Abstract
Sleep disturbances are common in critically ill patients and have been characterised by numerous studies using polysomnography. Issues regarding patient populations, monitoring duration and timing (nocturnal versus continuous), as well as practical problems encountered in critical care studies using polysomnography are considered with regard to future interventional studies on sleep. Polysomnography is the gold standard in objectively measuring the quality and quantity of sleep. However, it is difficult to undertake, particularly in patients recovering from critical illness in an acute-care area. Therefore, other objective (actigraphy and bispectral index) and subjective (nurse or patient assessment) methods have been used in other critical care studies. Each of these techniques has its own particular advantages and disadvantages. We use data from an interventional study to compare agreement between four of these alternative techniques in the measurement of nocturnal sleep quantity. Recommendations for further developments in sleep monitoring techniques for research and clinical application are made. Also, methodological problems in studies validating various sleep measurement techniques are explored.
Current Controlled Trials ISRCTN47578325.
Introduction
Sleep disturbances are common in critically ill patients and they contribute to patient morbidity. Polysomnography studies in both ventilated and non-ventilated critical care patients demonstrate that these sleep disturbances are characterised by severe fragmentation with frequent arousals and awakenings. Sleep architecture is disrupted with a dominance of stage-1 and -2 non-rapid eye movement (NREM) sleep with reduced deeper phases of sleep (slow-wave sleep [SWS] and rapid eye movement [REM]). Critical care patients' sleep traverses the day-night interface, with approximately half of total sleep time occurring in the daytime [1,2]. Inter- and intra-patient variability also occurs; this is not surprising given the multiple causes of sleep disruption in this patient group. These include environmental factors [3,4], medication [5], ventilator [6], stress response, inflammatory response, and circadian rhythm disturbance factors [2]. To control for these co-variables, studies should involve relatively large patient numbers and be conducted over multiple days and nights. Polysomnography is the gold standard for monitoring the quantity and quality of a patient's sleep. However, polysomnography is technically difficult, especially in critical care (due to environmental and patient considerations). Also, there is clearly no role for polysomnography in the clinical evaluation of patients' sleep on a daily basis. Ultimately, we will rely on clinical evaluation methods to assess individual patients' sleep before deciding whether interventions such as hypnotic therapy are warranted and subsequently to review their efficacy. The Society of Critical Care Medicine guidelines on sedation monitoring recommend that sleep assessment be undertaken [7]. The guidelines recommend patient self-report, but if this is not possible nurse observation could be used.
This review examines the variety of objective and subjective sleep monitoring techniques available for both research and clinical evaluation and discusses their merits and limitations. We complement this review by including comparisons of nocturnal sleep quantity data from a randomised clinical trial of exogenous melatonin in critical care patients which used a number of the techniques discussed.
Materials and methods
Literature review
MEDLINE (1966 to April 2007), EMBASE (1974 to April 2007), and CINAHL (1982 to April 2007) databases were searched using the following terms, both as MeSH (Medical Subject Heading) headings and text words: 'sleep', 'sleep disorders', 'sleep deprivation', 'actigraphy', 'actimetry' and 'polysomnography', in combination with 'critical illness', 'intensive care', 'critical care', and 'intensive care unit'. Reference lists of all identified papers were also scanned for other relevant publications. Papers were restricted to those pertaining to sleep measurement in adult patients during their critical care admission and published in full in English.
Study comparing different sleep measurement techniques
In the context of a small randomised trial on the effect of melatonin on sleep in critical care patients (unpublished data), we investigated nocturnal sleep in 24 patients studied over the span of 4 nights who were being weaned from mechanical ventilation. The study design and patient characteristics are presented in the Appendix.
Due to the lack of the facilities required by polysomnography (in terms of both staff and equipment), sleep was measured using actigraphy (Actiwatch; Cambridge Neurotechnology Ltd., Cambridge, UK), bispectral index (BIS) (BIS XP, Quattro sensor; Aspect Medical Systems, Inc., Norwood, MA, USA), and nurse assessment and patient assessment (Richards-Campbell Sleep Questionnaire [RCSQ]). BIS data were downloaded every 5 seconds into a personal computer, and patients were recorded as asleep if BIS values were less than 80 [9]. Actigraphy was continuously recorded over the whole study period from the non-dominant hand in 30-second epochs. Delirium-positive patients using the Confusion Assessment Method for the Intensive Care Unit [10] were excluded from RCSQ evaluation. Nurse assessment of nocturnal sleep was by direct observation using hourly epochs according to the critical care unit's routine sleep monitoring.
Results of the four techniques for nocturnal sleep were expressed using as a common measure the sleep efficiency index (SEI) (total sleep time expressed as a ratio of time available for nocturnal sleep) in order to compare them. We defined nocturnal sleep as the 9 hours between 10 p.m. and 7 a.m. These times coincided with the commencement of the nocturnal quiet time on the unit and ended with the morning nursing staff change over and lights on. Although the RCSQ provides a five-component rating of nocturnal sleep, a total score can be calculated from the mean of total scores in the five domains. This total score has been used as a measure of SEI and has been validated versus polysomnography [11].
A convenience sample of 12 of the 24 patients underwent dynamometric measurement of grip strength (Jamar hydraulic hand dynamometer; Asimov Engineering Company, Los Angeles, CA, USA) upon study completion to provide an indication of neuromuscular weakness. The mean of three recordings was used, and results were expressed as the percentage of normal values from age- and gender-matched controls [12].
Agreement
Agreement between techniques was evaluated using the limits-of-agreement method [13]. This approach compares two techniques at a time and consists of the following:
(a) Drawing a simple scatterplot of the results of the two techniques for each patient with the line of equality (y = x). If the techniques have perfect agreement, all points should fall along this line.
(b) Drawing a graph of the differences between the results of the two techniques plotted against the average measurement value (Bland-Altman plot). From this plot, it is possible not only to evaluate the magnitude of the differences and thus decide on its clinical acceptability, but also to see whether the magnitude of the differences varies with the magnitude of the measurements (for example, increase in the differences with increase in the average values).
(c) From the mean and standard deviation (SD) of these differences, calculating the 95% limits of agreement (that is, the range within which 95% of the differences should lie: mean - 1.96 × SD, mean + 1.96 × SD).
Results
Studies included in literature review
Both objective and subjective monitoring techniques have been used to study sleep in critical care patients. Objective techniques include polysomnography, processed electroencephalograms (EEGs), and actigraphy, whereas subjective assessment usually relies on methods of nurse observation or patient self-report. Individual monitoring techniques are summarised in Table 1.
Table 1.
Summary of methods used in critical care for sleep measurement
| Instrument | Validity and reliability | Advantages | Disadvantages | Clinical application |
| Polysomnography | Gold standard Inter-rater reliability in critical care kappa = 0.79–0.83 [11,20,26,29] | Monitors sleep quantity and quality | Sleep technician needed continually during monitoring and to score results Significant setup time required Rater subjectivity, especially when scoring stage-1 sleep Potential for monitoring electrodes to adversely affect sleep in non-sedated patients Few critical care studies over multiple days Cost – expensive setup and maintenance Prone to patient dislodgement Prone to electrical interference Critical illness (for example, delirium may affect EEG) |
Not practical for routine clinical use |
| Bispectral index | All patients with BIS values of less than 80 were asleep [9] | Can be used by non-specialists Sensor easily applied Continuous attendance of technician not required Low cost once monitor purchased Trend screen provides quick view of immediate sleep quantity |
Prone to patient dislodgement Prone to electrical interference Some patients may find sensor intrusive Electromyogram activity may raise BIS value Need to download into personal computer for complete evaluation Critical illness (for example, delirium may affect EEG) |
Not practical for routine clinical use Validation and algorithm development required |
| Actigraphy | Correlation 0.72 to 0.98 versus polysomnography for total sleep time [60] Not validated versus polysomnography in critical care patients |
Non-intrusive Can be used by non-specialists Low cost once device purchased Allows continuous measurement over days to weeks Some actigraphs have a facility to measure light exposure simultaneously Robust – unlikely to be removed by patient |
Neuromuscular weakness increases risk of overestimating sleep quantity Nursing staff may remove and not replace watch during washing, and so on Periods of inactivity such as watching television scored as sleep |
Yes – but only for circadian rhythm monitoring |
| Patient assessment |
1. Verran/Snyder-Halpern Sleep Scale Convergent validity (r = 0.39) only when polysomnography awakenings of more than 4 minutes scored [21] No significant difference in total sleep time results compared to actigraphy [36] |
1–4. If capable, patient can compare baseline quality with that currently experienced Relatively quick to complete | 1–4. Cannot be used in cognitively impaired patients Memory problems may limit accuracy Patient perception of nocturnal sleep may be adversely affected by circadian rhythm abnormalities |
1,2,4. Yes – but exclude patients with delirium/dementia and beware of obvious patient sleep-state misperception |
| Patient assessment |
2. Hospital Anxiety and Depression Scale (sleep component) Not validated versus polysomnography [41] 3. Sleep in the Intensive Care Unit Questionnaire Not validated versus polysomnography [3] 4. Richards-Campbell Sleep Questionnaire Reliability (Cronbach's alpha = 0.90) Correlation = 0.58 with polysomnography sleep efficiency index in critical care patients [11] |
|||
| Nurse assessment |
1. Direct observation Direct observation at 5-minute intervals Observation significantly overestimated polysomnography total sleep time [19] Direct observation at 15-minute intervals. Nurses assessment of sleep state compared to polysomnography correct 81.9% of the time [22]. 2. Echols' Patient Sleep Behavioural Observation Tool Direct observation at 5-minute intervals. Moderate convergent validity demonstrated with polysomnography awakenings. Single trained observer [21] No significant difference in total sleep time results compared to actigraphy [36] 3. Richards-Campbell Sleep Questionnaire Reliability versus patients (Cronbach's alpha = 0.83–0.95) [44,75] |
1,3. Relatively easy to incorporate into routine nursing care 2. Attempts to qualify wake, non-rapid eye movement, and rapid eye movement sleep |
1–3. Overestimates total sleep time Frequent assessment required Risk of data loss due to other direct and indirect nurse activities 3. Relies on nursing staff being able to make an accurate report of the patient's total sleep quality |
1. Yes – but even with frequent assessment likely to overestimate total sleep time. This may limit its practicality and a compromise between frequency and accuracy will be necessary. 2. No – extensive observation required of eyelid positioning, respiration, and eye and body motility and responses 3. Yes – potentially the most useful sleep assessment tool currently available for clinical use |
BIS, bispectral index; EEG, electroencephalogram.
Twenty-seven studies in critical care patients which used objective sleep measurement techniques were identified. These were predominantly polysomnography studies [4,6,11,14-33] (Table 2); the remainder used actigraphy [34-36] and the BIS [37] (Table 3). There were 10 subjective sleep measurement studies [3,38-46] and these used a variety of nurse and patient assessment techniques (Table 4).
Table 2.
Polysomnography studies of sleep in critical care patients
| Author(s) (year) | Number of patients | Critical care population | Duration | Sedation | Number ventilated | Intervention monitored | Practical difficulties |
| Johns et al. (1974) [14] | 4 | Surgical | Continuously for first few days | Opioids and nocturnal hypnotics | Not stated | No | None identified |
| Karacan et al. (1974) [15] | 4 | Medical | Continuous × 24 to 108 hours | Majority nocturnal hypnotics | None | No | None identified |
| Hilton (1976) [16] | 10 | Medical | Continuous × 48 hours | Not stated | Not stated | No | Data incomplete for 3/10 patients |
| Orr and Stahl (1977) [17] | 9 | Surgical | 3–4 nights | Majority nocturnal opioids and/or benzodiazepines | Not stated | No | Considerable muscle artifact across all recording channels |
| Broughton and Baron (1978) [18] | 12 (10 reported) | Medical | Majority 9 nights but up to 13 | Majority nocturnal benzodiazepines and/or barbiturates | None | No | Two patients withdrew due to inconvenience of monitoring |
| Aurell and Elmqvist (1985) [19] | 9 | Surgical | Continuous × approximately 72 hours | Opioid and local analgesia, some benzodiazepines | 2/9 | No | None identified |
| Richards and Bairnsfathera (1988) [20] | 10 | Medical | 1–3 nights | Not stated | None | No | One patient withdrew from study after EEG electrodes were positioned |
| Fontaine (1989) [21] | 20 | Trauma | 1 night | All received opioid and nocturnal benzodiazepine | 1/20 | No | None identified |
| Edwards and Schuring (1993) [22] | 21 | Medical | 1 night | 18/21 nocturnal benzodiazepine/barbiturate | 20/21 | No | None identified |
| Gottschlich et al. (1994) [23] | 11 | Burns | Continuous × 24 hours (repeated intervals) | Not stated | All | No | None identified |
| Aaron et al. (1996) [24] | 6 | Medical | Continuous × 24 hours 2/6, × 48 hours 4/6 | 3/6 received hypnotics/opioids | None | No – effect of environmental disturbances recorded | None identified |
| Richards et al.a(1996) [25] | 9 | Medical | 1 night | 3/9 received nocturnalbenzodiazepines | None | No | None identified |
| Richardsb(1998) [26] | 69 | Medical | 1 night | Minority received nocturnal hypnotics | None | Yes – relaxation techniques | Standard sensitivity and paper speed settings for polygraph unavailable and were therefore altered; 23/94 refused most commonly due to study/polysomnography being an additional stressor. Only one patient could be studied per night.c |
| Cooper et al. (2000) [27] (20 reported) | 26 | Medical | Continuous × 24 hours | Majority received opioids, benzodiazepines or haloperidol | All | No | Six patient records unable to score due to technical difficulties: electrical artifact (4), respiratory artifact (2). |
| Richards et al.b(2000) [11] | 70 | Medical | 1 night | Minority received nocturnalhypnotics | None | No – see above | cSee above entry |
| Freedman et al. (2001) [28] | 22 | Medical | Continuous × 24 hours 14/22, × 48 hours 8/22 | 8 of 22 intermittent benzodiazepine or opioid | 20/22 | No – effect of environmental disturbances recorded | Five patient records unable to be scored due to sepsis-induced alterations to EEG pattern |
| Parthasarathy and Tobin (2002) [6] | 11 | Medical | 1 night | All received sedatives | All | Yes – mode of ventilation | None identified |
| Richards et al.b(2002) [29] | 64 | Medical | 1 night | Minority received nocturnal hypnotics | None | No – see above | cSee above entry |
| Valente et al. (2002) [30] | 24 | Neuro-Trauma | Continuous × 24 hours | At least 24 hours post-sedation discontinued | Not stated | No | None identified |
| Gabor et al. (2003) [4] | 7 | Medical/Trauma | Continuous × 24 hours | Majority opioids, benzodiazepines, and/or antipsychotics | All | No – effect of environmental disturbances recorded | None identified |
| Cochen et al. (2005) [31] | 17 | Medical/Trauma | 1–2 nights, some daytime | None | All | No | 4/31 sleep recordings not scored due to electrical artifact |
| Hardin et al. (2006) [32] | 18 | Medical | Continuous × 24 hours | 6/18 received intermittent sedation only and were awake and alert, 12/18 received continuous sedation | All | No – group comparison between neuromuscular blocking agents, continuous sedation, and intermittent sedation | Modified delta criteria used. Unknown quantity of epochs scored as non-classifiable. Recorder malfunctioned in one patient |
| Bosma et al. (2007) [33] | 13 | Medical/Surgical | 2 nights (crossover study) | 3/13 received opioids and 2 received All haloperidol | Yes – pressure support versus proportional assist controlled ventilation and patient-ventilator dysynchrony | None identified |
a,bMultiple reports refer to a single polysomnography study. EEG, electroencephalogram.
Table 3.
Actigraphy and bispectral index studies of sleep in critical care patients
| Authors (year) | Number of patients | Critical care population | Duration | Sedation | Number ventilated | Intervention monitored | Method/Practical difficulties |
| Shilo et al. (1999) [34] | 14 | Medical | Continuous × 72 hours | Not stated, no opioids | Not stated | No | Actigraphy/None identified |
| Shilo et al. (2000) [35] | 8 | Medical | Continuous × 72 hours | None | 4/8 | Yes – exogenous melatonin | Actigraphy/None identified |
| Kroon and West (2000) [36] | 13 | Medical | 1 night | None | None | No | Actigraphy/None identified |
| Nicholson et al. (2001) [37] | 29 (27 reported) | Medical/Surgical | 1 night | 23/27 received morphine and midazolam or propofol | 17/27 | No | Bispectral index/Two patients withdrew early in study |
Table 4.
Subjective studies of sleep in critical care patients
| Author(s) (year) | Number of patients | Critical care population | Duration | Sedation | Number ventilated | Intervention monitored | Method/Practical difficulties |
| Woods (1972) [38] | 4 | Surgical | 8 nights | Not stated | Not stated | No | Nurse observation (10-minute intervals)/Not stated |
| Helton et al. (1980) [39] | 62 | Medical/Surgical | Continuous × 5 days | Not stated | Not stated | No | Nurse observation (15-minute intervals) – interruptions recorded/Not stated |
| Williamson (1992) [40] | 60 | Surgical | 3 nights | Not stated | Not stated | Yes – ocean sounds (white noise) | RCSQ/Not stated |
| Treggiari-Venzi et al. (1996) [41] | 40 (32 reported) | Trauma/Surgical | 3 nights | Midazolam or propofol only | None | Yes – midazolam versus propofol on sleep quality | Hospital Anxiety and Depression Scale/Not stated |
| Freedman et al. (1999) [3] | 203 | Medical/Surgical | 1 night | Not stated | 32/203 | No – assessed environmental aetiologies of sleep disturbances | Sleep in the Intensive Care Unit Questionnaire/Not stated |
| Olson et al. (2001) [42] | 239 (Glasgow Coma Scale ≥ 10) | Medical/Surgical | Daily during monitoring periods (2 × 2 months) | Not stated | Not stated | Yes – effect of environmental controls | Nurse observation × 8 at predefined times/Not stated |
| Nelson et al. (2001) [43] | 100 | Medical | Multiple days | Three quarters received sedatives | 74/100 | No – assessed frequency of difficulty sleeping and related degree of stress | Edmonton Symptom Assessment Scale/Used verbal descriptions due to difficulties with visual analogue scale. Only 50% of patients were able to complete questionnaire. |
| Frisk and Nordstrom (2003) [44] | 31 | Medical/Surgical/Trauma | 1–2 nights | 12/31 received hypnotics | Not stated | No – but RCSQ scores lower in patients receiving hypnotics | RCSQ/Half of eligible patients were unable to complete questionnaire |
| Richardson (2003) [45] | 36 | Medical/Surgical | 3 nights | Not stated | Not stated | Yes – combined relaxation and guided imagery | Verran/Snyder-Halpern Sleep Scale/Some patients required assistance with the visual analogue scale |
| Ibrahim et al. (2006) [46] | 32 (27 reported) | Not stated | Minimum 2 nights | 14 received extra sedation or haloperidol | All | Yes – exogenous melatonin | Nurse observation (frequency not stated)/Not stated |
RCSQ, Richards-Campbell Sleep Questionnaire.
Agreement between sleep measurement techniques in interventional study
On 91 nights, data were available for evaluation. Missing data from the four sleep measurement methods are summarised in Table 5. Patient grip strength was a mean of 23.0% (95% confidence interval [CI], 10.1% to 35.9%) compared to age-and gender-matched controls.
Table 5.
Summary of missing data from pharmacological intervention study
| Method | Nights missing data | Reasons |
| Bispectral index | 11/91 (12.1%) Average 11.8 minutes lost per 9-hour night studied (2.2%) |
Patient removed sensor (4) Signal quality index low (3) Hardware failure (2) Patient refused (2) |
| Patient assessment (Richards-Campbell Sleep Questionnaire) | 17/91 (18.7%) | Delirium (16) Patient unable to complete (1) |
| Nurse assessment | 23/91 (25.3%) | Unable to evaluate (too busy, forgot, or unsure of sleep status) |
| Actigraphy | 0/91 (0%) | Not applicable |
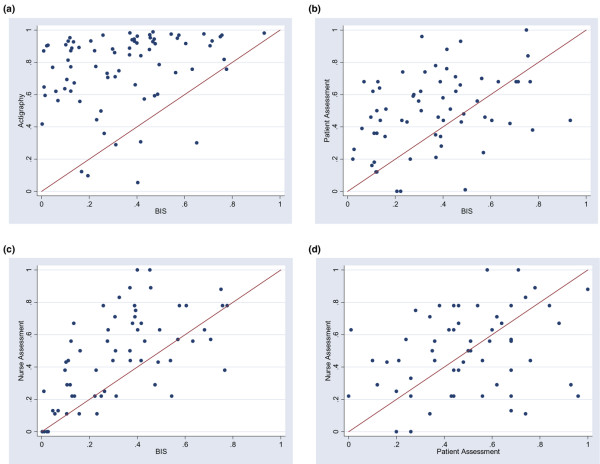
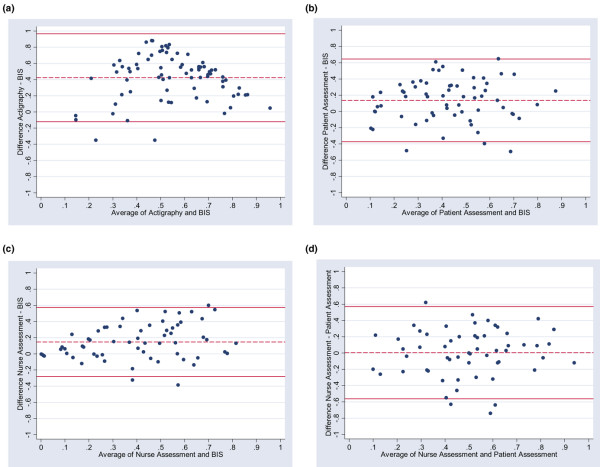
Agreement between sleep measurement techniques is graphically evaluated by scatterplots of the results of four different techniques used to measure nocturnal SEI in our intervention study (Figure 1) and Bland-Altman plots (Figure 2).
Figure 1.
Scatterplots of the results of four different techniques used to measure nocturnal sleep in our intervention studies: (a) bispectral index (BIS) quantity versus actigraphy, (b) BIS quantity versus patient assessment (Richards-Campbell Sleep Questionnaire), (c) BIS quantity versus nurse assessment, and (d) nurse assessment versus patient assessment.
Figure 2.
Bland-Altman plots. Horizontal lines are drawn at the mean difference and at the mean difference plus and minus 1.96 times the standard deviation of the differences: (a) bispectral index (BIS) quantity versus actigraphy, (b) BIS quantity versus patient assessment (Richards-Campbell Sleep Questionnaire), (c) BIS quantity versus nurse assessment, and (d) nurse assessment versus patient assessment.
Limits of agreement (upper limit and lower limit) for SEI for selected sleep measurement techniques were the following:
Actigraphy versus BIS (Figure 2a):
-0.12 (95% CI, -0.22 to -0.02) and 0.97 (95% CI, 0.87 to 1.06).
Patient assessment versus BIS (Figure 2b):
-0.37 (95% CI, -0.46 to -0.28) and 0.65 (95% CI, 0.56 to 0.74).
Nurse assessment versus BIS (Figure 2c):
-0.28 (95% CI, -0.36 to -0.21) and 0.57 (95% CI, 0.50 to 0.65).
Nurse assessment versus patient assessment (Figure 2d):
-0.56 (95% CI, -0.66 to -0.46) and 0.57 (95% CI, 0.47 to 0.67).
Objective measurements of sleep
Polysomnography
Polysomnography is the only method of sleep measurement that is capable of identifying individual sleep stages. It requires not only recording of the EEG but also polygraphic recording, including the electro-oculogram and electromyogram (EMG). With these recordings, it is possible to stage sleep using the Rechstaffen and Kales criteria into REM sleep and NREM or SWS (stages 1 to 4). However, the procedure is intensive on technician time and the equipment is costly. Precise and secure placement of the electrodes is required and normally takes a skilled technician approximately 1 hour. A trained physiological measurement technician should be available throughout the recording process to deal with technical problems, including replacing electrodes; interpretation and sleep staging of the recording can take up to 4 hours per sleep cycle. Computer programmes designed to perform time-saving sleep-stage analysis are commercially available. However, these programmes are generally considered inaccurate and manual sleep staging remains the preferred option. Even manual sleep staging may be subjective, particularly in identifying drowsiness and sleep onset in stage 1. All electrodes are glued to the skin with collodion, but the EMG electrodes (which are usually placed sub-mentally) are particularly vulnerable to dislodgement.
Reliability of the polysomnography recordings is reduced further in the hostile electrical environment of the critical care area. It can be difficult to eliminate 50-Hz electrical artifact caused by various essential items of electrical equipment being simultaneously used on the patient or indeed on other patients in the intensive care unit. Individuals subjected to polysomnography recording often find that the electrodes and recording equipment themselves have a disruptive effect on their sleep [11]. This is overcome in sleep laboratories by having acclimatisation nights. The latter have not been used routinely in critical care studies, and it could be argued that polysomnography equipment introduces yet another potential environmental disruption in non-sedated critical care patients. Additionally, they may impact on some nursing activities (for example, patient turning).
Critically ill patients frequently experience delirium [10], and therefore removal of one or more of the electrodes during the recording is a significant risk and the amount of sedation or antipsychotic therapy required in these patients may be increased by their presence. The support and financing of polysomnography in terms of sleep laboratory equipment and skilled staff, as well as the practical difficulties, have led investigators to adopt other techniques in critical care. Indeed, some studies have used portable polysomnographic equipment capable of providing simplified sleep characteristics such as total sleep time [47]. Such methods may provide a more feasible approach to future polysomnography studies in critical care patients. Since the advent of digital polygraphic recording, there is probably less variation in recording equipment used and modern equipment is less cumbersome than previously. The technical difficulties of undertaking polysomnography in critical care patients are frequently highlighted [1,27,36,37,46]. However, fewer than half of the studies using polysomnography identified any practical difficulties or loss of data (Table 2), which suggests that there is under-reporting of these difficulties in research studies.
The majority of polysomnography studies have been conducted in non-sedated critical care patients. Although there are some similarities between the states of sleep and sedation (for example, neurotransmitter pathways involved), there are also significant differences such as the lack of temporal or circadian cycling during sedation [2]. Despite these differences, a review of polysomnography sleep studies in critical care patients found reports of similar sleep disturbances in sedated and non-sedated populations [1].
The limitations of conventional sleep staging have been identified as a particular problem in critical care patients who demonstrate significantly disrupted sleep phases with complex electrophysiological changes [27,37]. The rapid fluctuations between EEG patterns of wakefulness, NREM 1 and 2 with REMs, and REM sleep without atonia are characteristic of status dissociaticus [31,48]. Status dissociaticus represents a significant breakdown in the clinical and polysomnographic markers of the three states of being (that is, REM sleep, NREM sleep, and wakefulness) [49]. It is possible that the combination of sleep disturbances and polypharmacy experienced by many critical care patients predisposes to this form of REM sleep behavioural disorder, which shares symptoms similar to delirium [50].
Studies published on the use of polysomnography in critical care patients tend to be very small, with only 1 out of 23 being completed in more than 25 critical care patients (published as three reports [11,26,29]) and the vast majority examining between 15 and 20 patients. In fact, only three polysomnography studies identified have examined the effect of an intervention [6,26,33]. The first large randomised controlled trial in 69 patients investigated back massage compared to standard nursing care [26] and suggested increased sleep quantity in the intervention group. A randomised crossover trial of 11 medical patients reported significant differences in the number of arousals and awakenings between pressure-support and assist-controlled ventilation modes within the same night [6]. Recently, another randomised crossover study in 13 patients found an increase in the number of nocturnal sleep arousals as a consequence of patient-ventilator dysynchrony [33]. The study by Bosma and colleagues [33] highlights the importance of study endpoint, as reduced ventilator dysynchrony improved sleep quality but had no effect on nocturnal sleep quantity.
Due to the known inter- and intra-patient variability in sleep, we are less clear as to the full benefits that might be observed if multiple nights were studied. In fact, less than half of the critical care studies reported multiple nights' data. Given the loss of the circadian rhythm of the sleep-wake cycle, continuous monitoring of sleep in these patients is important [15]. When the full 24 hours is considered, critical care patients may not have reduced total sleep time [1]. Five of the polysomnography studies [15,16,19,24,28] undertook continuous monitoring for 48 hours; only three studies [14,15,19] examined periods greater than this, totalling no more than 15 patients. Seven studies were undertaken in a single isolation room within the critical care unit [11,18,19,22,23,26,29] and may therefore be of limited applicability to general critical care practice.
In light of these studies, it is a significant challenge to design research studies examining the full effects of sleep interventions over multiple days, identifying appropriate endpoints and in a relatively large number of patients. Polysomnography is currently the definitive sleep monitoring technique, but it may not meet all our requirements for sleep research in critical care patients.
Bispectral index
A number of processed EEG monitoring devices have been developed for monitoring sedation in the anaesthesia and critical care environments. Of these, the BIS is the most studied for the measurement of sleep. The BIS is calculated from multiple analysis of the raw EEG waveform, including power spectral analysis, bispectral analysis, and time-based analysis for suppression/non-suppression. Multivariate statistical modelling of these key EEG factors was used to define an algorithm providing a scaled BIS value (index), which correlated with clinical depth of anaesthesia in volunteers. BIS values near 100 represent an 'awake' clinical state, whereas 0 equals EEG silence.
Studies of sleep using the BIS demonstrate that the BIS values fall during physiological sleep and rise during arousal but that there is significant overlap of values for a given sleep stage [9,51,52]. One group progressed to use BIS to investigate sleep in critical care patients [37]. They adopted BIS values from their previous study to classify patients as awake (more than 85), in light sleep (60 to 85), in SWS (less than 60), and in REM (BIS of more than 60 with reduced EMG). The study confirmed polysomnography findings that almost none of the intensive care patients displayed normal sleep. The sleep that did occur was reduced in quantity and that abnormal cyclical sleep occurred in approximately half of the patients studied [37].
BIS has been demonstrated to correlate with neurological status in non-sedated critically ill patients [53]. In patients with better neurological function, BIS values were higher. Therefore, neurological abnormalities (for example, traumatic brain injury) would be expected to reduce BIS values and therefore potentially provide an inaccurate indication of the patients' sleep characteristics. Also in studies of patients with dementia [54] and delirium [55], there is a decrease in fast-wave activity in the EEG and BIS values are reduced. Residual effects of sedative agents that may have accumulated in patients with renal and/or hepatic failure would also potentially affect any EEG-based analysis technique.
An advantage of BIS quantification of sleep versus polysomnography is that a technician does not need to be in attendance to ensure good recording. However, there are still potential problems with the practical application of BIS for this indication. Similar to traditional EEG, BIS is subject to electrical interference, and in a group of non-sedated patients, movement and particularly increased EMG activity adversely affect the signal quality index (SQI). Patient removal of the sensor remains a risk, although unlike polysom-nography electrodes, the sensor does not require a skilled technician to replace it.
Our missing data record demonstrates that transient drops in the SQI below 15 result in loss of some, albeit minimal (2%), BIS data in most patients. Three patients had insufficient SQIs that resulted in the loss of more than 2 hours of data and were excluded from analysis. Patient removal, refusal, and hardware failure also accounted for data loss on some nights.
A recent review concluded that the BIS is capable of detecting sleep, but the spread of overlap of BIS values for a given sleep stage prevents its current use as a depth-of-sleep monitor [56]. Nevertheless, it remains an attractive proposition as the continuous monitoring capabilities of BIS ultimately may better capture the dynamics of sleep [56]. It must be highlighted that algorithm developments for the BIS have been based primarily on depth of sedation in patients undergoing general anaesthesia. As previously noted, there are some similarities between sleep and sedation states, but also important differences [2]. Therefore, substantial algorithm development specifically in sleep monitoring is required before it can be used routinely in research studies for this purpose.
Actigraphy
An actigraph is a small wristwatch device that is capable of both sensing and storing information regarding patient movement. An accelerometer detects movement in two or three planes, which are then translated into digital counts during predefined epoch periods. The epoch length is the period of time over which the actigraphy data are averaged. The actigraph is capable of collecting data over extended periods before data are downloaded into a personal computer. Computer software based on validated algorithms translates the movement data into sleep-wake periods, which then can be analysed to provide data on various parameters such as the total sleep time, number and frequency of awakenings, and SEI. However, it does not provide any information related to the stage/quality of a patient's sleep.
A variety of commercial products exist as do the accompanying algorithms. Results from one actigraph/algorithm are not necessarily translatable to another [57]. Developments in actigraph hardware and software led the American Academy of Sleep Medicine to acknowledge its merit in measuring sleep variability over multiple nights and the efficacy of various interventions in insomniacs [58]. In healthy individuals, actigraphy is more accurate in recording total sleep time compared to subjective sleep assessment [59]. However, actigraphy still overestimates total sleep time compared to polysomnography, as it has a high sensitivity for detecting sleep, but is less reliable in detecting wakefulness (that is, reduced sleep specificity) [59]. That actigraphy overestimates total sleep time is not unexpected as it commences at an earlier phase of the sleep-onset process compared to polysomnography [60].
Compared to polysomnography, there are relatively few studies of sleep in critical care patients using actigraphy. In common with polysomnography studies, one report found that sleep was fragmented and limited to short periods of naps throughout the 24 hours [34]. Actigraphy also has been used to monitor the effects of a pharmacological intervention on the sleep characteristics of intensive care patients [35]. No studies have compared actigraphy versus polysomnography in measuring sleep quantity in critical care patients. It seems reasonable to expect that technology that detects movement and uses a predefined algorithm to convert into various sleep parameters may be less accurate in critical care patients. In fact, intensive care-acquired abnormalities of the neuromuscular system are associated with sepsis, certain drugs such as steroids [61], neuromuscular blockers, and severity of illness. Although these abnormalities may affect nerves, muscles, or both, myopathy is probably the most important problem. The reported occurrence of neuromuscular abnormality varies widely, from 33% to 82% [62-68], probably due to the variability in the methods used to diagnose the problem. Clinical studies such as that of De Jonghe and colleagues [61] used the Medical Research Council scale to clinically evaluate weakness when the patients were awake and found severe weakness in 25% of patients. Similarly, 26% of patients with two-organ failure due to sepsis or systemic inflammatory response syndrome developed severe weakness [69]. The incidence of mild or moderate weakness was far higher. Though limited, our grip strength data provided an estimate of the degree of neuromuscular weakness experienced by the critical care patients we studied. Hence, there is a significant risk that actigraphy will overestimate sleep quantity variables in the critical care population. We found that actigraphy overestimated the SEI compared to BIS, nurse, and patient assessments (data not shown). We therefore conclude that actigraphy should not be used with currently available technology to measure sleep in this population. However, actigraphy is particularly suited to patient rest-activity rhythm monitoring in this environment over protracted periods of time [59,70], where we are interested primarily in movement timing as opposed to amplitude.
Subjective measurements of sleep
Compared to polysomnography studies, reports of sleep assessment in critical care patients using subjective methods have evaluated much larger patient numbers, over more prolonged periods, and studied more interventions. In clinical practice, they offer the only real means of assessing the efficacy of interventions in attempting to improve individual patients' sleep.
Patient assessment
Using the patients' own assessment of their sleep during their critical care stay is attractive because the patient is best placed to be able to relate their chronic sleep quality and quantity with their acute illness. Indeed, sleep diaries are an important measure of many chronic sleep disturbances and their use in combination with actigraphy provides an assessment of sleep comparable to polysomnography [59]. However, the use of sleep diaries in critically ill patients is limited by the cognitive and physical capabilities of the patient. For these reasons, sleep diaries have not been adopted for critical care assessment of sleep and other measures of subjective sleep such as those based on visual analogue scales (VASs) have been developed.
Patients using the Verran/Snyder-Halpern Sleep Scale demonstrated a comparable assessment of their total sleep time when compared to actigraphy [36], but another patient group was found to be able to reliably judge only their frequency of awakenings compared to polysomnography when wake periods in excess of 4 minutes were evaluated [21].
The RCSQ [11] comprises five VASs. These cover the sleep domains of depth, latency, awakenings, percentage time awake, and quality of sleep. There was a moderate correlation between RCSQ and polysomnography SEI in one critical care group [11].
Patient sleep perception has been used as the endpoint in three interventional studies in critical care patients [40,41,45]. Patients in a critical care area who received nocturnal ocean sounds (white noise) rated their sleep by the RCSQ significantly better than those exposed to ambient sounds [40]. A comparison of overnight midazolam or propofol sedation reported no significant differences in sleep quality between the agents using the Hospital Anxiety and Depression Scale [41]. A combination of a relaxation and guided imagery intervention did not demonstrate a statistically significant benefit on critical care patients' self-report of sleep quality [45].
A problem with RCSQ when used in a critical care setting is that patients might not be able to complete the questionnaire, with reported failing rates up to 50% [44]. In our interventional study, almost 20% of patients were unable to complete the RCSQ primarily due to the presence of delirium. Also, some patients struggle to use VASs [71] and verbal descriptions have been adopted in another assessment of patients' sleep for this reason [43].
In our intervention study, we found that patient perception of sleep grossly differed from SEI by any other measures even when we excluded patients deemed unable to complete the RCSQ. Compared with BIS, RCSQ tended to overestimate nocturnal sleep efficiency. Patient assessment of sleep did not agree well with direct nurse observations either, which is in line with the findings of a previous report [38]. Patient sleep misperception is encountered in chronic insomniacs, and even non-delirious critical care patients may be particularly prone to perceptual difficulties due to memory problems. The complex pharmacokinetics and pharmacodynamics of the sedative drug regimes these patients receive, in tandem with multiple organ failure, have the potential to adversely affect patient assessment. Critical care patients may have memory problems as a direct consequence of sedative exposure [72], and even in patients with memories, these may be delusional [72,73]. Interestingly, memory processing appears to be sleep-dependent [74] and therefore critical care patients with their documented sleep disturbances may be particularly vulnerable to poor recall of their own sleep quality and quantity. Furthermore, patients may lack time cues for day and night and therefore struggle to identify when they actually slept. Finally, the circadian rhythm abnormalities these patients exhibit may further compound their difficulties in subjectively assessing their own nocturnal sleep.
Although patient assessment of sleep has been recommended [7], caution is required to exclude patients with acute cognitive dysfunction and obvious perceptual problems. This limits the application of tools such as the RCSQ in a significant number of critical care patients.
Nurse assessment
Nurse assessment of a patient's sleep is often the trigger used to identify patients with significant sleep disturbances in the clinical environment. Research studies in critical care have used direct nurse observation as well as a variety of scales and questionnaires. The frequency of sleep recording by direct observation has ranged from every 5 minutes to 8 times per day. Direct nurse observation has been used to assess sleep in two intervention studies [42,46]. During periods of reduced environmental noise and disturbances, patients were reported to have increased sleep quantity [42]. In the other study, exogenous melatonin was reported to have no effect on nocturnal or diurnal total sleep time [46]. Another study found that even at 5-minute intervals, nursing staff observation of total sleep time was significantly different compared to polysomnography and provided an overestimate [19]. In our study, we also found that direct nurse observation overestimated sleep efficiency in patients compared to BIS results. It is therefore possible that studies that purely rely on direct nurse observation may not be sensitive enough to detect some changes in sleep quantity due to a given intervention. In regard to the comparison of nurse assessment with patient RCSQ, there was no evidence of a tendency toward either overestimation or underestimation, but the agreement was poor (Figures 1d and 2d). Hourly sleep assessment by nurse observation forms part of our critical care unit's routine nocturnal observations. However, the reality of other direct and indirect nursing care activities will obviously affect the reliability of results. Due to frequent awakenings in these patients (particularly in those receiving mechanical ventilation), intensive observation is probably required for precise recording of sleep quantity [21]. Also, as emphasised by our missing data, there are occasions when the nursing staff experience difficulties in judging the patients' sleep status. Compared to polysomnography, nurses have been shown to correctly assess patients' sleep status 82% of the time [22]. However, this study also found that nurses were too busy or could not tell in almost 20% of the observations even over the relatively short period of the study (4 hours) [22]. Having the nursing staff use a sleep assessment tool such as the RCSQ may well be a better indicator of sleep parameters than purely relying on approximations of sleep quantity. In a study in which RCSQ was used by both patients and nurses, nurses have been shown to rate the RCSQ slightly higher than patients do, but the difference was not statistically significant, although comparison was made in only 13 patients [44]. The coefficient for reliability (Cronbach's alpha) for nurses using the RCSQ has been reported to be between 0.83 and 0.95 [44,75]. Use of the RCSQ by nurses may avoid the common limitations that critical care patients have in undertaking the scale accurately and may improve nurse assessment, but further validation is necessary.
Methodological problems of reviewed studies
A methodological pitfall common to almost all method comparison studies we reviewed relates to the statistical approach used to compare different techniques, and in particular the use of correlation coefficients. Although in medical literature the correlation coefficient (r) between the results of two measurement methods is often chosen as a measure of agreement, this approach has been shown to be inappropriate for a number of reasons [13]. First, r measures the strength of association between two variables and not their agreement. The hypothesis being tested is that there is no association, precisely no linear relationship (r = 0), between the measurements by the two methods, so that a very small p value indicates that indeed these measurements are related. However, it would be very surprising if they were not, given that they are designed to measure the same quantity, so that the statistical significance of their correlation is irrelevant to the question of agreement. Second, large values of r do not necessarily imply high agreement. As an extreme example, if a method tends to give values that are double those of the other method, the correlation between the measurements by the two methods would be very high but of course the agreement would not. Moreover, correlation depends on the range of the true quantity in the sample, with wide ranges giving greater correlations than narrow ranges, which has nothing to do with whether the true agreement is high or low.
What is the appropriate approach that should be taken when analysing results from method comparison studies on sleep? The answer mainly depends on the nature of the comparison, which can be either of the following:
(a) Comparison of two methods for measuring sleep, neither of which can be regarded as providing the true value (that is, both methods provide an approximate measure of sleep). This is the case for the comparisons in our interventional study in which all four techniques were approximate measures of sleep, and the best analytical approach is that based on the limits-of-agreement method as described above. The calculation of the limits of agreement assumes approximate normal distribution of the differences, which can be assessed graphically by drawing a histogram of the differences. More importantly, this calculation assumes that the mean and SD of the differences are constant (that is, do not depend on the magnitude of the measurement, which can be assessed graphically in the Bland-Altman plots). If indeed a trend is present, alternative methods have to be used [76].
(b) Comparison of a simpler approximate method with a very precise one, with the aim of assessing whether the two methods agree sufficiently for the simpler method to replace the precise one. In this case, the nature of the question is calibration of the simpler method against the 'exact' method rather than agreement. Standard regression analysis can be used to predict the measurement obtained by the reference method from the measurement obtained by the simpler method.
Conclusion
Polysomnography undoubtedly remains the gold standard for qualifying and quantifying sleep. However, the critical care environment provides many unique challenges and this has led to the use of alterative sleep assessment methods in research studies. All of these techniques have limitations and these should be anticipated in future interventional study designs. Of the alternative objective techniques, the BIS has particular advantages over actigraphy in this patient group. Further algorithm development of the BIS as a measure of sleep quantity may be a useful compromise and facilitate larger research studies over multiple days in critical care.
Clinically, patient self-assessment is attractive, though potentially misleading, and should be regarded with appropriate caution. Perhaps nurse assessment using a tool such as the RCSQ provides the most attractive way forward at this time. Clearly, there is room for further developments in the techniques for measuring sleep in the critical care patient. Concurrent assessment of sleep and delirium is particularly important if we are to appropriately guide pharmacological and non-pharmacological therapies.
The statistical methodology of future method comparison studies for sleep measurement should also be improved, and in particular the use of correlation coefficients should be avoided, in order to provide stronger evidence on the performance of difference methods.
Abbreviations
BIS = bispectral index; CI = confidence interval; EEG = electroencephalogram; EMG = electromyogram; NREM = non-rapid eye movement; RCSQ = Richards-Campbell Sleep Questionnaire; REM = rapid eye movement; SD = standard deviation; SEI = sleep efficiency index; SQI = signal quality index; SWS = slow-wave sleep; VAS = visual analogue scale.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
RSB conceived the clinical study, enrolled patients, collated the data, analysed and interpreted results, and conceived and undertook the literature review. CM completed the statistical analysis and contributed to the planning of the review. GHM participated in design of the clinical study and data analysis. RK provided methodological advice regarding EEG monitoring. All authors contributed to, read, and approved the final manuscript.
Appendix
Pharmacological intervention study design and patient characteristics
Randomised double-blind placebo-controlled trial in 24 intensive care patients. Patients were randomly assigned to melatonin 10 mg, administered enterally at 9 p.m. for 4 nights, or to matching placebo.
Inclusion criteria
Patients admitted to the adult general intensive care unit with acute respiratory failure, requiring mechanical ventilation and a tracheostomy to assist weaning.
Exclusion criteria
Expected length of stay of less than 5 further days, pre-admission treatment of sleep disturbances, not receiving target enteral feed volume or aspirates greater than 200 ml, previous history of convulsions or psychiatric or neurological disease, excessive alcohol consumption (more than or equal to 50 units per week), recreational drug use, sleep apnoea, and severe heart failure (New York Heart Association III/IV).
Sedative infusions were discontinued for at least 24 hours (propofol and alfentanil) or more than 36 hours (morphine and midazolam) with a Sedation Agitation Score (SAS) of greater than or equal to 4 [8]. No hypnotics were allowed during the study period; however, haloperidol was administered in patients with an SAS of greater than or equal to 6 (very agitated). Ear plugs and eye masks were made available for use at the patients' discretion each night.
A locally derived scale was used to provide details of environmental disturbances, and nurses subjectively ranked the noise level each night. Staff meetings and posters were employed to encourage staff to minimise environmental, nursing, and clinical disturbances during the nocturnal study periods. Baseline nocturnal illuminance at the head of each patient bed when all lights were off was recorded using a light meter (Luxmeter PU150; Eagle International, Wembley, UK). Mode of ventilation was also recorded hourly and ranked as low-flow/high-flow oxygen, external continuous positive airway pressure (CPAP), CPAP assisted spontaneous breathing, and bi-level positive airway pressure. Baseline characteristics of the study population are reported in Table A1.
Table A1.
Baseline characteristics of patients
| Baseline characteristics | Results |
| Male, number (percentage) | 11 (45.8) |
| Age in years, mean (SD) | 64.3 (13.28) |
| APACHE II at study entry, mean (SD) | 17.0 (3.55) |
| Actual body weight in kilograms, median (IQR) | 67.0 (61.0; 72.5) |
| Ideal body weight in kilograms, mean (SD) | 58.6 (6.70) |
| Body mass index, mean (SD) | 24.8 (3.91) |
| Normal sleep duration in hours, mean (SD) | 6.4 (1.81) |
| Time ventilated prior to study in days, mean (IQR) | 15.0 (10.0; 20.5) |
| Time since sedation stopped prior to study in days, mean (SD) | 7.0 (3.84) |
| Length of intensive care unit stay prior to study in days, median (IQR) | 16.5 (11.5; 21.0) |
| Delirium at baseline, number (percentage) | 5 (20.8) |
| Delirium at end of study, number (percentage) | 4 (16.7) |
| Ventilated at baseline, number (percentage) | 19 (79.2) |
| Ventilated at end of study, number (percentage) | 14 (58.3) |
Results are presented as number and percentage, mean and standard deviation (SD), or median and interquartile range (IQR), as appropriate. APACHE II, Acute Physiology and Chronic Health Evaluation II.
Acknowledgments
Acknowledgements
The randomised controlled trial was funded by the Sheffield Teaching Hospitals Department of Pharmacy and Medicines Management and Small Grants Scheme.
See related commentary by Watson, http://ccforum.com/content/11/4/159
Contributor Information
Richard S Bourne, Email: richard.bourne@sth.nhs.uk.
Cosetta Minelli, Email: cosetta.minelli@imperial.ac.uk.
Gary H Mills, Email: gary.mills@sth.nhs.uk.
Rosalind Kandler, Email: rosalind.kandler@sth.nhs.uk.
References
- Parthasarathy S, Tobin MJ. Sleep in the intensive care unit. Intensive Care Med. 2004;30:197–206. doi: 10.1007/s00134-003-2030-6. [DOI] [PubMed] [Google Scholar]
- Weinhouse GL, Schwab RJ. Sleep in the critically ill patient. Sleep. 2006;29:707–716. doi: 10.1093/sleep/29.5.707. [DOI] [PubMed] [Google Scholar]
- Freedman NS, Kotzer N, Schwab RJ. Patient perception of sleep quality and etiology of sleep disruption in the intensive care unit. Am J Respir Crit Care Med. 1999;159:1155–1162. doi: 10.1164/ajrccm.159.4.9806141. [DOI] [PubMed] [Google Scholar]
- Gabor JY, Cooper AB, Crombach SA, Lee B, Kadikar N, Bettger HE, Hanly PJ. Contribution of the intensive care unit environment to sleep disruption in mechanically ventilated patients and healthy subjects. Am J Respir Crit Care Med. 2003;167:708–715. doi: 10.1164/rccm.2201090. [DOI] [PubMed] [Google Scholar]
- Bourne RS, Mills GH. Sleep disruption in critically ill patients – pharmacological considerations. Anaesthesia. 2004;59:374–384. doi: 10.1111/j.1365-2044.2004.03664.x. [DOI] [PubMed] [Google Scholar]
- Parthasarathy S, Tobin MJ. Effect of ventilator mode on sleep quality in critically ill patients. Am J Respir Crit Care Med. 2002;166:1423–1429. doi: 10.1164/rccm.200209-999OC. [DOI] [PubMed] [Google Scholar]
- Jacobi J, Fraser GL, Coursin DB, Riker RR, Fontaine D, Wittbrodt ET, Chalfin DB, Masica MF, Bjerke HS, Coplin WM, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30:119–141. doi: 10.1097/00003246-200201000-00020. [DOI] [PubMed] [Google Scholar]
- Riker RR, Picard JT, Fraser GL. Prospective evaluation of the Sedation-Agitation Scale for adult critically ill patients. Crit Care Med. 1999;27:1325–1329. doi: 10.1097/00003246-199907000-00022. [DOI] [PubMed] [Google Scholar]
- Tung A, Lynch JP, Roizen MF. Use of the BIS monitor to detect onset of naturally occurring sleep. J Clin Monit Comput. 2002;17:37–42. doi: 10.1023/A:1015404803637. [DOI] [PubMed] [Google Scholar]
- Ely EW, Margolin R, Francis J, May L, Truman B, Dittus R, Speroff T, Gautam S, Bernard GR, Inouye SK. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) Crit Care Med. 2001;29:1370–1379. doi: 10.1097/00003246-200107000-00012. [DOI] [PubMed] [Google Scholar]
- Richards KC, O'Sullivan PS, Phillips RL. Measurement of sleep in critically ill patients. J Nurs Meas. 2000;8:131–144. [PubMed] [Google Scholar]
- Mathiowetz V, Kashman N, Volland G, Weber K, Dowe M, Rogers S. Grip and pinch strength: normative data for adults. Arch Phys Med. 1985;66:69–74. [PubMed] [Google Scholar]
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- Johns MW, Large AA, Masterton JP, Dudley HA. Sleep and delirium after open heart surgery. Br J Surg. 1974;61:377–381. doi: 10.1002/bjs.1800610513. [DOI] [PubMed] [Google Scholar]
- Karacan I, Green JR, Jr, Taylor WJ, Williams JC, Eliot RS, Williams RL, Thornby JI, Salis PJ. Sleep in post-myocardial infarction patients. In: Eliot RS, editor. Stress and the Heart. New York, NY: Futura Publishing Co; 1974. pp. 163–195. [Google Scholar]
- Hilton BA. Quantity and quality of patients' sleep and sleep-disturbing factors in a respiratory intensive care unit. J Adv Nurs. 1976;1:453–468. doi: 10.1111/j.1365-2648.1976.tb00932.x. [DOI] [PubMed] [Google Scholar]
- Orr WC, Stahl ML. Sleep disturbances after open heart surgery. Am J Cardiol. 1977;39:196–201. doi: 10.1016/S0002-9149(77)80191-4. [DOI] [PubMed] [Google Scholar]
- Broughton R, Baron R. Sleep patterns in the intensive care unit and on the ward after acute myocardial infarction. Electroencephalogr Clin Neurophysiol. 1978;45:348–360. doi: 10.1016/0013-4694(78)90187-6. [DOI] [PubMed] [Google Scholar]
- Aurell J, Elmqvist D. Sleep in the surgical intensive care unit: continuous polygraphic recording of sleep in nine patients receiving postoperative care. Br Med J (Clin Res Ed) 1985;290:1029–1032. doi: 10.1136/bmj.290.6474.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards KC, Bairnsfather L. A description of night sleep patterns in the critical care unit. Heart Lung. 1988;17:35–42. [PubMed] [Google Scholar]
- Fontaine DK. Measurement of nocturnal sleep patterns in trauma patients. Heart Lung. 1989;18:402–410. [PubMed] [Google Scholar]
- Edwards GB, Schuring LM. Pilot study: validating staff nurses' observations of sleep and wake states among critically ill patients, using polysomnography. Am J Crit Care. 1993;2:125–131. [PubMed] [Google Scholar]
- Gottschlich MM, Jenkins ME, Mayes T, Khoury J, Kramer M, Warden GD, Kagan RJ. The 1994 Clinical Research Award. A prospective clinical study of the polysomnographic stages of sleep after burn injury. J Burn Care Rehabil. 1994;15:486–492. doi: 10.1097/00004630-199411000-00005. [DOI] [PubMed] [Google Scholar]
- Aaron JN, Carlisle CC, Carskadon MA, Meyer TJ, Hill NS, Millman RP. Environmental noise as a cause of sleep disruption in an intermediate respiratory care unit. Sleep. 1996;19:707–710. doi: 10.1093/sleep/19.9.707. [DOI] [PubMed] [Google Scholar]
- Richards KC, Curry N, Lyons W, Todd B. Cardiac dysrhythmia during sleep in the critically ill: a pilot study. Am J Crit Care. 1996;5:26–33. [PubMed] [Google Scholar]
- Richards KC. Effect of a back massage and relaxation intervention on sleep in critically ill patients. Am J Crit Care. 1998;7:288–299. [PubMed] [Google Scholar]
- Cooper AB, Thornley KS, Young GB, Slutsky AS, Stewart TE, Hanly PJ. Sleep in critically ill patients requiring mechanical ventilation. Chest. 2000;117:809–818. doi: 10.1378/chest.117.3.809. [DOI] [PubMed] [Google Scholar]
- Freedman NS, Gazendam J, Levan L, Pack AI, Schwab RJ. Abnormal sleep/wake cycles and the effect of environmental noise on sleep disruption in the intensive care unit. Am J Respir Crit Care Med. 2001;163:451–457. doi: 10.1164/ajrccm.163.2.9912128. [DOI] [PubMed] [Google Scholar]
- Richards KC, Anderson WM, Chesson AL, Jr, Nagel CL. Sleep-related breathing disorders in patients who are critically ill. J Cardiovasc Nurs. 2002;17:42–55. doi: 10.1111/j.0889-7204.2002.00927.x. [DOI] [PubMed] [Google Scholar]
- Valente M, Placidi F, Oliveira AJ, Bigagli A, Morghen I, Proietti R, Gigli GL. Sleep organization pattern as a prognostic marker at the subacute stage of post-traumatic coma. Clin Neurophysiol. 2002;113:1798–1805. doi: 10.1016/S1388-2457(02)00218-3. [DOI] [PubMed] [Google Scholar]
- Cochen V, Arnulf I, Demeret S, Neulat ML, Gourlet V, Drouot X, Moutereau S, Derenne JP, Similowski T, Willer JC, et al. Vivid dreams, hallucinations, psychosis and REM sleep in Guillain-Barre syndrome. Brain. 2005;128:2535–2545. doi: 10.1093/brain/awh585. [DOI] [PubMed] [Google Scholar]
- Hardin KA, Seyal M, Stewart T, Bonekat HW. Sleep in critically ill chemically paralyzed patients requiring mechanical ventilation. Chest. 2006;129:1468–1477. doi: 10.1378/chest.129.6.1468. [DOI] [PubMed] [Google Scholar]
- Bosma K, Ferreyra G, Ambrogio C, Pasero D, Mirabella L, Braghiroli A, Appendini L, Mascia L, Ranieri VM. Patient-ventilator interaction and sleep in mechanically ventilated patients: pressure support versus proportional assist ventilation. Crit Care Med. 2007;35:1048–1054. doi: 10.1097/01.CCM.0000260055.64235.7C. [DOI] [PubMed] [Google Scholar]
- Shilo L, Dagan Y, Smorjik Y, Weinberg U, Dolev S, Komptel B, Balaum H, Shenkman L. Patients in the intensive care unit suffer from severe lack of sleep associated with loss of normal melatonin secretion pattern. Am J Med Sci. 1999;317:278–281. doi: 10.1097/00000441-199905000-00002. [DOI] [PubMed] [Google Scholar]
- Shilo L, Dagan Y, Smorjik Y, Weinberg U, Dolev S, Komptel B, Shenkman L. Effect of melatonin on sleep quality of COPD intensive care patients: a pilot study. Chronobiol Int. 2000;17:71–76. doi: 10.1081/CBI-100101033. [DOI] [PubMed] [Google Scholar]
- Kroon K, West S. 'Appears to have slept well': assessing sleep in an acute care setting. Contemp Nurse. 2000;9:284–294. doi: 10.5172/conu.2000.9.3-4.284. [DOI] [PubMed] [Google Scholar]
- Nicholson T, Patel J, Sleigh JW. Sleep patterns in intensive care unit patients: a study using the Bispectral Index. Crit Care Resusc. 2001;3:86–91. [PubMed] [Google Scholar]
- Woods NF. Patterns of sleep in postcardiotomy patients. Nurs Res. 1972;21:347–352. doi: 10.1097/00006199-197207000-00012. [DOI] [PubMed] [Google Scholar]
- Helton MC, Gordon SH, Nunnery SL. The correlation between sleep deprivation and the intensive care unit syndrome. Heart Lung. 1980;9:464–468. [PubMed] [Google Scholar]
- Williamson J. The effects of ocean sounds on sleep after coronary artery bypass graft surgery. Am J Crit Care. 1992;1:91–97. [PubMed] [Google Scholar]
- Treggiari-Venzi M, Borgeat A, Fuchs-Buder T, Gachoud JP, Suter PM. Overnight sedation with midazolam or propofol in the ICU: effects on sleep quality, anxiety and depression. Intensive Care Med. 1996;22:1186–1190. doi: 10.1007/BF01709334. [DOI] [PubMed] [Google Scholar]
- Olson D, Borel C, Laskowitz D, Moore D, McConnell E. Quiet time: a nursing intervention to promote sleep in neurocritical care units. Am J Crit Care. 2001;10:74–78. [PubMed] [Google Scholar]
- Nelson JE, Meier DE, Oei EJ, Nierman DM, Senzel RS, Manfredi PL, Davis SM, Morrison RS. Self-reported symptom experience of critically ill cancer patients receiving intensive care. Crit Care Med. 2001;29:277–282. doi: 10.1097/00003246-200102000-00010. [DOI] [PubMed] [Google Scholar]
- Frisk U, Nordstrom G. Patients' sleep in an intensive care unit – patients' and nurses' perception. Intensive Crit Care Nurs. 2003;19:342–349. doi: 10.1016/S0964-3397(03)00076-4. [DOI] [PubMed] [Google Scholar]
- Richardson S. Effects of relaxation and imagery on the sleep of critically ill adults. Dimens Crit Care Nurs. 2003;22:182–190. doi: 10.1097/00003465-200307000-00009. [DOI] [PubMed] [Google Scholar]
- Ibrahim MG, Bellomo R, Hart GK, Norman TR, Goldsmith D, Bates S, Egi M. A double-blind placebo-controlled randomised pilot study of nocturnal melatonin in tracheostomised patients. Crit Care Resusc. 2006;8:187–191. [PubMed] [Google Scholar]
- Resta O, Guido P, Foschino Barbaro MP, Picca V, Talamo S, Lamorgese V. Sleep-related breathing disorders in acute respiratory failure assisted by non-invasive ventilatory treatment: utility of portable polysomnographic system. Respir Med. 2000;94:128–134. doi: 10.1053/rmed.1999.0682. [DOI] [PubMed] [Google Scholar]
- Mahowald MW, Schenck CH. Status dissociatus – A perspective on states of being. Sleep. 1991;14:69–79. doi: 10.1093/sleep/14.1.69. [DOI] [PubMed] [Google Scholar]
- Mahowald M, Schenck C. Dissociated states of wakefulness and sleep. Neurology. 1992;42:44–51. [PubMed] [Google Scholar]
- Mahowald MW. Parasomnias. Med Clin North Am. 2004;88:669–678. doi: 10.1016/j.mcna.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Sleigh JW, Andrzejowski J, Steyn Ross A, Steyn Ross M. The bispectral index: a measure of depth of sleep? Anesth Analg. 1999;88:659–661. doi: 10.1097/00000539-199903000-00035. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuijs D, Coleman EL, Douglas NJ, Drummond GB, Dahan A. Bispectral index values and spectral edge frequency at different stages of physiologic sleep. Anesth Analg. 2002;94:125–129. doi: 10.1097/00000539-200201000-00024. [DOI] [PubMed] [Google Scholar]
- Gilbert TT, Wagner MR, Halukurike V, Paz HL, Garland A. Use of bispectral electroencephalogram monitoring to assess neurologic status in unsedated, critically ill patients. Crit Care Med. 2001;29:1996–2000. doi: 10.1097/00003246-200110000-00024. [DOI] [PubMed] [Google Scholar]
- Renna M, Handy J, Shah A. Low baseline bispectral index of the electroencephalogram in patients with dementia. Anesth Analg. 2003;96:1380–1385. doi: 10.1213/01.ANE.0000059223.78879.0F. [DOI] [PubMed] [Google Scholar]
- Ely EW, Truman B, Manzi DJ, Sigl JC, Shintani A, Bernard GR. Consciousness monitoring in ventilated patients: bispectral EEG monitors arousal not delirium. Intensive Care Med. 2004;30:1537–1543. doi: 10.1007/s00134-004-2298-1. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuijs DJF. Processed EEG in natural sleep. Baillieres Best Pract Res Clin Anaesthesiol. 2006;20:49–56. doi: 10.1016/j.bpa.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Lichstein KL, Stone KC, Donaldson J, Nau SD, Soeffing JP, Murray D, Lester KW, Aguillard RN. Actigraphy validation with insomnia. Sleep. 2006;29:232–239. [PubMed] [Google Scholar]
- Littner M, Kushida CA, Anderson WM, Bailey D, Berry RB, Davila DG, Hirshkowitz M, Kapen S, Kramer M, Loube D, et al. Practice parameters for the role of actigraphy in the study of sleep and circadian rhythms: an update for 2002. Sleep. 2003;26:337–341. doi: 10.1093/sleep/26.3.337. [DOI] [PubMed] [Google Scholar]
- Ancoli IS, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- Tryon WW. Issues of validity in actigraphic sleep assessment. Sleep. 2004;27:158–165. doi: 10.1093/sleep/27.1.158. [DOI] [PubMed] [Google Scholar]
- De Jonghe B, Sharshar T, Lefaucheur J-P, Authier F-J, Durand-Zaleski I, Boussarsar M, Cerf C, Renaud E, Mesrati F, Carlet J, et al. Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA. 2002;288:2859–2867. doi: 10.1001/jama.288.22.2859. [DOI] [PubMed] [Google Scholar]
- Witt N, Zochodne D, Bolton C, Grand'Maison F, Wells G, Young G, Sibbald W. Peripheral nerve function in sepsis and multiple organ failure. Chest. 1991;99:176–184. doi: 10.1378/chest.99.1.176. [DOI] [PubMed] [Google Scholar]
- Coakley JH, Nagendran K, Honavar M, Hinds CJ. Preliminary observations on the neuromuscular abnormalities in patients with organ failure and sepsis. Intensive Care Med. 1993;19:323–328. doi: 10.1007/BF01694705. [DOI] [PubMed] [Google Scholar]
- Leijten FS, Harinck-de Weerd JE, Poortvliet DC, de Weerd AW. The role of polyneuropathy in motor convalescence after prolonged mechanical ventilation. JAMA. 1995;274:1221–1225. doi: 10.1001/jama.274.15.1221. [DOI] [PubMed] [Google Scholar]
- Leijten FS, De Weerd AW, Poortvliet DC, De Ridder VA, Ulrich C, Harink-De Weerd JE. Critical illness polyneuropathy in multiple organ dysfunction syndrome and weaning from the ventilator. Intensive Care Med. 1996;22:856–861. doi: 10.1007/BF02044107. [DOI] [PubMed] [Google Scholar]
- Berek K, Margreiter J, Willeit J, Berek A, Schmutzhard E, Mutz NJ. Polyneuropathies in critically ill patients: a prospective evaluation. Intensive Care Med. 1996;22:849–855. doi: 10.1007/BF02044106. [DOI] [PubMed] [Google Scholar]
- Coakley JH, Nagendran K, Yarwood GD, Honavar M, Hinds CJ. Patterns of neurophysiological abnormality in prolonged critical illness. Intensive Care Med. 1998;24:801–807. doi: 10.1007/s001340050669. [DOI] [PubMed] [Google Scholar]
- de Letter MA, Schmitz PI, Visser LH, Verheul FA, Schellens RL, Op de Coul DA, van der Meche FG. Risk factors for the development of polyneuropathy and myopathy in critically ill patients. Crit Care Med. 2001;29:2281–2286. doi: 10.1097/00003246-200112000-00008. [DOI] [PubMed] [Google Scholar]
- Bednarik J, Lukas Z, Vondracek P. Critical illness polyneuromyopathy: the electrophysiological components of a complex entity. Intensive Care Med. 2003;29:1505–1514. doi: 10.1007/s00134-003-1858-0. [DOI] [PubMed] [Google Scholar]
- Vinzio S, Ruellan A, Perrin AE, Schlienger JL, Goichot B. Actigraphic assessment of the circadian rest-activity rhythm in elderly patients hospitalized in an acute care unit. Psychiatry Clin Neurosci. 2003;57:53–58. doi: 10.1046/j.1440-1819.2003.01079.x. [DOI] [PubMed] [Google Scholar]
- Simpson T, Lee ER. Individual factors that influence sleep after cardiac surgery. Am J Crit Care. 1996;5:182–189. [PubMed] [Google Scholar]
- Samuelson K, Lundberg D, Fridlund B. Memory in relation to depth of sedation in adult mechanically ventilated intensive care patients. Intensive Care Med. 2006;32:660–667. doi: 10.1007/s00134-006-0105-x. [DOI] [PubMed] [Google Scholar]
- Jones C, Griffiths RD, Humphris G. Disturbed memory and amnesia related to intensive care. Memory. 2000;8:79–94. doi: 10.1080/096582100387632. [DOI] [PubMed] [Google Scholar]
- Walker MP, Stickgold R. Sleep, memory, and plasticity. Annu Rev Psychol. 2006;57:139–166. doi: 10.1146/annurev.psych.56.091103.070307. [DOI] [PubMed] [Google Scholar]
- Richards K. Techniques for measurement of sleep in critical care. Focus Crit Care. 1987;14:34–40. [PubMed] [Google Scholar]
- Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8:135–160. doi: 10.1191/096228099673819272. [DOI] [PubMed] [Google Scholar]