Abstract
While Dicer alone has been shown to form stable complexes with double-stranded RNAs and short interfering RNAs, its interactions with single-stranded RNAs (ssRNAs) have not been characterized. Here, we show that recombinant human Dicer alone can bind 21-nt ssRNAs in vitro, independent of their sequence and structure. We also demonstrate that Dicer binds ssRNAs having a 5’-phosphate with greater affinity versus those with a 5’-hydroxyl. In addition, 3’-biotinylated ssRNAs are bound by Dicer with lower affinity than 3’-hydroxyl ssRNAs. The stability of ssRNA-Dicer complexes was found to depend on divalent cations. Together, our results suggest a role for the PAZ domain of Dicer in binding ssRNAs and may indicate roles for Dicer in cellular function beyond those currently known.
Introduction
RNA interference (RNAi) is an evolutionarily conserved mode of gene regulation and host defense in eukaryotes [1,2]. This mechanism of gene silencing is induced either by endogenously-generated precursor microRNAs (pre-miRNAs) or exogenously-introduced double stranded RNAs (dsRNAs). Both pre-miRNAs and exogenous dsRNAs serve as substrates for Dicer, a cytoplasmic RNase III family protein [3,4], that cleaves them into ~21 nt miRNAs and short interfering RNAs (siRNAs), respectively. Unlike Drosophila, in which separate Dicer proteins generate miRNAs and siRNAs [5], humans have one Dicer enzyme that performs both functions [4]. Human Dicer is a multi-domain protein with an N-terminal RNA helicase/ATPase domain, a domain of unknown function (DUF 283), a Piwi Argonaute Zwille (PAZ) domain, two RNAse III domains (RIIIa and RIIIb), and a double-stranded RNA binding domain (dsRBD) [6,7]. The functions of the DUF 283 domain, which has a fold similar to a dsRBD [8], and the helicase/ATPase domain have not yet been described. The RNase domains cleave the target dsRNA into siRNAs and miRNAs with characteristic 2 nt overhangs at each 3’ end [9,10]. The C-terminal dsRBD and the PAZ domain orient the dsRNA for cleavage at the proper locations to generate the characteristic miRNA/siRNA structure [10].
The PAZ domain is highly conserved and is found only in Dicer and Argonaute family proteins, including Ago2, the key catalytic component of RISC [11-14]. The PAZ domain of Dicer plays a central role in dsRNA substrate recognition and binding, resulting in the preference of Dicer for substrate dsRNAs possessing 3’ overhangs as compared to blunt end substrates [10,15,16]. Structural studies using an isolated Drosophila Ago2 PAZ domain have revealed an oligonucleotide-binding fold that is known to bind single-stranded nucleic acids [17-19]. Binding studies have demonstrated PAZ domain binding to siRNAs and also 21-nt single-stranded RNAs (ssRNAs) [19]. PAZ domains from different human Argonaute proteins have also been shown to bind to ssRNAs [20]. As the Dicer PAZ domain has been suggested to bind to the single-stranded 3’ ends of dsRNA and the Argonaute PAZ domain binds ssRNAs, we investigated the ability of the full-length, purified human Dicer to bind 21-nt ssRNAs. We show that Dicer alone can bind 21-nt ssRNAs, independent of their sequence and predicted secondary structure.
Materials and methods
RNA and labeling
HPLC purified RNAs were purchased from Invitrogen (Carlsbad, CA) or Dharmacon (Lafayette, CO). Lyophilized RNAs were resuspended to 100 μM in TE (pH 8.0) and stored at -80°C. RNAs were 5’-labeled with 33P-γ-ATP (Perkin Elmer Life and Analytical Sciences, Boston, MA) using T4 polynucleotide kinase (New England Biolabs, Ipswich, MA). Labeled strands were purified from unincorporated label using G-25 Sephadex columns (Roche Applied Science, Indianapolis, IN). For nonisotopic labeling, unlabeled ATP (Roche Applied Science, Indianapolis, IN) was substituted for 33P-γ-ATP. The ssRNA and siRNA sequences used were: siRNA, 5’–GCUGACCCUGAAGUUCAUCUU-3’ (Sense strand), 5’-GAUGAACUUCAGGGUCA GCUU-3’ (Antisense strand); ssRNA-SS1 5’ –GUCACAUUGCCCAAGUCUCTT-3’; ssRNA- SS2, 5’- UUUUUUUUUUUUUUUUUUUTT-3’. For experiments requiring 3’-biotinylated ssRNAs, SS2 was ordered with a 3’-biotin. Secondary structures and melting temperatures for ssRNAs were predicted using mfold [21,22].
Dicer-RNA binding assays
Recombinant human Dicer (Invitrogen) was used in all the binding reactions. Dicer binding assays were carried out in 30 mM Tris-HCl (pH=8.0), 250 mM NaCl, 2.5 mM MgCl2, and 0.02 mM EDTA. Labeled ssRNA (9 nM) or siRNA (9 nM) and Dicer (200 nM, unless otherwise specified) (Invitrogen) were incubated at 4°C for 2 hours in 10 μl reaction volumes. For antibody supershift assays, either rabbit polyclonal antibody to Dicer (Abcam, Cambridge, MA) or control antibody to NF-kB was incubated with Dicer at 4°C for 3 hours after which labeled RNA was added and incubated for 2 additional hours. Samples were electrophoresed at 150 V for 1.5 hours at 4°C on 4-20 % TBE gels (Bio-Rad). Gels were dried under vacuum at 80°C, exposed to storage phosphor screens, and imaged on a Storm 860 imager (GE Healthcare/Amersham Biosciences, Piscataway, NJ). Binding reactions to test divalent cation dependence were carried out with the appropriate divalent cation substitution for Mg2+ in the binding buffer. Mass spectrometry was performed on gel-purified protein by the Michigan State University Research Technology Support Facility.
Results
ssRNA-Dicer complex formation in vitro
Dicer is known to form stable complexes with dsRNAs and siRNAs [23-25], though for siRNAs some conflicting reports exist [24,26]. To study whether Dicer would also form stable complexes with ssRNAs, binding reactions were set up with 5’-33P ssRNAs and Dicer at 4°C (Fig. 1A). As a control, we verified complex formation of Dicer with an siRNA (Fig. 1A, lane 2). Two bands were seen in the case of siRNA-Dicer binding under identical binding conditions, suggesting the possible presence of ssRNA in the siRNA preparation. Faster migration of the siRNA-Dicer complex relative to the ssRNA-Dicer complex is due to the increase in negative charge on the complex (due to the backbone phosphates of the second strand) with a relatively small change in molecular size/weight [27].
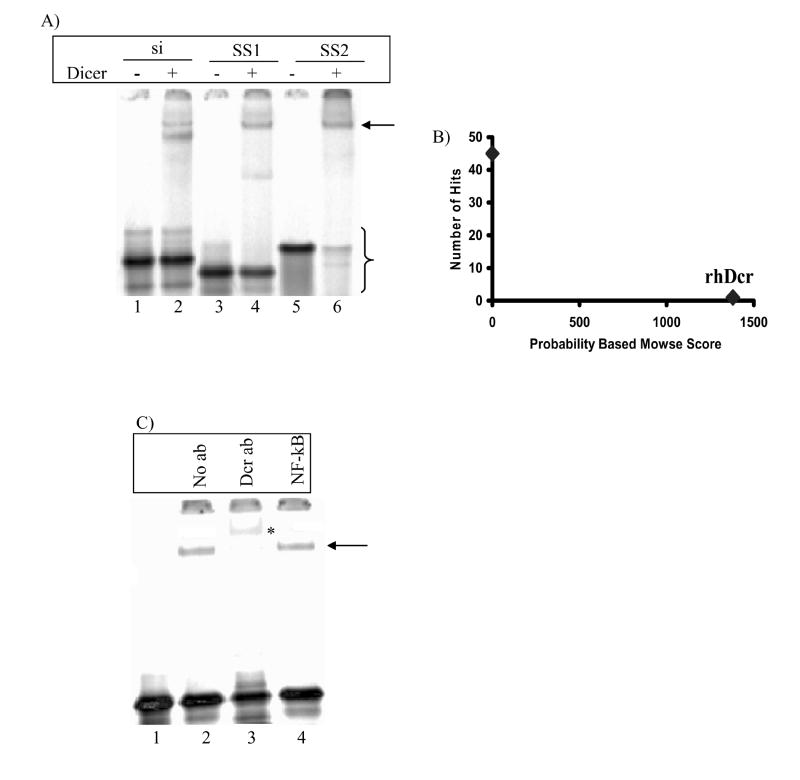
Fig. 1. In vitro binding of Dicer with siRNA and ssRNAs.
(A) Electrophoretic mobility shift assay of the complex formed between Dicer and, siRNA (lane 2), structured ssRNA SS1 (lane 4), and unstructured ssRNA SS2 (lane 6). The arrow indicates the position of the Dicer-ssRNA complex. Curly brace indicates unbound siRNAs and ssRNAs. (B) Mass spectrometry of the protein-ssRNA complex denoted by the arrow in (A) showed only the presence of Dicer in the complex. (C) Electrophoretic mobility shift assay of Dicer-SS2 complex in the presence of Dicer antibody (lane 3) and antibody against NF-kB (lane 4). The asterisk denotes the supershifted complex. Bands running ahead of unbound ssRNAs may reflect some RNase contamination in the binding reactions.
Because Dicer is naturally a dsRNA-binding protein, the impact of secondary structure of an ssRNA on the ability of Dicer to bind it was expected to be significant. We tested an ssRNA predicted to have a weak secondary structure at 4°C (SS1) and a polyuridine ssRNA (SS2) that is presumably unable to form any significant secondary structure even at 4°C. The reduced electrophoretic motility of unbound SS2 relative to unbound SS1 suggests the general absence of structure in SS2 (Fig. 1A, compare lanes 3 and 5), supporting our expectations from the Tm calculations (Table 1). Both SS1 and SS2 are bound stably by Dicer (Fig. 1A, lanes 4 and 6) as are other 21-nt ssRNA sequences (Table 1). Identical ssRNA-Dicer complexes were also formed with 12 and 15-nt ssRNAs (data not shown).
Table 1.
Predicted ΔG and Tm for tested sequences*.
| Name | Sequence | ΔG, kcal/mole | Tm, °C |
|---|---|---|---|
| SS1 | GUC ACA UUG CCC AAG UCU CTT | 0.7 | 20 |
| SS2 | UUU UUU UUU UUU UUU UUU UTT | - | - |
| SS3 | GCU AAA AAA AAA AAA AAA ATT | 1.94 | -19.3 |
| SS4 | GUC AAA AAA AAA AAA AAA ATT | 1.94 | -19.3 |
| SS5 | GCU GAC CCU GAA AUU GAU CTT | -0.61 | 32.2 |
| SS6 | GAG ACU UGG GCA AUG UGA CUT | -1.62 | 36.7 |
Though not shown due to space limitations and redundancy, binding results for sequences SS3-SS6 were similar to results for SS1 and SS2.
The ssRNA-Dicer complex formed by SS1 with Dicer was analyzed by mass spectrometry to confirm the presence of Dicer as the single, major protein component (Fig. 1B). To confirm further that the complex contained Dicer, an antibody supershift assay was performed. Adding Dicer antibody subsequent to Dicer-RNA binding did not produce any shift in the complex, so assays were performed by prior incubation of the antibody with Dicer followed by addition of SS1. This implies that this antibody is a competitive inhibitor of the binding by Dicer of ssRNAs. In the presence of the Dicer antibody, the characterized complex is nearly completely retarded as compared to negative and non-specific antibody controls (Fig. 1C., compare lane 3 with lanes 2 and 4).
Divalent cation dependence of ssRNA-Dicer complex formation
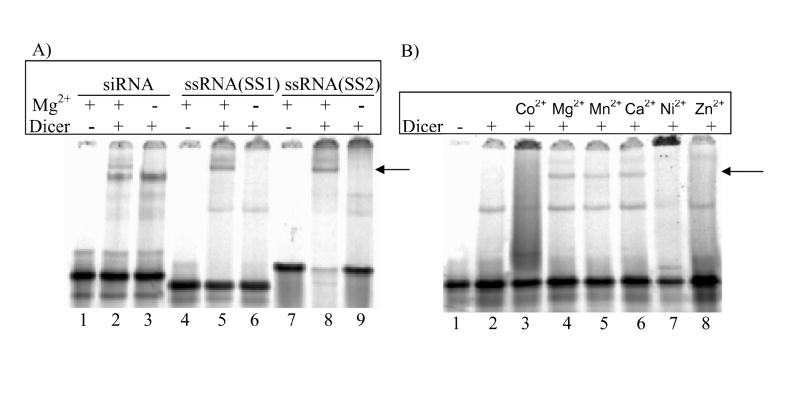
Though Dicer requires the presence of Mg2+ to be catalytically-active [23], it has been shown to form stable complexes with 100-130 bp dsRNAs even in the absence of Mg2+ [23,24]. Other divalent cations like Mn2+ and Co2+ can substitute for Mg2+ in supporting the catalytic activity of Dicer [28]. In E. coli RNase III, Mg2+ and Ca2+ act to stabilize complex formation of the enzyme with the bacteriophage T7 R1.1 RNA, which has a hairpin structure [29]. To test if ssRNA-Dicer complex formation depends on the presence of divalent cations, binding reactions were performed in Mg2+-free buffers. In the absence of Mg2+, Dicer did not stably bind either ssRNA (Fig. 2A, lanes 6 and 9, arrow). Complex formation was restored when Mn2+ or Ca2+ were added to the buffer but not Co2+, Ni2+, or Zn2+ (Fig. 2B, arrow). Similar divalent cation dependence was not observed for the formation of a stable siRNA-Dicer complex (Fig. 2A, lane 3). Both in the presence and absence of divalent cations, ssRNAs appear to form smaller complexes (Fig. 2B, lane 2 and 8), but this binding could not be assigned to a specific protein after analysis by mass spectrometry (Fig. S1).
Fig. 2. Divalent cation dependence of Dicer-ssRNA complex formation.
(A) Dicer-ssRNA complexes formed in the presence of Mg2+ (lanes 5 and 8) and not in its absence (lanes 6 and 9). siRNA-Dicer complex formation was not cation dependent (lanes 2 and 3). (B) Dicer-ssRNA(SS1) complex formation can occur in the presence of Mg2+,Mn2+, and Ca2+ but not Co2+, Ni2+, or Zn2+.
Possible contribution of the PAZ domain to ssRNA binding by Dicer
Chemically synthesized siRNAs possess a 5’-OH moiety. Upon entry into cells, these siRNAs are immediately phosphorylated by the human RNA kinase hClp1 [30,31]. siRNAs with a chemical modification that prevents 5’ phosphorylation are bound by Dicer with significantly lower affinity [25]. To assess the impact of 5’-phosphates on complex formation between ssRNAs and Dicer, we performed a competition assay between labeled ssRNAs in the presence of an excess of unlabeled ssRNAs and siRNAs. At a 100-fold excess concentration, neither 5’-OH ssRNAs nor 5’-OH siRNAs could displace 5’-PO3 ssRNA SS2 from its complex with Dicer (Fig. 3, lanes 3 and 4). However, phosphorylated strands effectively displaced bound SS2 (Fig. 3, lanes 5 and 6).
Fig. 3. Impact of 5’-phosphate on Dicer binding affinity for ssRNAs and siRNAs.
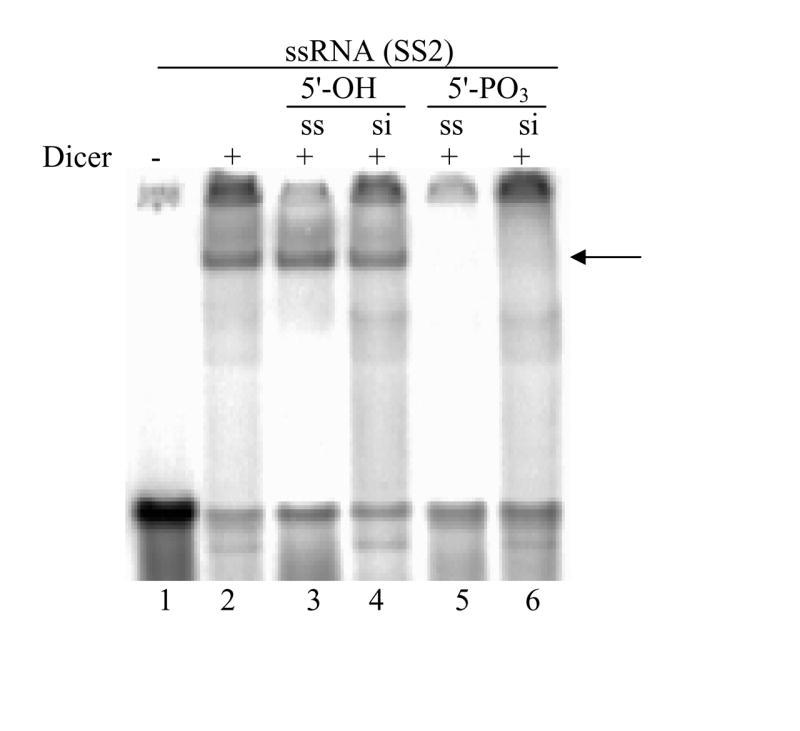
Dicer-ssRNA binding reactions were performed in the presence of 100-fold excess unlabeled ssRNA (lanes 3 and 5) or siRNA (lanes 4 and 6). In lanes 3 and 4, competition was performed with 5’-hydroxyl RNAs. In lanes 5 and 6, competition was performed with 5’-phosphate RNAs. The arrow indicates the position of the Dicer-ssRNA complex.
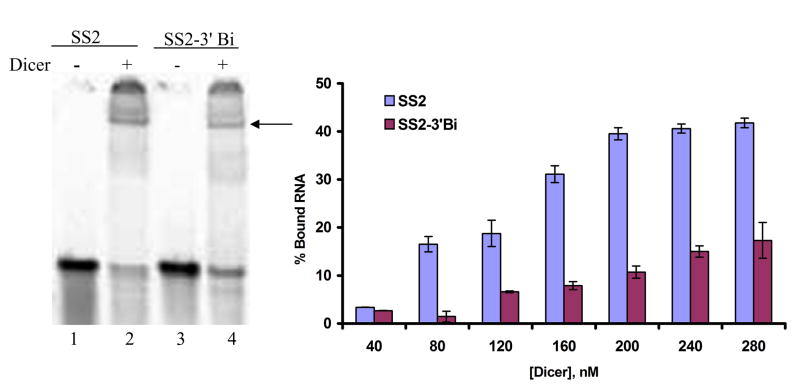
Of the domains in Dicer, only the PAZ domain is known to possess a greater affinity for 5’-PO3 ssRNAs as compared to 5’-OH ssRNAs [20]. To explore if the higher affinity for 5’-PO3 substrates seen in ssRNA-Dicer binding was due to the influence of the Dicer PAZ domain, binding to SS2 with a 5’-PO3 ssRNA and a 3’-biotin was tested. It is known that this modification disturbs PAZ domain-RNA interactions [20]. We also find that, at multiple Dicer concentrations, Dicer has a lower affinity for this sequence relative to 3’-OH SS2 (Fig. 4), further suggesting a role for the PAZ domain in binding to the ssRNAs.
Fig. 4. Dicer has lower affinity for ssRNAs having a 3’-biotin.
(A) Representative figure showing Dicer-ssRNA binding with ssRNA having a 3’-OH (lane 2) and ssRNA having a 3’-biotin (lane 4). (B) Dependence on Dicer concentration of Dicer binding to ssRNA having a 3’-biotin or a 3’-OH. Percentage of bound RNA was calculated by normalizing intensity of ssRNA-Dicer complexes to their respective unbound RNA at each concentration of Dicer.
Discussion
We have shown that recombinant human Dicer alone can bind ssRNAs in vitro and that binding is independent of the sequence and secondary structure of the ssRNA. In all experiments, the ssRNAs tested were far shorter than hairpin structures with stems of at least 29 bp in length that are known to be Dicer substrates [32]. Nonetheless, the results from our competition experiments suggest that Dicer binding of both siRNAs and ssRNAs may occur at the same site. Though our data strongly suggest that the PAZ domain is involved in binding, the other characterized domains in Dicer may also participate in the interaction.
We have demonstrated that binding of ssRNAs by Dicer depends upon the presence of divalent cations. Dicer and other RNase III family enzymes need one Mg2+ ion for each of the RNase domains to be catalytically active [33]. Moreover, recent structural evidence suggests that two metal ions may be required for maximum activity [28,34]. The second metal ion is suggested to bind to the highly negative catalytic valley of RNase III enzymes and thereby stabilize the interaction between the enzyme and the negatively charged phosphate backbone of substrate RNA [34]. It may therefore be that the divalent cation dependence we see for ssRNA binding is related to an interaction of the RNase domains of Dicer with the ssRNAs. Each of these domains may simultaneously, and possibly cooperatively, contribute to the affinity of Dicer for ssRNAs. Recent evidence for dsRNA-Dicer complex formation supports this possibility [16].
It is unclear if binding of ssRNAs to Dicer would occur in vivo, and, if so, what the biological relevance of such an interaction would be. One necessary feature of active silencers is the presence of a 5’-phosphate on the guide strand [31]. siRNAs possessing a 5’ end that is impaired for phosphorylation lack the ability to bind Dicer or induce RNAi [25,31]. The ability of Dicer to distinguish phosphorylated and non-phosphorylated targets may be a mechanism for controlling the loading of RISC. It may also suggest a pathway by which ssRNAs can enact RNAi [35,36]. In those cases, only ssRNAs possessing a 5’-phosphate, or that are 5’-phosphorylated upon entering the cell, act as potent inhibitors. These would be bound more tightly by Dicer, enhancing their association with other RISC proteins such as Ago 2, TRBP, and PACT [26,37]. Another possibility is that Dicer binding to ssRNAs protects them from degradative RNases and thereby improves their half-life and likelihood to be incorporated into RISC. Recent evidence also points to the possible involvement of Dicer or Drosha in the processing of single-stranded RNA substrates into PIWI-interacting RNAs (piRNAs) [38,39], though this is still up for debate [40].
Both the RNA binding domains of Dicer, the PAZ domain and the dsRBD, are involved in the binding of siRNAs and longer dsRNAs with overhangs [10]. The PAZ domain binds to the overhangs and positions the dsRBD and concomitantly the RNase III domains [23,25]. It has been reported that the dsRBD can only bind dsRNAs [41,42]. This suggests that ssRNA complex formation with Dicer is mediated at least in part by the PAZ domain. 5’-phosphate dependence of the 21-nt ssRNAs for binding with Dicer shown in this research is identical to the binding behavior of the 5-nt ssRNAs to the Ago2 PAZ domain [20]. Though we cannot currently rule out other interactions, our studies strongly indicate the involvement of the Dicer PAZ domain in binding ssRNAs.
Supplementary Material
Only proteins of human origin (i.e., contamination due to material and sample handling) could be identified in the lower, faster moving bands.
Acknowledgments
We thank all the members of Chan and Walton Lab for their advice and support, and Doug Whitten from MSU Proteomics Facility for assistance with the mass spectrometry. Financial support for this work was provided in part by the National Science Foundation (#0425821), the National Institutes of Health (#CA126136, #GM079688), and Michigan State University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–31. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 2.Cullen BR. RNA interference: antiviral defense and genetic tool. Nat Immunol. 2002;3:597–9. doi: 10.1038/ni0702-597. [DOI] [PubMed] [Google Scholar]
- 3.Ketting RF, Fischer SE, Bernstein E, Sijen T, Hannon GJ, Plasterk RH. Dicer functions in RNA interference and in synthesis of small RNA involved in developmental timing in C. elegans. Genes Dev. 2001;15:2654–9. doi: 10.1101/gad.927801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409:363–6. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- 5.Lee YS, Nakahara K, Pham JW, Kim K, He Z, Sontheimer EJ, Carthew RW. Distinct roles for Drosophila Dicer-1 and Dicer-2 in the siRNA/miRNA silencing pathways. Cell. 2004;117:69–81. doi: 10.1016/s0092-8674(04)00261-2. [DOI] [PubMed] [Google Scholar]
- 6.Hammond SM. Dicing and slicing: the core machinery of the RNA interference pathway. FEBS Lett. 2005;579:5822–9. doi: 10.1016/j.febslet.2005.08.079. [DOI] [PubMed] [Google Scholar]
- 7.Cook A, Conti E. Dicer measures up. Nat Struct Mol Biol. 2006;13:190–2. doi: 10.1038/nsmb0306-190. [DOI] [PubMed] [Google Scholar]
- 8.Dlakic M. DUF283 domain of Dicer proteins has a double-stranded RNA-binding fold. Bioinformatics. 2006;22:2711–4. doi: 10.1093/bioinformatics/btl468. [DOI] [PubMed] [Google Scholar]
- 9.Lee Y, Jeon K, Lee JT, Kim S, Kim VN. MicroRNA maturation: stepwise processing and subcellular localization. EMBO J. 2002;21:4663–70. doi: 10.1093/emboj/cdf476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang H, Kolb FA, Jaskiewicz L, Westhof E, Filipowicz W. Single processing center models for human Dicer and bacterial RNase III. Cell. 2004;118:57–68. doi: 10.1016/j.cell.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 11.Meister G, Landthaler M, Dorsett Y, Tuschl T. Sequence-specific inhibition of microRNA- and siRNA-induced RNA silencing. RNA. 2004;10:544–50. doi: 10.1261/rna.5235104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rivas FV, Tolia NH, Song JJ, Aragon JP, Liu J, Hannon GJ, Joshua-Tor L. Purified Argonaute2 and an siRNA form recombinant human RISC. Nat Struct Mol Biol. 2005;12:340–9. doi: 10.1038/nsmb918. [DOI] [PubMed] [Google Scholar]
- 13.Miyoshi K, Tsukumo H, Nagami T, Siomi H, Siomi MC. Slicer function of Drosophila Argonautes and its involvement in RISC formation. Genes Dev. 2005;19:2837–48. doi: 10.1101/gad.1370605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sen GL, Blau HM. Argonaute 2/RISC resides in sites of mammalian mRNA decay known as cytoplasmic bodies. Nat Cell Biol. 2005;7:633–6. doi: 10.1038/ncb1265. [DOI] [PubMed] [Google Scholar]
- 15.Vermeulen A, Behlen L, Reynolds A, Wolfson A, Marshall WS, Karpilow J, Khvorova A. The contributions of dsRNA structure to Dicer specificity and efficiency. RNA. 2005;11:674–82. doi: 10.1261/rna.7272305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacRae IJ, Zhou K, Doudna JA. Structural determinants of RNA recognition and cleavage by Dicer. Nat Struct Mol Biol. 2007;14:934–40. doi: 10.1038/nsmb1293. [DOI] [PubMed] [Google Scholar]
- 17.Song JJ, Smith SK, Hannon GJ, Joshua-Tor L. Crystal structure of Argonaute and its implications for RISC slicer activity. Science. 2004;305:1434–7. doi: 10.1126/science.1102514. [DOI] [PubMed] [Google Scholar]
- 18.Theobald DL, Mitton-Fry RM, Wuttke DS. Nucleic acid recognition by OB-fold proteins. Annu Rev Biophys Biomol Struct. 2003;32:115–33. doi: 10.1146/annurev.biophys.32.110601.142506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lingel A, Simon B, Izaurralde E, Sattler M. Structure and nucleic-acid binding of the Drosophila Argonaute 2 PAZ domain. Nature. 2003;426:465–9. doi: 10.1038/nature02123. [DOI] [PubMed] [Google Scholar]
- 20.Yan KS, Yan S, Farooq A, Han A, Zeng L, Zhou MM. Structure and conserved RNA binding of the PAZ domain. Nature. 2003;426:469–74. doi: 10.1038/nature02129. [DOI] [PubMed] [Google Scholar]
- 21.Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003;31:3406–15. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathews DH, Sabina J, Zuker M, Turner DH. Expanded sequence dependence of thermodynamic parameters improves prediction of RNA secondary structure. J Mol Biol. 1999;288:911–40. doi: 10.1006/jmbi.1999.2700. [DOI] [PubMed] [Google Scholar]
- 23.Zhang H, Kolb FA, Brondani V, Billy E, Filipowicz W. Human Dicer preferentially cleaves dsRNAs at their termini without a requirement for ATP. EMBO J. 2002;21:5875–85. doi: 10.1093/emboj/cdf582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Provost P, Dishart D, Doucet J, Frendewey D, Samuelsson B, Radmark O. Ribonuclease activity and RNA binding of recombinant human Dicer. EMBO J. 2002;21:5864–74. doi: 10.1093/emboj/cdf578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pellino JL, Jaskiewicz L, Filipowicz W, Sontheimer EJ. ATP modulates siRNA interactions with an endogenous human Dicer complex. RNA. 2005;11:1719–24. doi: 10.1261/rna.2102805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chendrimada TP, Gregory RI, Kumaraswamy E, Norman J, Cooch N, Nishikura K, Shiekhattar R. TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature. 2005;436:740–4. doi: 10.1038/nature03868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu D, Regnier FE. Native protein separations and enzyme microassays by capillary zone and gel electrophoresis. Anal Chem. 1993;65:2029–35. doi: 10.1021/ac00063a017. [DOI] [PubMed] [Google Scholar]
- 28.MacRae IJ, Zhou K, Li F, Repic A, Brooks AN, Cande WZ, Adams PD, Doudna JA. Structural basis for double-stranded RNA processing by Dicer. Science. 2006;311:195–8. doi: 10.1126/science.1121638. [DOI] [PubMed] [Google Scholar]
- 29.Li H, Nicholson AW. Defining the enzyme binding domain of a ribonuclease III processing signal. Ethylation interference and hydroxyl radical footprinting using catalytically inactive RNase III mutants. EMBO J. 1996;15:1421–33. [PMC free article] [PubMed] [Google Scholar]
- 30.Weitzer S, Martinez J. The human RNA kinase hClp1 is active on 3’ transfer RNA exons and short interfering RNAs. Nature. 2007;447:222–6. doi: 10.1038/nature05777. [DOI] [PubMed] [Google Scholar]
- 31.Nykanen A, Haley B, Zamore PD. ATP requirements and small interfering RNA structure in the RNA interference pathway. Cell. 2001;107:309–21. doi: 10.1016/s0092-8674(01)00547-5. [DOI] [PubMed] [Google Scholar]
- 32.Siolas D, Lerner C, Burchard J, Ge W, Linsley PS, Paddison PJ, Hannon GJ, Cleary MA. Synthetic shRNAs as potent RNAi triggers. Nat Biotechnol. 2005;23:227–31. doi: 10.1038/nbt1052. [DOI] [PubMed] [Google Scholar]
- 33.Sun W, Pertzev A, Nicholson AW. Catalytic mechanism of Escherichia coli ribonuclease III: kinetic and inhibitor evidence for the involvement of two magnesium ions in RNA phosphodiester hydrolysis. Nucleic Acids Res. 2005;33:807–15. doi: 10.1093/nar/gki197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ji X. Structural basis for non-catalytic and catalytic activities of ribonuclease III. Acta Crystallogr D Biol Crystallogr. 2006;62:933–40. doi: 10.1107/S090744490601153X. [DOI] [PubMed] [Google Scholar]
- 35.Schwarz DS, Hutvagner G, Haley B, Zamore PD. Evidence that siRNAs function as guides, not primers, in the Drosophila and human RNAi pathways. Mol Cell. 2002;10:537–48. doi: 10.1016/s1097-2765(02)00651-2. [DOI] [PubMed] [Google Scholar]
- 36.Holen T, Amarzguioui M, Babaie E, Prydz H. Similar behaviour of single-strand and double-strand siRNAs suggests they act through a common RNAi pathway. Nucleic Acids Res. 2003;31:2401–7. doi: 10.1093/nar/gkg338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kok KH, Ng MH, Ching YP, Jin DY. Human TRBP and PACT Directly Interact with Each Other and Associate with Dicer to Facilitate the Production of Small Interfering RNA. J Biol Chem. 2007;282:17649–57. doi: 10.1074/jbc.M611768200. [DOI] [PubMed] [Google Scholar]
- 38.Aravin A, et al. A novel class of small RNAs bind to MILI protein in mouse testes. Nature. 2006;442:203–7. doi: 10.1038/nature04916. [DOI] [PubMed] [Google Scholar]
- 39.MacRae IJ, Doudna JA. Ribonuclease revisited: structural insights into ribonuclease III family enzymes. Curr Opin Struct Biol. 2007;17:138–45. doi: 10.1016/j.sbi.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 40.Lin H. piRNAs in the germ line. Science. 2007;316:397. doi: 10.1126/science.1137543. [DOI] [PubMed] [Google Scholar]
- 41.Ryter JM, Schultz SC. Molecular basis of double-stranded RNA-protein interactions: structure of a dsRNA-binding domain complexed with dsRNA. EMBO J. 1998;17:7505–13. doi: 10.1093/emboj/17.24.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bevilacqua PC, George CX, Samuel CE, Cech TR. Binding of the protein kinase PKR to RNAs with secondary structure defects: role of the tandem A-G mismatch and noncontiguous helixes. Biochemistry. 1998;37:6303–16. doi: 10.1021/bi980113j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Only proteins of human origin (i.e., contamination due to material and sample handling) could be identified in the lower, faster moving bands.