Hepatocytes.
Hepatocytes were prepared by the collagenase perfusion method followed by repeated centrifugations according to Seglen (17).
Radioimmunoassay (RIA).
Serum IGF-I was measured by double antibody IGFBP-blocked RIA according to Blum and Breier (18). Mouse GH was measured by using an RIA (RPA 551; Amersham), with a detection range of 1.3–100 ng/ml. It is known that the GH secretion in rodents is sexually dimorphic (19). In this study, the proportions of females were similar in LI-IGF-I −/− mice and control group (8/12 and 18/29, respectively).
Western Ligand Blotting of IGF Binding Protein 3.
Western ligand blotting was performed as previously described (20). The band corresponding to IGF binding protein 3 was analyzed by densitometric scanning with a PhosphorImager.
RESULTS
Characterization of the Efficacy and Specificity of IGF-I Inactivation in LI-IGF-I −/− Mice.
IFN induction of mice with the Cre recombinase under the control of an IFN-responsive promoter (Mx-Cre) has previously been shown to cause almost 100% recombination in the liver and a partial recombination in the spleen, whereas the recombination in peripheral tissues such as muscle, fat, kidney, heart, and bone is low (15, 21). Mx-Cre mice were intercrossed with mice with exon 4 of the IGF-I gene flanked with loxP sites. Mice homozygous for loxP and heterozygous for Mx-Cre were induced with IFN at 24–28 days of age. Neither Cre expression nor IFN treatment by itself regulated any of the parameters described in the present study.
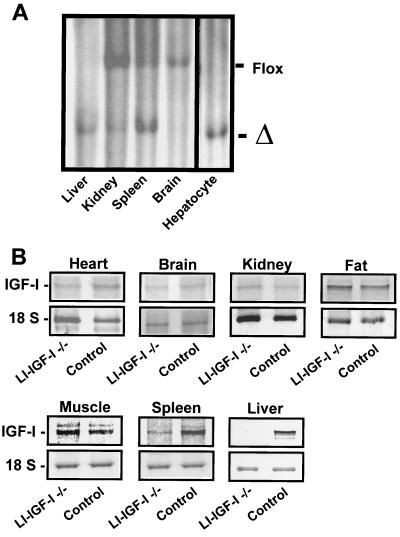
The efficiency of recombination was studied by Southern blotting. There was an approximately 90% recombination in the liver, which consists of both the hepatocytes (with a high proportion of binuclear and polyploid cells) and nonparenchymal cells (22). In purified hepatocytes of LI-IGF-I −/− mice the IGF-I gene was completely recombined (Fig. 1A). In all other tissues the recombination, as determined by Southern blotting, was less than 20%, except for the spleen where the recombination was 65% (Fig. 1A). No recombination was found in Cre mice not induced with IFN (data not shown). IGF-I mRNA levels in LI-IGF-I −/− mice, as determined by RNase-protection assay, were decreased by more than 95% in liver and by approximately 60% in spleen, whereas no significant effect was seen in other tissues, including fat, muscle, kidney, brain, and heart (Fig. 1B and Table 1). Thus, the LI-IGF-I −/− mice have a high inactivation of IGF-I expression in the liver. In contrast, no significant inactivation was seen in extrahepatic tissues of relevance for body growth.
Figure 1.
Effects of Cre-mediated deletion of exon 4 of the IGF-I gene. Mice were induced with IFN at 24–28 days of age. Various tissues from LI-IGF-I −/− mice were analyzed 53–60 days later. (A) Southern blotting was used to determine the deletion of exon 4 of the IGF-I gene in various tissues and in an enriched hepatocyte preparation from LI-IGF-I −/− mice. The band corresponding to exon 4 of IGF-I flanked with loxP sites (Flox) and the band corresponding to deleted exon 4 (Δ) are indicated. (B) RNase-protection assay demonstrating IGF-I mRNA expression in various tissues from control and LI-IGF-I −/− mice. The IGF-I mRNA expression was normalized to the levels of 18S rRNA.
Table 1.
IGF-I mRNA levels
| Tissue | mRNA
|
|
|---|---|---|
| LI-IGF-I −/− | Control | |
| Brain | 125 ± 20 | 100 ± 25 |
| Kidney | 80 ± 26 | 100 ± 38 |
| Fat | 136 ± 30 | 100 ± 25 |
| Muscle | 89 ± 18 | 100 ± 5 |
| Spleen | 38 ± 10** | 100 ± 4 |
| Liver | 5 ± 1** | 100 ± 9 |
Mice were induced with IFN at 24–28 days of age as described in the legend of Fig. 2. IGF-I mRNA levels in various tissues were measured by RNase-protection assay. The IGF-I mRNA expression was normalized to the level of 18S mRNA. Values are expressed as percent of control and are given as mean ± SEM. ∗∗, P < 0.01, LI-IGF-I −/− versus control (Student’s t test). Four or five animals were included in each group.
Effects on Serum IGF-I Levels.
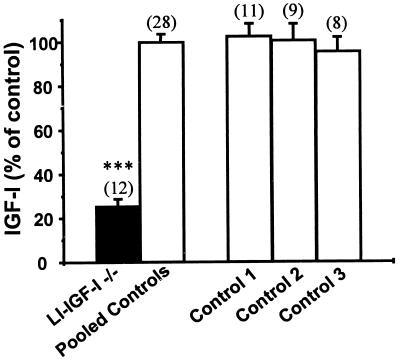
Serum IGF-I levels decreased dramatically in the LI-IGF-I −/− mice. The effect was seen within 1 week after IFN induction at 24 days of age and was still present 53 days later (Fig. 2; LI-IGF −/− 89 ± 12 ng/ml, pooled control 350 ± 17 ng/ml). Therefore 75% of all IGF-I in mouse serum was derived from the liver. Thus, liver-derived IGF-I is the main determinant of serum IGF-I levels. This finding is in line with the results of previous indirect calculations based on the IGF-I production rate in isolated rat liver (23). The decreased serum IGF-I levels were, as expected, associated with a similar magnitude of decrease in serum levels of IGF binding protein 3 (LI-IGF-I −/− 19% ± 5%, pooled control 100% ± 11%; P < 0.01 by Student’s t test) as measured with ligand blotting.
Figure 2.
Serum IGF-I levels in LI-IGF −/− mice. Mice were induced with IFN at 24–28 days of age. Serum levels of IGF-I were measured 53 days later by RIA. We also investigated three different groups of control mice: mice that had been treated with IFN but lack the Mx-cre transgene (Control 1), Mx-cre transgenic mice not treated with IFN (Control 2), and mice without Mx-cre transgene and not treated with IFN (Control 3). All three control groups were homozygous for loxP sequences flanking exon 4 of the IGF-I gene (flox/flox). Because the results did not differ among the three control groups, the results were pooled (Pooled Controls). Serum IGF-I levels are expressed as percent of control and presented as mean ± SEM. The number of observations in each group is indicated within parentheses. ∗∗∗, P < 0.001.
Effects on Body Growth.
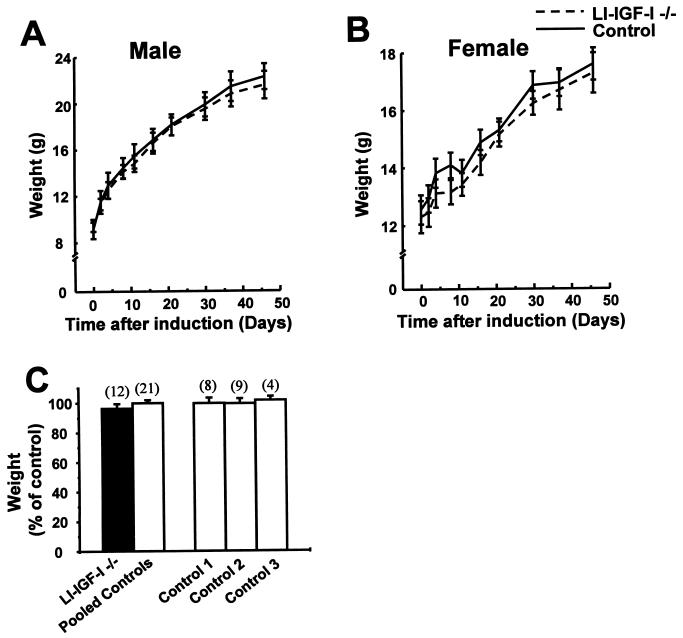
LI-IGF −/− mice were induced with IFN at 24–28 days of age and the body growth was monitored during the following 53 days. No significant difference in weight gain was seen between LI-IGF −/− mice and control mice (Fig. 3). Similar results were seen in male and female mice. There was no significant difference in the length of the tibia and the height of the lumbar spine, although a small decrease in the femoral length was seen in LI-IGF −/− mice compared with control mice 53 days after IFN induction (Table 2).
Figure 3.
Body growth in LI-IGF −/− mice. The litter sizes were similar in the LI-IGF-I mice and the control mice. Mice were induced with IFN at 24–28 days of age as described in the legend of Fig. 2. The weights of male (A) and female (B) mice at various times after IFN induction are indicated. The final weights of mice from three different experiments expressed as percent of control are indicated in C. The control groups are defined in the legend of Fig. 2. Values are expressed as mean ± SEM. The number of observations in each group is indicated within parentheses. Neither Cre expression nor IFN treatment by itself regulated any of the given parameters.
Table 2.
Sizes of bones
| Measurement | Relative size
|
|
|---|---|---|
| LI-IGF-I −/− | Control | |
| Tibia length | 98.6 ± 0.5 (n = 12) | 100 ± 0.4 (n = 21) |
| Femur length | 96.3 ± 0.5*(n = 12) | 100 ± 0.6 (n = 21) |
| Spine height (L1 to L5) | 98.8 ± 0.7 (n = 9) | 100 ± 0.5 (n = 8) |
Mice were induced with IFN at 24–28 days of age as described in the legend of Fig. 2. Lengths of tibia and femur and height of spine (lumbral vertebrae L1 to L5) 53 days after IFN induction are given. Neither Cre expression nor IFN treatment by itself regulated any of the given parameters. Values are expressed as percent of control and are given as mean ± SEM. ∗, P < 0.05 LI-IGF-I −/− versus control (Student’s t test). The number of observations in each group (n) is indicated within parentheses.
Effects on Organ Growth.
Some visceral organs from mice with IGF-I inactivated from day 24 to day 77 were weighed and related to total body weight. The relative spleen and heart sizes were unchanged in LI-IGF-I −/− mice compared with control mice (Table 3). Interestingly, the kidneys were smaller and the livers larger in LI-IGF-I −/− mice than in control mice.
Table 3.
Organ weight
| Organ | Relative weight
|
|
|---|---|---|
| LI-IGF-I −/− (n = 12) | Control (n = 21) | |
| Liver | 113.7 ± 1.99** | 100 ± 1.44 |
| Spleen | 98.3 ± 3.06 | 100 ± 2.43 |
| Kidney | 88.0 ± 3.04** | 100 ± 2.07 |
| Heart | 105.8 ± 3.04 | 100 ± 2.06 |
Mice were induced with IFN at 24–28 days of age as described in the legend of Fig. 2. Wet weights of various organs 53 days after IFN induction are given. Wet weights of the organs were normalized to body weight. Neither Cre expression nor IFN treatment by itself regulated any of the given parameters. Values are expressed as percent of control and are given as mean ± SEM. ∗∗, P < 0.01, LI-IGF-I −/− versus control (Student’s t test). The number of observations in each group is indicated within parentheses.
Compensatory Increase in GH Concentration.
Measurements of the GH concentration in the serum of control and LI-IGF-I −/− mice showed that the hormone concentration was higher in the LI-IGF-I −/− mice (Table 4). In the control mice, 66% of values were below 15 ng/ml, whereas in the mutant mice, 84% of values were greater than 15 ng/ml. The geometric mean value was 8 ng/ml for the controls and 25 ng/ml for the mutants.
Table 4.
Serum levels of GH
| Serum GH, ng/ml | Frequency, %
|
|
|---|---|---|
| LI-IGF-I −/− (n = 12) | Control (n = 35) | |
| <1.5 | 17 | 29 |
| 1.5–15 | 0 | 37 |
| 15–50 | 42 | 20 |
| >50 | 42 | 14 |
| Total | 100 | 100 |
Frequency distributions (in percent of all values) of serum GH levels in LI-IGF-I −/− and control mice are shown. Four class intervals were chosen by dividing the serum GH levels from all mice into quartiles (33). P = 0.02 LI-IGF-I −/− versus control (χ2 test). The number of mice in each group is given in parentheses.
DISCUSSION
Liver-Derived IGF-I Is Not Required for Postnatal Body Growth.
Body growth was normal in the LI-IGF-I −/− mice, demonstrating that liver-derived IGF-I is not required for postnatal growth, a direct challenge to the somatomedin hypothesis. According to the original somatomedin hypothesis, GH stimulates body growth by stimulating liver production of somatomedins (IGFs), which in turn stimulate longitudinal bone growth in an endocrine manner (3, 23). In contrast, the present data show that body growth is maintained at a rate not significantly different from controls when hepatic IGF-I production is abolished and the serum levels drop to 25% of the control value. This finding indicates that autocrine/paracrine IGF-I but not liver-derived IGF-I is the major determinant of postnatal body growth. An effect of autocrine/paracrine IGF-I on postnatal body growth is also supported by the finding that GH injected directly into the rat tibia growth plate stimulates longitudinal bone growth at the site of the injection (24) and that this effect is abolished when antibodies to IGFs are co-administered locally with GH (25). It has also been shown that extrahepatic tissue levels of IGF-I are regulated by GH (26). Therefore, the present study indicates that autocrine/paracrine IGF-I rather than endocrine IGF-I is the major determinant of GH-induced body growth. A decrease in total circulating IGF-I might reflect a decrease of inactive IGF-I in large molecular weight complexes (27). This idea is supported by a decrease in IGF binding protein 3 in the LI-IGF-I −/− mice. However, the findings that the feedback on plasma GH-levels, the relative organ size, and the levels of IGF binding protein 3 itself were affected indicate that the levels of biologically active IGF-I were decreased in the LI-IGF-I −/− mice. One may also speculate that IGF-II can compensate for the decreased IGF-I stimulation in the LI-IGF-I −/− mice. However, as recently discussed by Stewart and Rotwein (28), it is uncertain whether a putative increase in IGF-II can substitute for IGF-I in maintaining somatic growth in rodents. In humans, a patient with complete IGF-I gene deletion was severely growth retarded although the serum IGF-II levels were moderately increased (13). Thus, both animal studies and a clinical study indicate that IGF-II cannot replace IGF-I in stimulating normal postnatal body growth.
Lack of liver-Derived IGF-I Results in Disproportional Organ Growth.
The endocrine status in the LI-IGF-I −/− mice with very low serum IGF-I levels and increased serum levels of GH is associated with normal total body growth but disproportionate organ growth. It was demonstrated earlier that GH but not IGF-I increases liver size in proportion to body weight (4, 5, 29, 30). Thus, one might speculate that the increased liver size in the LI-IGF-1 −/− mice is a result of the increased GH levels. Furthermore, since there is no IGF-I expression in the livers of LI-IGF-I −/− mice, the enlarged liver size in LI-IGF-I −/− mice is probably a non-IGF-I-mediated direct GH effect. We speculate that the liver could be subject to a classic feedback regulation exerted by an IGF-I–GH loop in which liver-derived circulating IGF-I modulates GH secretion, which in turn regulates liver size. Systemic delivery of IGF-I has been shown to increase the kidney size (4, 5), indicating that the decreased kidney size in LI-IGF-I −/− mice is a result of the decreased serum IGF-I levels.
Significance of the Compensatory Increase in Serum GH.
The LI-IGF-I −/− mice had increased serum levels of GH. This result extends earlier findings by indicating that circulating, liver-derived IGF-I exerts a negative feedback regulation of the pulsatile GH secretory pattern. It has been known for a long time that treatment with IGF-I suppresses GH secretion (31). Results from a patient with complete IGF-I gene deletion showed enhanced GH secretion (13), an effect that could have been due to either lack of local IGF-I production in the hypothalamopituitary region (32) or lack of circulating IGF-I. Although the present results do not exclude a local IGF-I effect, they indicate a physiological role for liver-derived circulating IGF-I in the negative-feedback regulation of GH secretion. In the present study on liver-specific IGF-I inactivation, the much reduced serum IGF-I levels had virtually no effect on body growth. However, compensatory mechanism(s) at various levels may have been activated in the LI-IGF-I −/− mice. For example, compensatory increase in GH secretion may have activated extrahepatic production of IGF-I, although we saw no significant increase in IGF-I mRNA levels in various organs (Fig. 1B and Table 1).
In conclusion, liver-derived IGF-I is the main determinant of serum IGF-I but is not required for postnatal growth, indicating that autocrine/paracrine-produced IGF-I is more important than liver-derived IGF-I for body growth. Thus, the present study indicates that the “classical” somatomedin hypothesis needs revision.
Acknowledgments
Excellent technical assistance was provided by Petra Strand, Maud Pettersson, and Lotta Uggla. We thank R. Kuhn and C. Rajewsky for the Mx-Cre31 transgenic mouse strain and C. Weissmann for IFN-α2/α1. This study was supported by the Swedish Medical Research Council, the Bergvall Foundation, the Lundberg Foundation, the Nordic Insulin Pharma, the Swedish Medical Society, the Göteborg Medical Society, Pharmacia–Upjohn, the Novo Nordisk Foundation, the Swedish Cancer Foundation, and the Swedish Association Against Rheumatic Disease.
ABBREVIATIONS
- GH
growth hormone
- IGF-I
insulin-like growth factor I
- LI-IGF-I −/− mice
mice with liver-specific inducible inactivation of IGF-I
- IFN
interferon
References
- 1.Ohlsson C, Bengtsson B A, Isaksson O G, Andreassen T T, Slootweg M C. Endocr Rev. 1998;19:55–79. doi: 10.1210/edrv.19.1.0324. [DOI] [PubMed] [Google Scholar]
- 2.Isaksson O G, Lindahl A, Nilsson A, Isgaard J. Endocr Rev. 1987;8:426–438. doi: 10.1210/edrv-8-4-426. [DOI] [PubMed] [Google Scholar]
- 3.Daughaday W H, Rotwein P. Endocr Rev. 1989;10:68–91. doi: 10.1210/edrv-10-1-68. [DOI] [PubMed] [Google Scholar]
- 4.Holder A T, Spencer E M, Preece M A. J Endocrinol. 1981;89:275–282. doi: 10.1677/joe.0.0890275. [DOI] [PubMed] [Google Scholar]
- 5.Guler H P, Zapf J, Scheiwiller E, Froesch E R. Proc Natl Acad Sci USA. 1988;85:4889–4893. doi: 10.1073/pnas.85.13.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker J L, Ginalska-Malinowska M, Romer T E, Pucilowska J B, Underwood L E. N Engl J Med. 1991;324:1483–1488. doi: 10.1056/NEJM199105233242107. [DOI] [PubMed] [Google Scholar]
- 7.Laron Z, Anin S, Klipper-Aurbach Y, Klinger B. Lancet. 1992;339:1258–1261. doi: 10.1016/0140-6736(92)91594-x. [DOI] [PubMed] [Google Scholar]
- 8.Backeljauw P F, Underwood L E. J Clin Endocrinol Metab. 1996;81:3312–3317. doi: 10.1210/jcem.81.9.8784089. [DOI] [PubMed] [Google Scholar]
- 9.Baker J, Liu J P, Robertson E J, Efstratiadis A. Cell. 1993;75:73–82. [PubMed] [Google Scholar]
- 10.Liu J P, Baker J, Perkins A S, Robertson E J, Efstratiadis A. Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- 11.Powell-Braxton L, Hollingshead P, Warburton C, Dowd M, Pitts-Meek S, Dalton D, Gillett N, Stewart T A. Genes Dev. 1993;7:2609–2617. doi: 10.1101/gad.7.12b.2609. [DOI] [PubMed] [Google Scholar]
- 12.Liu J L, Grinberg A, Westphal H, Sauer B, Accili D, Karas M, LeRoith D. Mol Endocrinol. 1998;12:1452–1462. doi: 10.1210/mend.12.9.0162. [DOI] [PubMed] [Google Scholar]
- 13.Woods K, Camacho-Hubner C, Savage M, Clark A. N Engl J Med. 1996;335:1363–1367. doi: 10.1056/NEJM199610313351805. [DOI] [PubMed] [Google Scholar]
- 14.Gu H, Marth J D, Orban P C, Mossmann H, Rajewsky K. Science. 1994;265:103–106. doi: 10.1126/science.8016642. [DOI] [PubMed] [Google Scholar]
- 15.Kuhn R, Schwenk F, Aguet M, Rajewsky K. Science. 1995;269:1427–1429. doi: 10.1126/science.7660125. [DOI] [PubMed] [Google Scholar]
- 16.Sauer B. Methods. 1998;14:381–392. doi: 10.1006/meth.1998.0593. [DOI] [PubMed] [Google Scholar]
- 17.Seglen P O. Exp Cell Res. 1972;74:450–454. doi: 10.1016/0014-4827(72)90400-4. [DOI] [PubMed] [Google Scholar]
- 18.Blum W F, Breier B H. Growth Regul. 1994;4, Suppl. 1:11–19. [PubMed] [Google Scholar]
- 19.Jansson J O, Eden S, Isaksson O G. Endocr Rev. 1985;6:128–150. doi: 10.1210/edrv-6-2-128. [DOI] [PubMed] [Google Scholar]
- 20.Hossenlopp P, Seurin D, Segovia-Quinson B, Hardouin S, Binoux M. Anal Biochem. 1986;154:138–143. doi: 10.1016/0003-2697(86)90507-5. [DOI] [PubMed] [Google Scholar]
- 21.Rohlmann A, Gotthardt M, Hammer R E, Herz J. J Clin Invest. 1998;101:689–695. doi: 10.1172/JCI1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schulte-Hermann R, Hoffmann V, Landgraf H. Chem–Biol Interact. 1980;31:301–311. doi: 10.1016/0009-2797(80)90018-6. [DOI] [PubMed] [Google Scholar]
- 23.Froesch E R, Schmid C, Schwander J, Zapf J. Annu Rev Physiol. 1985;47:443–467. doi: 10.1146/annurev.ph.47.030185.002303. [DOI] [PubMed] [Google Scholar]
- 24.Isaksson O G, Jansson J O, Gause I A. Science. 1982;216:1237–1239. doi: 10.1126/science.7079756. [DOI] [PubMed] [Google Scholar]
- 25.Schlechter N L, Russell S M, Spencer E M, Nicoll C S. Proc Natl Acad Sci USA. 1986;83:7932–7934. doi: 10.1073/pnas.83.20.7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D’Ercole A J, Stiles A D, Underwood L E. Proc Natl Acad Sci USA. 1984;81:935–939. doi: 10.1073/pnas.81.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones J I, Clemmons D R. Endocr Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 28.Stewart C E, Rotwein P. Physiol Rev. 1996;76:1005–1026. doi: 10.1152/physrev.1996.76.4.1005. [DOI] [PubMed] [Google Scholar]
- 29.Shea B T, Hammer R E, Brinster R L. Endocrinology. 1987;121:1924–1930. doi: 10.1210/endo-121-6-1924. [DOI] [PubMed] [Google Scholar]
- 30.Behringer R R, Lewin T M, Quaife C J, Palmiter R D, Brinster R L, D’Ercole A J. Endocrinology. 1990;127:1033–1040. doi: 10.1210/endo-127-3-1033. [DOI] [PubMed] [Google Scholar]
- 31.Frohman L A, Jansson J O. Endocr Rev. 1986;7:223–253. doi: 10.1210/edrv-7-3-223. [DOI] [PubMed] [Google Scholar]
- 32.Olchovsky D, Song J, Gelato M C, Sherwood J, Spatola E, Bruno J F, Berelowitz M. Mol Cell Endocrinol. 1993;93:193–198. doi: 10.1016/0303-7207(93)90123-2. [DOI] [PubMed] [Google Scholar]
- 33.Jansson J O, Ekberg S, Isaksson O G, Edén S. Endocrinology. 1984;114:1287–1294. doi: 10.1210/endo-114-4-1287. [DOI] [PubMed] [Google Scholar]