Abstract
Moenomycin inhibits bacterial growth by blocking the transglycosylase activity of class A penicillin-binding proteins (PBPs), which are key enzymes in bacterial cell wall synthesis. We compared the binding affinities of moenomycin A with various truncated PBPs by using surface plasmon resonance analysis and found that the transmembrane domain is important for moenomycin binding. Full-length class A PBPs from 16 bacterial species were produced, and their binding activities showed a correlation with the antimicrobial activity of moenomycin against Enterococcus faecalis and Staphylococcus aureus. On the basis of these findings, a fluorescence anisotropy-based high-throughput assay was developed and used successfully for identification of transglycosylase inhibitors.
Keywords: penicillin-binding proteins, transglycosylase inhibitors, fluorescence anisotropy
Many common bacterial pathogens, such as Staphylococcus aureus, Streptococcus pneumoniae, and Enterococcus faecalis, have become multidrug-resistant and have emerged as a public health concern, creating an urgent need for new antibiotics.
The bacterial cell wall, or its peptidoglycan synthesis pathway, has been targeted for the development of antibacterial agents (1). The synthesis of peptidoglycan consists of several steps, including the formation of lipid I and lipid II, followed by the final transglycosylation and transpeptidation of lipid II to form peptidoglycan (1, 2). Many current antibiotics are β-lactam derivatives that target transpeptidation. To our knowledge, no medicines have yet been developed to inhibit the transglycosylation process. The only known potent inhibitors for transglycosylase (TG) are moenomycin complexes (flavomycin), including moenomycin A (Moe A) (Fig. 1A, compound 1), A12, C1, C3, and C4 (3, 4). Among these, Moe A is the most abundant agent in its family (3, 4). The unique antibacterial properties of Moe A have prompted chemists to synthesize moenomycin fragments and derivatives (5–7) in an attempt to develop new antibiotics. Recently, the total synthesis of Moe A (8), and of its biosynthesis pathway (9), has been reported. However, due to poor bioavailability, flavomycin is currently used only as a growth promoter in animal feeds (10).
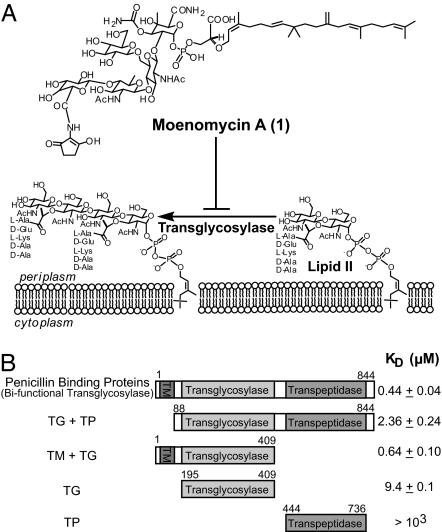
Fig. 1.
Inhibition of TG by moenomycin and binding affinities of truncated PBP variants. (A) Moe A (1) inhibits the transglycosylation step in bacterial cell wall synthesis. (B) Schematic representation of PBP variants used for moenomycin binding studies. The full-length PBP (TM + TG + TP) contains amino acid residues 1–844 of the E. coli PBP1b P02919. TG + TP (residues 88–844), TM + TG (residues 1–409), TG (residues 195–409), and TP (residues 444–736) are truncated variants with that portion of the full-length PBP1b. The moenomycin binding constant (KD) for these variants was determined by using SPR.
The characterization of class A penicillin-binding proteins (PBPs) and the identification of TG inhibitors require functional PBP and lipid II as the substrate for the enzyme. However, the limited availability of lipid II has hampered the development of effective enzymatic assays for identification of inhibitors. As a result, the majority of the screening methods that are used to search for TG inhibitors, including the low-throughput methods that use surface plasmon resonance (SPR) (12) or radioactive assays (13–16), rely mainly on moenomycin (11). Development of a TG activity assay that is amenable to high-throughput screening (HTS) is thus desirable for inhibitor identification.
In this study, we compared the binding of moenomycin to various truncated PBPs and concluded that the transmembrane (TM) domain is critical for moenomycin binding. Consequently, the full-length PBP was used to develop a high-throughput assay. We devised a general method for expressing and purifying class A PBPs from 16 bacterial species and characterized their moenomycin binding activity. A fluorescence anisotropy (FA)-based assay was developed by using the PBP that had higher binding affinity and was successfully used for the HTS of TG inhibitors.
Results
Domain Requirement of Moenomycin Binding to Class A PBPs (Bifunctional TGs).
Transglycosylation is mainly catalyzed by bifunctional class A PBPs (17). PBPs are the major enzymes responsible for last-step cell wall formation by polymerization of N-acetylglucosamine-N-acetylmuramyl pentapeptide, and cross-linking between pentapeptides, and thus have been considered as one of the important targets for antibiotics discovery. These membrane-anchored enzymes consist of three distinct protein domains—TM, TG, and transpeptidase (TP)—from their amino-to-carboxyl termini. The recent description of x-ray crystal structures of the PBP2 extracellular domain from S. aureus (18) and the TG domain of PBP1a from Aquifex aeolicus (19) have provided invaluable structural insights into the plausible mechanism of cell wall peptidoglycan polymerization. Lacking, however, is information regarding the role of the TM domain of PBPs in catalysis, although it has been speculated to interact with the lipid moiety of moenomycin or lipid II (20, 21). To address this question, protein constructs containing different domains of PBP1b from Escherichia coli were expressed and purified with suitable detergents [see supporting information (SI) Materials and Methods and SI Fig. 7] and used for analysis of moenomycin binding by using SPR. Five E. coli PBP1b variants were expressed and purified: (i) the full-length protein containing the TM, TG, and TP domains (TM + TG + TP), (ii) TG + TP, (iii) TM + TG, (iv) TG alone, and (v) TP alone (Fig. 1B). After immobilization of the target proteins on the sensor chip, different concentrations of moenomycin were passed through the surface, and the binding affinities were determined. As shown in Fig. 1B, the binding affinity of moenomycin to PBP without the TM domain (TG + TP) was ≈5-fold lower than with the full-length PBP1b. On the other hand, the binding affinity of TM + TG is similar to that of the full-length protein. These results indicate that the TM domain plays an important role in moenomycin binding.
Correlation of PBP Binding Activities and MIC of Moenomycin.
Because the TM domain contributes significantly to moenomycin binding, we set out to prepare full-length PBPs from 16 bacteria (10 Gram-negative and 6 Gram-positive) (Table 1) for moenomycin binding studies. The genes of class A PBPs were identified by using the National Center for Biotechnology Information database (www.ncbi.nlm.nih.gov/BLAST/), and all were confirmed to have TG and TP motifs (22, 23) (see SI Fig. 6). The target genes were amplified from respective genomic DNA from each individual bacterial species and cloned into expression vectors for recombinant protein production using E. coli as host. The enzymatic activities of the purified proteins were confirmed by lipid II polymerization (data not shown) and moenomycin binding. As shown in Table 1, varied steady-state affinity (KD) values were found among PBPs from different species. Nonetheless, all of the measured KD values fell into the range of 10−7 M, close to the reported inhibition concentration for the transglycosylation process (12). Interestingly, among the Gram-positive bacteria we tested, the KD values of PBP from E. faecalis and S. aureus correlate with the MIC values, suggesting that the moenomycin binding site of PBP may be a good target for development of antibiotics against these species.
Table 1.
Correlation of antimicrobial activity and PBP binding affinity of moenomycin for 16 bacterial strains
| Species | MIC, μM* | Reported values† |
Gene sequence ID‡ | SPR_ KD, nM§ | FA_ KD, nM¶ | |
|---|---|---|---|---|---|---|
| MIC, μM | Citation | |||||
| Gram-negative | ||||||
| Bordetella pertussis | 0.011–0.021 | — | PBP1a (NP_882163) | 1,270 | 594 ± 230 | |
| Citrobacter freudii | — | 352 | 28 | PBP1b (CAA90232) | 728 | — |
| Escherichia coli | 80 | 55–110 | 28 | PBP1b (NP_414691) | 440 | 54 ± 17 |
| Haemophilus influenzae | — | — | PBP1b (AAX88775) | 966 | 174 ± 13 | |
| Helicobacter pylori | — | 1.3 | 29 | PBP1a (NP_207392) | 334 | 25 ± 14 |
| Klebsiella pneumoniae | 80 | 13.75–27.5 | 28 | PBP1b (NTUH-2044) | 819 | 78 ± 24 |
| Neisseria gonorrhoeae | — | 1.69 | 28 | PBP1 (YP_207272) | 450 | 47 ± 26 |
| Pseudomonas aeruginosa | 40 | 55–110 | 28 | PBP1b (YP_793163) | 866 | 42 ± 29 |
| Salmonella enterica | 160 | 13.75–110 | 28 | PBP1b (YP_149541) | 561 | 138 ± 113 |
| Shigella flexneri | 40 | 27.5–110 | 28 | PBP2 (YP688236) | 290 | — |
| Gram-positive | ||||||
| Bacillus subtilis | 0.3–0.6 | 0.07–0.43 | 30 | PBP1a/1b (NP_390113) | 1,690 | 197 ± 80 |
| Clostridium difficile | >160 | 220–440 | 30 | PBP (CAJ67615) | 582 | 37 ± 33 |
| Enterococcous faecalis | 0.075–0.3 | 0.1 | 31 | PBP2a (NP_814430) | 619 | 276 ± 14 |
| Enterococcous faecium | — | >40.5 | 10 | PBP1 (EAN08787) | 94 | 56 ± 11 |
| Staphylococcus aureus | 0.075 | 0.02–0.12 | 10 | PBP2 (NP_371974) | 393 | 30 ± 29 |
| Streptococcus pneumoniae | 0.625 | — | PBP1b (NP_359500) | 900 | 38 ± 24 | |
*MIC of moenomycin against different bacterial species.
†MIC values of moenomycin from the literature.
‡Sequence ID of class A PBP genes from individual bacterial strains. Homologs of class A PBPs were identified by using BLAST against the NCBI database.
§KD of PBP homologs and moenomycin using SPR. Average values are shown.
¶KD of PBP homologs and moenomycin using our FA-based assay.
Development of an FA-Based Assay for Identification of TG Inhibitors.
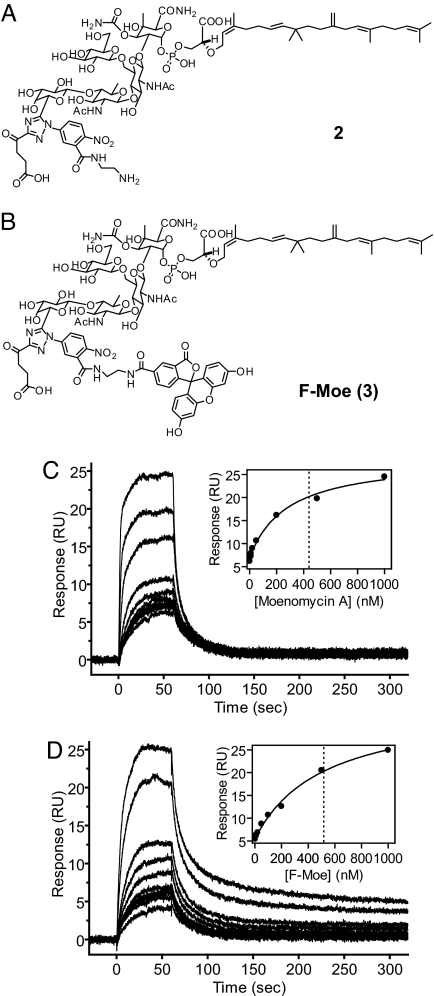
Based on the conclusion that the full-length proteins (TM + TG + TP) should be used for moenomycin binding studies, we designed an FA-based assay to monitor the binding affinities of small molecules toward TG. Previous studies on the complex crystal structure (18) and the structure–activity relationship (6) between moenomycin and TG suggested that modification of compound 1 to compound 2 (Fig. 2A) will not dramatically reduce binding and antibacterial activities. Furthermore, the amine moiety in 2 can be conjugated with any fluorophore and used as a probe for binding studies (24, 25). Indeed, compound 2 was linked with fluorescein by using 6-carboxyfluorescein N-hydroxysuccinimide ester to prepare the fluorescent probe 3 (F-Moe, Fig. 2B). To investigate whether the fluorophore or the structural modification interferes with the binding between the targeted protein and the small molecule, the PBP binding affinities for Moe A and the fluorescent probe were compared by using SPR. The determined steady-state affinity (KD) values are very similar (4.4 × 10−7 for Moe A vs. 5.2 × 10−7 M for F-Moe) (Fig. 2 C and D), suggesting that F-Moe is a valid fluorescent probe that is useful for the FA-based binding assay.
Fig. 2.
Design of an FA assay for TG. (A and B) Chemical structures of modified Moe A (2) and fluorescein-labeled moenomycin, F-Moe (3). (C and D) Results of SPR analysis of the binding activity of Moe A (C) and F-Moe (D) to E. coli PBP1b. Shown are responses for moenomycin binding to immobilized E. coli PBP1b. The data were analyzed by using steady-state affinity and fitted to a 1:1 interaction model (Insets). The KD values deduced from the intercepts of the x axis and the dotted lines are 440 and 520 nM for Moe A and F-Moe, respectively.
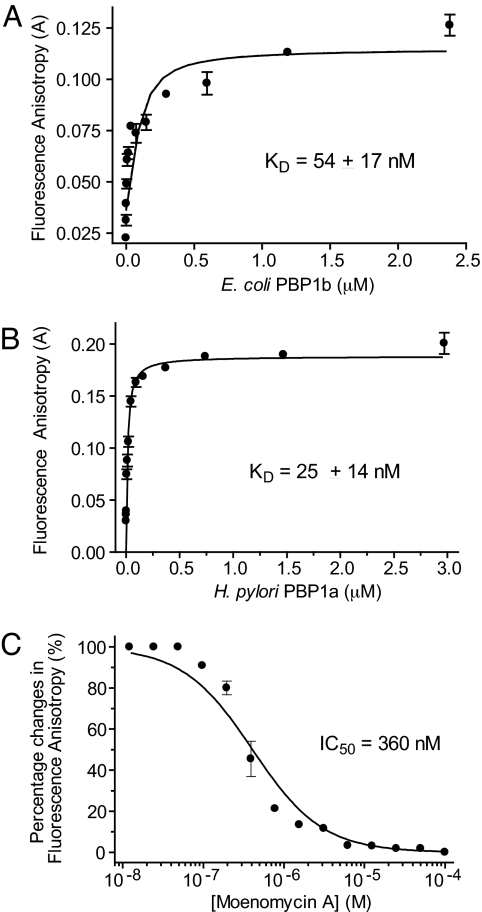
The anisotropy of F-Moe increased significantly during incubation with E. coli PBP1b, presumably because of the formation of an F-Moe–PBP1b complex (Fig. 3A). In contrast, the anisotropy of F-Moe was unchanged when incubated with BSA, up to 100 μM (data not shown). The protein concentrations were further titrated down to establish the dose-dependency of the anisotropy changes. The deduced dissociation constant (KD) for F-Moe was in the nanomolar range (54 ± 17 nM) for E. coli PBP1b (Fig. 3A). However, the signal of the FA assay using the E. coli PBP1b is only medium, with an anisotropy of 0.12. We thus screened different class A PBPs to improve the assay, i.e., to obtain higher anisotropy. Of all homologs tested, the FA assay using Helicobacter pylori PBP1a produced the best signal-to-noise ratio with KD of 25 ± 14 nM and with anisotropy reaching 0.2 upon binding to F-Moe (Fig. 3B). To develop an assay for inhibitor screening, F-Moe was preincubated with H. pylori PBP1a, and unlabeled Moe A was then added at various concentrations. A decrease in anisotropy was observed as the concentration of Moe A increased, and from this competition analysis the inhibition constant (KI) and IC50 values were determined as 0.47 ± 0.10 μM and 0.36 μM, respectively (Fig. 3C). This result validates the FA assay, which can be used to screen for inhibitors that compete with moenomycin for binding to PBP.
Fig. 3.
Development of a high-throughput FA assay for TG. (A) Concentration-dependent changes in FA were observed when E. coli PBP1b bound to F-Moe. (B) Improved FA assay with H. pylori PBP1a. The concentration-dependent FA changes were performed similarly to A but using the H. pylori PBP1a. The maximum anisotropy value was 0.2. (C) Displacement of the PBP1a-bound F-Moe complex by unlabeled moenomycin at various concentrations. The changes in FA are defined as [(Aobs − Amin)/(Amax − Amin) × 100%]. The KD and IC50 values were calculated as described in SI Materials and Methods.
HTS for TG Inhibitors.
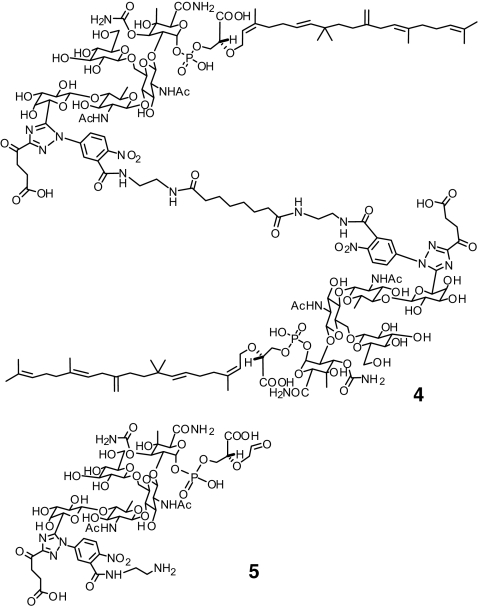
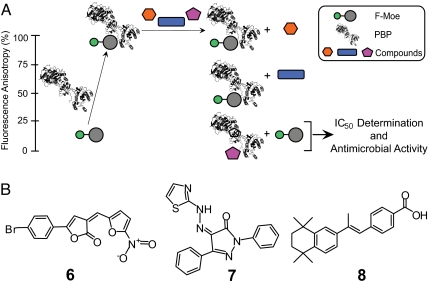
The FA assay was used to screen a collection of 57,000 small molecules, along with Moe A derivatives (Fig. 4), at 50 μM. High controls are assays with 1 μM moenomycin, and low controls are assays with 2.5% DMSO. Other proteins, such as BSA, were included as a control to confirm that the anisotropy increase is not the result of nonspecific binding. The Z′ value, a statistical parameter ranging from 0 to 1 used to evaluate the robustness of HTS (26), was determined as 0.895 from >100 independent experiments. Eleven possible hits that showed at least 75% inhibition in the screening were selected for additional studies involving antimicrobial assays and IC50 determinations for PBP binding (Fig. 5A). Among these hits, two moenomycin analogues (2 and 4) and three small molecules (compounds 6–8) were confirmed to have both antibacterial and TG binding inhibition activities (Table 2).
Fig. 4.
Chemical structures of moenomycin derivatives (4 and 5).
Fig. 5.
Screening for small molecules as TG inhibitors using the class A PBPs. (A) HTS for TG inhibitors using the FA assay. Protein structure graphics were created from Protein Data Bank (www.pdb.org) ID code 2OLV (12). (B) Chemical structures of the HTS hits (compounds 6–8).
Table 2.
Inhibition of TG activity and the antibacterial determinations of selected hits
| Compound* | IC50, μM† | MIC, μM‡ |
|||
|---|---|---|---|---|---|
| B. subtilis (ATCC23857) | E. faecalis (ATCC29212) | S. aureus (ATCC29213) | S. pneumoniae (ATCC49619) | ||
| 1 | 0.36 | 0.33 | 0.04 | <0.01 | 0.33 |
| 2 | 2.10 | 2.50 | 10.0 | 1.25 | 20.0 |
| 4 | 0.92 | 1.25 | 5.0 | 0.625 | 20.0 |
| 5 | 125.00 | — | — | — | — |
| 6 | 34.00 | 0.25 | 0.25 | 1.0 | 4.0 |
| 7 | 3.70 | 0.25 | 1.0 | 4.0 | — |
| 8 | 9.30 | 4.0 | >4.0 | >4.0 | >4.0 |
*Compounds 2, 4, and 5 are moenomycin derivatives. Compounds 6–8 were HTS hits.
†IC50 values were determined by using the fluorescence anisotropy assay shown in Fig. 5A.
‡The MICs of moenomycin against different bacterial species were determined as described in Materials and Methods. ATCC, American Type Culture Collection.
Discussion
We have shown that the TM domain is important for Moe A binding. A general method was thus devised for preparing the full-length class A PBPs from various bacterial strains. We found that the binding affinity of Moe A to PBP correlated with its antibiotic activity for E. faecalis and S. aureus. By using the full-length PBP1b, an FA-based HTS assay was developed for identification of TG inhibitors as antibacterial agents.
We were concerned about the nonspecific binding of the C25 lipid of Moe A. By using a TP variant as a control, it was confirmed that the determined binding affinities of moenomycin to the PBP1b variants are significant and specific. Binding studies using truncated PBP1b variants further showed that the binding affinity of the TM + TG variant is similar to that of the full-length protein. The role of the TM domain in Moe A binding was revealed in the observation that the binding affinity of the TG + TP variant decreased 5-fold compared with that of full-length PBP1b (Fig. 1B). These studies suggest that one should use the full-length PBP, or the truncated PBP containing the TM and TG domains, for moenomycin binding studies and inhibitor discovery and that the TM domain may be important for the structure of the enzyme.
In the course of our study, several moenomycin analogues (2, 4, and 5) were prepared to evaluate the reliability of the FA-based assay. As shown in Table 2, the differential binding affinities (IC50 values) of these moenomycin analogues by the FA-based assay are comparable with published results (12, 27). The modification of Moe A to 2 resulted in a slight decrease in TG binding affinities. Dimerization of 2 by means of an eight-carbon spacer produced 4, with a 2-fold increase in both PBP binding and antimicrobial potency, although 4 is still less potent than Moe A (1). More significantly, compound 5, without the C25 lipid moiety, could barely displace the F-Moe probe 3, indicating that the hydrophobic part of moenomycin is crucial for its activity. It is noteworthy that the order of inhibition potency is in agreement with the MIC values (Table 2).
Although moenomycin and its analogues are potent against Gram-positive bacteria, their poor pharmacokinetic properties demand unique approaches for antibiotic development. An efficient and economical TG assay in an HTS format may facilitate the identification of new TG inhibitors. Using our FA-based assay, a hit rate of 0.02% was achieved with a Z′ value of 0.895, suggesting that this assay could be used as a robust primary screen to quickly identify potential hits from large compound libraries. The selected hits would be further screened by using antibacterial assay and lipid II polymerizing activity analysis, to identify leads. As with all fluorescence-based assays, our FA-based assay cannot be used to screen fluorescent compounds for TG inhibitors. Nonetheless, the FA-based PBP binding assay has been demonstrated to be a powerful HTS assay for the discovery of new antibacterial agents.
Materials and Methods
SPR Analysis.
Purified PBP and its variants were immobilized onto CM3 sensor chips (GE Healthcare) to the level of ≈1,500–2,000 relative units via amine coupling. The chips were then passed over with different concentrations of Moe A (0–2,000 nM). Immobilization and data collection were performed with BIAcore T100 (GE Healthcare) at 25°C.
FA Measurements.
FA measurements were carried out in triplicate in 384-well plates by using laser fluorimetry (IsoCyte; Blueshift Biotech). Various buffers, salts, pH values, and divalent cations (Ca2+, Mg2+, Co++) were optimized for FA measurements. KD and KI determinations were carried out in100 mM NaCl, 10 mM Tris, pH 8.0 (see SI Materials and Methods for details).
HTS for TG Inhibitors.
The FA assay was used to screen 50,000 purchased small molecules (ChemBridge) and 7,000 from our proprietary collections. The compounds were transferred to 96-well plates (Freedom Evo; Tecan Schweiz) and then to 384-well plates, using a multidispenser (Labcyte) to prepare the compound plates for screening. The H. pylori PBP1a (10 μg/ml) in 100 nM F-Moe, 10 mM Tris, 100 mM NaCl, pH 8.0, at a final volume of 40 μl was added to 384-well plates (Freedom Evo 150; Tecan). One microliter of 2 mM stock solution of compound was added to wells by using a multidispenser (Labcyte). The last two columns of every plate were controls containing 10 μM moenomycin and 2.5% DMSO, respectively. After a 30-min incubation, changes in FA were determined with Isocyte (Blueshift Biotech). Hits that showed >75% reduction compared with the control anisotropy values were selected for further confirmation.
Determination of MIC.
The MIC of tested compounds was determined in accordance with the National Committee for Clinical Laboratory standard. The experiments were conducted in 96-well microtiter plates using 2-fold dilutions in Muller–Hilton broth with (S. pneumoniae) or without (Bacillus subtilis, E. faecalis, S. aureus) blood. Exponentially growing cells at 5 × 105 cells per milliliter were incubated with test compounds at various concentrations. After 18- to 24-h incubation at 37°C, MIC was determined as the minimal concentration of the compound that prevents bacterial growth.
Supplementary Material
ACKNOWLEDGMENTS.
We thank Drs. Evan F. Cromwell and Steve Miller of Blueshift Biotechnologies, Inc., for help with FA detection and Dr. Andrij Buchynskyy for chemistry discussions. This work was supported in part by National Health Research Institute, Taiwan, Grant NHRI-EX95-9515SC (to C.M.) and National Science Council, Taiwan, Grant NSC95-2113-M-001-019-MY2 (to W.-C.C.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0710868105/DC1.
References
- 1.Silver LL. Novel inhibitors of bacterial cell wall synthesis. Curr Opin Microbiol. 2003;6:431–438. doi: 10.1016/j.mib.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Ostash B, Walker S. Bacterial TG inhibitors. Curr Opin Chem Biol. 2005;9:459–466. doi: 10.1016/j.cbpa.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 3.Zehl M, Pittenauer E, Rizzi A, Allmaier G. Characterization of moenomycin antibiotic complex by multistage MALDI-IT/RTOF-MS and ESI-IT-MS. J Am Soc Mass Spectrom. 2006;17:1081–1090. doi: 10.1016/j.jasms.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 4.Eichhorn P, Aga DS. Characterization of moenomycin antibiotics from medicated chicken feed by ion-trap mass spectrometry with electrospray ionization. Rapid Commun Mass Spectrom. 2005;19:2179–2186. doi: 10.1002/rcm.2044. [DOI] [PubMed] [Google Scholar]
- 5.Adachi M, et al. Degradation and reconstruction of moenomycin A derivatives: Dissecting the function of the isoprenoid chain. J Am Chem Soc. 2006;128:14012–14013. doi: 10.1021/ja065905c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Welzel P. Syntheses around the transglycosylation step in peptidoglycan biosynthesis. Chem Rev. 2005;105:4610–4660. doi: 10.1021/cr040634e. [DOI] [PubMed] [Google Scholar]
- 7.Welzel P. A long research story culminates in the first total synthesis of moenomycin A. Angew Chem Int Ed Engl. 2007;46:4825–4829. doi: 10.1002/anie.200700765. [DOI] [PubMed] [Google Scholar]
- 8.Taylor JG, Li X, Oberthür M, Zhu W, Kahne DE. The total synthesis of moenomycin A. J Am Chem Soc. 2006;128:15084–15085. doi: 10.1021/ja065907x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ostash B, Saghatelian A, Walker S. A streamlined metabolic pathway for the biosynthesis of moenomycin A. Chem Biol. 2007;14:257–267. doi: 10.1016/j.chembiol.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butaye P, Devriese LA, Haesebrouck F. Antimicrobial growth promoters used in animal feed: Effects of less well known antibiotics on Gram-positive bacteria. Clin Microbiol Rev. 2003;16:175–188. doi: 10.1128/CMR.16.2.175-188.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Guilmi AM, Dessen A, Dideberg O, Vernet T. Bifunctional penicillin-binding proteins: Focus on the glycosyltransferase domain and its specific inhibitor moenomycin. Curr Pharm Biotechnol. 2002;3:63–75. doi: 10.2174/1389201023378436. [DOI] [PubMed] [Google Scholar]
- 12.Stembera K, Vogel S, Buchynskyy A, Ayala JA, Welzel P. A surface plasmon resonance analysis of the interaction between the antibiotic moenomycin A, penicillin-binding protein 1b. ChemBioChem. 2002;3:559–565. doi: 10.1002/1439-7633(20020603)3:6<559::AID-CBIC559>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 13.Vollmer W, Holtje JV. A simple screen for murein TG inhibitors. Antimicrob Agents Chemother. 2000;44:1181–1185. doi: 10.1128/aac.44.5.1181-1185.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jha RK, de Sousa SM. Microplate assay for inhibitors of the transpeptidase activity of PBP1b of Escherichia coli. J Biomol Screen. 2006;11:1005–1014. doi: 10.1177/1087057106294364. [DOI] [PubMed] [Google Scholar]
- 15.Ravishankar S, et al. Scintillation proximity assay for inhibitors of Escherichia coli MurG and, optionally, MraY. Antimicrob Agents Chemother. 2005;49:1410–1418. doi: 10.1128/AAC.49.4.1410-1418.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chandrakala B, et al. High-throughput screen for inhibitors of TG and/or transpeptidase activities of Escherichia coli penicillin binding protein 1b. Antimicrob Agents Chemother. 2004;48:30–40. doi: 10.1128/AAC.48.1.30-40.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macheboeuf P, Contreras-Martel C, Job V, Dideberg O, Dessen A. Penicillin binding proteins: Key players in bacterial cell cycle and drug resistance processes. FEMS Microbiol Rev. 2006;30:673–691. doi: 10.1111/j.1574-6976.2006.00024.x. [DOI] [PubMed] [Google Scholar]
- 18.Lovering AL, de Castro LH, Lim D, Strynadka NC. Structural insight into the transglycosylation step of bacterial cell-wall biosynthesis. Science. 2007;315:1402–1405. doi: 10.1126/science.1136611. [DOI] [PubMed] [Google Scholar]
- 19.Yuan Y, et al. Crystal structure of a peptidoglycan glycosyltransferase suggests a model for processive glycan chain synthesis. Proc Natl Acad Sci USA. 2007;104:5348–5353. doi: 10.1073/pnas.0701160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye XY, et al. Better substrates for bacterial TGs. J Am Chem Soc. 2001;123:3155–3156. doi: 10.1021/ja010028q. [DOI] [PubMed] [Google Scholar]
- 21.Anikin A, et al. Membrane anchoring and intervesicle transfer of a derivative of the antibiotic moenomycin A. Angew Chem Int Ed. 1999;38:3703–3707. doi: 10.1002/(sici)1521-3773(19991216)38:24<3703::aid-anie3703>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 22.Barrett DS, Chen L, Litterman NK, Walker S. Expression and characterization of the isolated glycosyltransferase module of Escherichia coli PBP1B. Biochemistry. 2004;43:12375. doi: 10.1021/bi049142m. [DOI] [PubMed] [Google Scholar]
- 23.Di Guilmi AM, Dessen A, Dideberg O, Vernet T. Functional characterization of penicillin-binding protein 1b from Streptococcus pneumoniae. J Bacteriol. 2003;185:1650–1658. doi: 10.1128/JB.185.5.1650-1658.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buchynskyy A, et al. Synthesis of fluorescent derivatives of the antibiotic moenomycin A. Eur J Org Chem. 2002;7:1149–1162. [Google Scholar]
- 25.Donnerstag A, Marzian S, Müller D, Welzel P. A structurally and biogenetically interesting moenomycin antibiotic. Tetrahedron. 1995;51:1931–1940. [Google Scholar]
- 26.Zhang JH, Chung TD, Oldenburg KR. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 27.Marzian S, et al. Moenomycin A: Reactions at the lipid part. New structure–activity relations. Tetrahedron. 1994;50:5299–5308. [Google Scholar]
- 28.von Wasielewski E, Mushaweck R, Schütze E. Meonomycin, a new antibiotic. 3. Biological properties. Antimicrob Agents Chemother. 1966;5:743–748. [PubMed] [Google Scholar]
- 29.Riess G, Seibert G, Hedtmann U. US Patent 6. 2001;242:424. [Google Scholar]
- 30.Page SW. The Role of Enteric Antibiotics in Livestock Production. Canberra, Australia: Avcare; 2003. Chap 7, Bambermycin. [Google Scholar]
- 31.Baizman ER, et al. Antibacterial activity of synthetic analogues based on the disaccharide structure of moenomycin, an inhibitor of bacterial transglycosylase. Microbiology. 2000;146:3129–3140. doi: 10.1099/00221287-146-12-3129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.