Abstract
Improvement of local germplasm through artificial selection is regarded as the main force behind maize evolution and diversity in Mexico, the crop's center of origin. This perspective neglects the larger social context of maize evolution. Using a theoretical approach and Mexico-wide data, we show that farmer-led evolution of maize is largely driven by a technological diffusion and appropriation process that selectively integrates nonlocal germplasm into local seed stocks. Our approach construes farmer practices as events in the life history of seed to build a demographic model. The model shows how random and systematic differences in management combine to structure maize seed populations into subpopulations that can spread or become extinct, in some cases independently of visible agronomic advantages. The process involves continuous population bottlenecks that can lead to diversity loss. Nonlocal germplasm thus might play a critical role in maintaining diversity in individual localities. Empirical estimates show that introduction of nonlocal seed in Central and Southeastern Mexico is rarer than previously thought; prompt replacement further prevents new seed from spreading. Yet introduced seed perceived as valuable diffuses rapidly, contributing variation in the form of type diversity or through introgression into local seed. Maize seed dynamics and evolution are thus part of a complex social process driven by farmers' desire to appropriate the value in maize farming, not always achieved by preserving or improving local seed stocks.
Keywords: crop diversity and evolution, metapopulations, technological diffusion
Recent years have witnessed an exciting discussion on the evolution of maize (Zea mays L.). The crop's origin has been deciphered, but questions about the pace and form of evolution remain, largely because human and crop populations interact in complex ways (1–3). Farmers' management of seed has an important influence on the demography of maize and thus on gene flows and frequencies. The role of artificial selection in shaping the genetic makeup of maize is well recognized, yet other farmer practices have been neglected and their link to crop population genetics left unexplored (3). Existing research on seed management is designed to explain maize diversity on individual farms, but it sheds no light on maize seed demography across farms and its diffusion through seed systems. Because there is no formal quantitative framework integrating management practices with maize demography, practices are often analyzed and interpreted separately. Hence, reports that 11% of seed in Cuzalapa, an indigenous community in western Mexico, was introduced each year were taken as proof that landrace populations are exceedingly open systems (4–6). However, the spread of seed into local stocks depends not only on their introduction but also on other management practices. Farmers do not perform these practices haphazardly, but, rather, combine them to achieve specific objectives. Different farmers might have different objectives, but the interactions among farmers are not necessarily random. The effects of farmer management on maize populations thus can only be deciphered with the use of a demographic model transcending the farm and cutting across localities.
We propose a theoretical model in which the practices of diverse farmers combine to determine the growth of particular seed populations. We estimate model parameters, including germplasm diffusion rates across localities, using data from the nationally representative 2002 Mexico Rural Household Survey [Encuesta Nacional a Hogares Rurales de México (ENHRUM)]. Our findings indicate that case studies have offered an incomplete and often-biased description of maize seed dynamics in the crop's Mesoamerican center of origin and diversity. We describe these dynamics and discuss the effect of different management practices on the composition of local seed stocks.
Results
In our model, the rate of growth or displacement (λ) of seed populations within a metapopulation depends on the rates of replacement (1 − p) and diffusion (q) of seed lots in each population and on the rate of introduction (r) of nonlocal seed lots (Eqs. 1 and 3). Any characteristic that systematically influences these rates, such as seed type and source, can define a population, which might consist of a single seed lot in some cases.
Seed Replacement.
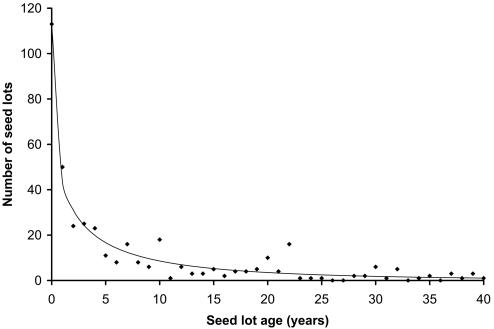
A log-linear model was used to test seed type and source effects on seed replacement rates nationwide (7). G tests for goodness of fit reveal significant interactions of replacement with seed type (P = 0.005) and source (P < 0.001) (Table 1); Freeman-Tukey deviates show no differences between local types with respect to replacement. A separate model (Table 1) reveals significant regional differences in replacement (P < 0.001). Híbridos are replaced least in the center region and criollos in the center and southeast. The age distribution of local híbridos approximates a Deevey type II constant survivorship curve [supporting information (SI) Table 4 and ref. 8]. Other seed types more closely resemble a type III curve with early mortality (i.e., replacement) (Fig. 1). First-year seed is replaced at twice the rate of all age categories combined. A separate log-linear replacement model was fitted for first-year seed (Table 1). A significant interaction between replacement and seed source was found (P = 0.001). Local seed is replaced less than introduced seed. No interaction with seed type is present (P = 0.93). Survival curves were fitted for the population over 2 years of age for seed types with a large enough sample (SI Table 4). Curves for the total and local criollo populations suggest a constant replacement rate after the second year.
Table 1.
Rates of maize seed replacement (1 − p) in Mexico in 2002
| Seed type | All ages by source (N = 476) | All ages by region (N = 476) | First-year seed by source (N = 151) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Local | Introduced | Total | G type effect | Southeast | Center | West-Center | Northwest-Northeast | Total | G type effect | Local | Introduced | Total | G type effect | |
| Criollo | 0.21 | 0.50 | 0.24 | 0.16 | 0.14 | 0.60 | 0.47 | 0.24 | 0.42 | 0.91 | 0.53 | |||
| Híbrido | 0.18 | 0.91 | 0.79 | 10.6* (2 df) | 0.75 | 0.58 | 0.81 | 0.89 | 0.79 | 39.2* (4 df) | 0.50 | 0.93 | 0.86 | 0.1 (2 df) |
| Total | 0.21 | 0.73 | 0.32 | 0.18 | 0.18 | 0.68 | 0.59 | 0.32 | 0.42 | 0.92 | 0.64 | |||
| G source or region effect | 20.2* (2 df) | 58.4* (6 df) | 13.3* (2 df) | |||||||||||
Significance at the 0.05 level is indicated by ∗.
Fig. 1.
Survival curve for criollo maize seed in Mexico in 2002.
Seed Diffusion.
Seed diffusion varies widely: 1% of lots multiplied 10-fold in 5 years, whereas 60% did not diffuse at all. Seed type and source effects on seed diffusion were analyzed with a log-linear model (Table 2). A test of complete independence cannot be rejected (P = 0.51; G = 3.3, 4 df), but large Freeman–Tukey deviates show that introduced híbridos diffuse well below statistical expectations. Pooling of híbridos and criollos allows analysis of a larger population that includes seed of unknown type. Separate goodness-of-fit tests reveal diffusion-rate differences between local (q = 0.22) and introduced seed (q = 0.13; P = 0.02, G = 5.3, 1 df), and between saved (q = 0.23) and new seed (q = 0.14; P = 0.004, G = 8.4, 1 df). A log-linear model revealed no significant association of diffusion with seed source or ownership for this sample (Table 2). A test of complete independence is also not significant (P = 0.26, G = 5.2, 4 df) but reveals two significant deviations: saved seed acquired locally diffuses more than expected, and new introduced seed diffuses less.
Table 2.
Rates of maize seed diffusion (q) in Mexico in 2002
| Seed source | Seed type (N = 476) | Seed ownership (N = 739) | Region (N = 739) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Criollo | Híbrido | Total | G source effect | Saved | New | Total | G source effect | Southeast | Center | West-Center | Northwest-Northeast | Total | G source effect | |
| Local | 0.23 | 0.17 | 0.23 | 0.23 | 0.16 | 0.22 | 0.24 | 0.19 | 0.35 | 0.04 | 0.22 | |||
| Introduced | 0.19 | 0.09 | 0.13 | 0.6 (2 df) | 0.19 | 0.10 | 0.13 | 1.0 (2 df) | 0.33 | 0.18 | 0.10 | 0.03 | 0.13 | 10.4* (4 df) |
| Total | 0.23 | 0.11 | 0.21 | 0.23 | 0.14 | 0.20 | 0.25 | 0.19 | 0.25 | 0.04 | 0.20 | |||
| G type or region effect | 1.2 (2 df) | 2.6 (2 df) | 28.9* (6 df) | |||||||||||
Significance at the 0.05 level is indicated by ∗.
A separate log-linear model (Table 2) reveals significant regional effects on diffusion (P < 0.001). Seed in the northeast and northwest diffuses below expectation. Local seed in the west-center and introduced seed in the south diffuses above expectation. Source effects are significant (P = 0.03) but largely restricted to the west-center, where local seed is diffused 3.5 times as much as introduced seed. Regional effects persist if new seed is excluded from the analysis (P < 0.001), but source effects vanish (P = 0.43).
A zero-inflated-Poisson regression model (9) reveals that diffusion involves two stages: (i) the decision of whether or not to diffuse a seed lot, and (ii) the number of diffusion events involved (SI Table 5). A Vuong test supports use of this model over a single-stage Poisson model (P < 0.001). The probability of diffusion is greater for criollos than for híbridos but does not increase with age, so only 1/3 of seed lots ever diffuse. Among those that do diffuse, the expected number of copies is largest for newly acquired and introduced seed lots and then accumulates with age.
Seed Introduction and Mixing.
A log-linear model analyzes regional and type differences in the rate of introduction (Table 3). Significant type (P < 0.001) and region interactions are present (P < 0.001). Introduction of criollos is very low in the southeast and center. Híbridos are introduced more often than criollos in every region. Rate differences across regions for híbridos reflect the strong presence of commercial seed in the northeast and northwest and creolized varieties in southeast and central Mexico.
Table 3.
Rates of maize seed introduction (r) and mixing in Mexico in 2002
| Seed type | Introduction (N = 476) | Mixing (N = 476) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Southeast-Center | West-Center | Northeast-Northwest | Total | G type effect | Local | Introduced | Total | G type effect | |
| Criollo | 0.01 | 0.23 | 0.16 | 0.05 | 0.19 | 0.14 | 0.18 | ||
| Híbrido | 0.60 | 0.78 | 0.89 | 0.76 | 112.4* (4 df) | 0.50 | 0.07 | 0.14 | 2.9 (2 df) |
| Total | 0.05 | 0.43 | 0.38 | 0.15 | 0.20 | 0.10 | 0.18 | ||
| G source or region effect | 42.8* (4 df) | 5.2 (2 df) | |||||||
Significance at the 0.05 level is indicated by ∗.
Separate goodness-of-fit tests show significant differences in mixing rates between local and introduced híbridos (P = 0.002; G = 9.6, 1 df) but not criollos (P = 0.40; Table 3). A log-linear model yields no significant association of mixing with type (P = 0.23) or source (P = 0.07). Although complete independence is not rejected (P = 0.14; G = 5.5, 3 df), significant deviations exist: local híbridos are mixed more often than expected, and introduced híbridos are mixed less often.
Discussion
It is likely that farmers throughout history have replaced seeds (10). Replacement brings to mind the spread of foreign seed into the household, displacement of local seed, and genetic erosion. Scholars believe that farmers demand and maintain crop diversity for lack of market alternatives but will sacrifice diversity to adopt improved varieties (IVs) as markets develop (11–14). It is increasingly clear that this description does not apply to maize in Mexico: loss of diversity has not been demonstrated even where markets are well developed (6, 15, 16), and only 27% of maize area was sown to unrecycled IVs in 2002 nationwide (17). Moreover, the majority of Mexican farmers do not demand maize diversity: >2/3 of farmers in the southeast grow a single maize variety, and the nationwide average is only 1.38 varieties per household. Communities hold up to five times more diversity than individual households (15). Studies have alluded to collective action as a mechanism that could maintain maize diversity, but no such mechanism has been found (18). We posit instead that diversity at the locality level is the unintended result of individual farmers' actions. In the absence of a regulatory mechanism, the system is continuously at risk of genetic erosion, although not necessarily as a result of replacement by nonlocal seed. Introduced germplasm might in fact contribute to maintain diversity in local maize populations. We now describe the resulting system.
According to ENHRUM data, ≈1/3 of maize seed lots are replaced nationwide every year (Table 1), but rates vary nearly 5-fold across regions. Rates reported for Cuzalapa (1 − p = 0.47) are normal for western Mexico, but they are >2.5 times the average in the southeast and central region and up to 10 times higher than in some localities (4, 6). Regional differences are explained by seed age and source and associated with the predominance of IVs: híbridos are replaced nearly four times more than criollos. Farmers have varying reasons to replace seed (6, 18, 19). Commercial growers who use IVs replace seed continuously to maximize yields. Most of those who prefer landraces save and sow their own seed, perhaps out of expediency. High revenue is of less concern to subsistence farmers, who deal with a larger set of issues and overwhelmingly prefer landraces. These differences notwithstanding, most farmers are willing to adopt new seed if it offers them a significant advantage. New seed does not always perform well, especially nonlocal types acquired through informal seed systems. Farmers test seed and discard ill-adapted and inferior types. Most introduced seed is replaced after its first year, more than twice the rate of local seed. A considerable drop in replacement rates after the second year suggests that trial of new seed is fairly prompt, but seed remains subject to a constant probability of replacement (Fig. 1). At 25 years of age, 18% of criollo seed lots in the southeast can be bequeathed across generations. Considerable seed replacement could nevertheless lead to a large fraction of the population being displaced, perhaps by IVs.
One way to assess the status of landrace use nationwide is to determine whether its populations are stable, i.e., whether diffusion offsets replacement (Eq. 1). The rate of diffusion of criollo seed nationwide is significantly higher than that of híbridos (Table 2), and its population growth rate is estimated at λ = 1.03 (Eq. 1). But even if there were no replacement of criollo seed with IVs (or other crops) on a national scale in 2002, differences across regions would be evident (Table 1). Relatively low replacement rates and moderate diffusion of landrace populations imply λ = 1.13 in southeast and central Mexico, but high diffusion rates fail to offset even higher replacement rates in the west. An estimated λ = 0.79 for this region does not imply a declining population, but rather, an open one: 1/4 of seed in western Mexico comes from institutional sources (e.g., the seed industry) or is acquired in stores as grain destined for consumption. These two sources raise λ to 1.02. Outside sources are even more important in the northeast and northwest, where diffusion of local seed is low. Regional rates conceal great heterogeneity across localities within regions. However, the fact that maize acreage in Mexico was relatively constant in 2002, even as IV sales declined 3% (17), suggests that the landrace population nationwide was in fact stable.
A negative association between the spread of IVs and crop diversity has long been maintained (12), but lack of spread does not necessarily imply conservation of maize diversity. Random differences in replacement and diffusion would lead to the spread of some seed types and loss of others in the same way that alleles are lost to genetic drift. Systematic differences can also lead to diversity loss: ≈2/3 of seed lots are not likely to diffuse at any point, whereas the rest diffuse early on and frequently thereafter, 15 times or more in 5 years, 2.9 times on average. These two “states” are evident for every seed category studied. Seed in the diffusing state could be in high demand by farmers who identify it as a distinct and valuable type. Alternatively, farmers might identify not seed but specific seed suppliers, who could have a considerable influence on seed diversity. A frequent supplier of seed might be a farmer whose seed is faithful to a type. His seed line will be well represented locally or even fixed (Eq. 4). Another farmer that keeps a stock of maize might be known as a sure supplier of seed when others are lacking. His seed might not be preferable to others' but might still become locally predominant if the seed population is small. If the population is large, the demographic outcome depends not only on the rate at which he gives out seed but also on how long he keeps it.
Consider a strictly local landrace such as chianquiahuitl, the second most common and “most conservatively” managed landrace in Cuzalapa, where few farmers express any “allegiance” to their own seed (4). At the observed replacement rate (1 − p = 0.26), random fixation of a single seed line in a demographically unstructured population could take a long time, but if at least one farmer has an allegiance and saves seed painstakingly, he will give structure to the maize population. Letting i represent that farmer's seed lot and J the rest of the local population, equilibrium is given by niJ = (qiJ/qJi)0.5 (Eq. 5). Because qJi = 0 by construction, the only possible stable outcome is one in which the farmer's seed becomes fixed in the population. If this farmer is also a sought-after supplier of seed, i.e., qiJ is large, a bottleneck is a distinct possibility. Hence, differences in replacement and diffusion rates across farmers can lead to the predominance of certain seed types independently of their agronomic properties. Unobserved differences caused by natural or artificial selection might be lost or ear characteristics might become uniform even absent an ideotype (6). For example, dominant maize types in highland Chiapas exhibit morphological differences across localities but do not always show clear agronomic superiority in their own locality (20). Other processes involving seed replacement can generate more severe bottlenecks.
Households are said not to maintain specific seed lines but a revolving portfolio of maize diversity (13). A variety that is now grown across a locality presumably began as a “minor” variety held by few farmers for testing or use in specialty dishes (4, 6). Minor varieties often originate from a few seed lines or even a single ear of maize and may be grown on small plots for long periods, susceptible to random genetic change (19, 21), e.g., accessions of specialty seeds show the lowest genetic variation in maize (22). As a minor seed type spreads and replaces another, its genetic variation depends as much on the number of seed lines diffused as on pollen exchange across types. Accidental gene flow might nevertheless be limited if diffusion is fast, if type diversity is not uncommonly high (as it is in Cuzalapa) or if types are segregated in different environments. Hence, varietal replacement could entail the loss of the variation that has emerged among aging seed populations. This process would add to losses caused by other practices, including seed selection. When more variation within a locality is lost than created, an external source is required to maintain diversity.
A foreign source of diversity contradicts the belief that introduced seed is a threat to local diversity (23, 24). Introductions into an unstructured population can displace local seed if both are replaced and diffused at the same rate (Eq. 2). That these conditions apply is implicit in arguments that seed exchange homogenizes widespread landrace populations (20). In Cuzalapa, for instance, ≈1/2 of all introduced seed consists of so-called local varieties (4). Local and introduced seed of these varieties often is considered equivalent and presumably saved and diffused at the same rates. Consider blanco, the main landrace. At present rates (p = 0.48; q = 0.37), introduced blanco (r = 0.15) could displace local blanco entirely within a generation (Eq. 2), which is one of the reasons researchers now describe landraces (e.g., Bolita in Oaxaca, Chalqueño in central Mexico, and Comiteco/Olotón in Chiapas) as open systems (5, 6, 20). But the introduction rate of blanco is unusually high. It is three times the national rate for criollos and >10 times the rate in the southeast and central regions (Table 3). In principle, any rate of introduction could displace local seed if all seed is equivalent (Eq. 2), but there is often a lack of consensus on the properties of seed. If at least one farmer recognizes differences between local (l) and introduced (i) seed, as in Zoatecpan, Mexico (SI Table 6), diversity is maintained in a stable equilibrium given by nli = (qliC/(qilC + ril))0.5 (Eq. 5), because rli = 0 by construction. Introduced seed could eventually be taken for local if type recognition depends on information passed between farmers. Cross-replacement rates (i.e., qil and qli) might then change with relative type abundance and lead to displacement, but coexistence results when differences between local and introduced seed are observable and rates constant. Cross-replacement is also restricted when seed exchange depends on the identity of suppliers. Thus, in theory (Eq. 5), several subpopulations can be maintained when seed exchange is mostly among kin or within ethnic groups (18, 20). The nature and geographical scope of seed systems is bound to determine the genetic diversity of populations within and across localities, but more detailed information on seed exchange might be needed to explain observed genetic structure (5, 20).
Overall, widely distributed maize landraces might not satisfy the conditions implicit in Eq. 2. Differences in management of local and introduced seed can prevent the homogenization of any particular seed type. They can also prevent replacement of local types by so-called foreign varieties. It is argued that foreign varieties tend to complement rather than substitute local diversity, i.e., their spread is restricted to a niche (4, 13). Eq. 4 lends theoretical support to this complementarity hypothesis: various replacement patterns can prevent displacement of local types. An empirical test is not possible because the parameters of Eq. 3 have never been estimated. Our results nevertheless suggest that introduced seed is spreading, albeit not in all cases. The growth differential between introduced and local seed is approximately equal to the rate of introduction weighted by the survival rate of first-year introductions: λi − λl = r·pi,1. In the criollo population nationwide this amounts to 0.05 (0.08) = 0.004. In the southeast and central regions the differential is <0.001. Although this average rate of spread is negligible, some nonlocal germplasm spreads swiftly. In fact, the fastest growing segment in maize populations is new introduced seed of either a criollo or híbrido type (SI Table 5). A single lot can produce an average of 6.6 copies in 5 years, >1/2 of these within 1 year of introduction. As diffusion progresses and seed of a nonlocal type becomes available from local sources, the rate of spread of individual sources probably decreases, but the number of sources increases.
It is unlikely that introduced seed is displacing local types systematically. Accounts of displacement of major varieties are infrequent, e.g., tehuacan for pepitilla in Cuautla (6). Moreover, the conditions for displacement in Eq. 4 assume that introduced seed is kept as a distinct type, when in fact 1/2 of all locally acquired híbridos and one in six introduced criollos are hybridized (Table 3). It is not possible to determine at this point the precise consequences of hybridization for seed populations. More must be learned before this practice can be integrated into a demographic model. The key issue is whether mixed seed is considered a distinct nonlocal type that has been adapted to local conditions. Alternatively, mixed seed might be considered a local type that has been “renewed” or has acquired a new trait through hybridization (6, 19). In 2002, 1/5 of local criollo lots had been mixed at least once by current owners. If they were mixed with introduced materials, the latter probably ceased to exist as a type. The fate of introduced germplasm thus depends on type recognition. Local seed lines improved through hybridization are likely to diffuse, spreading introduced germplasm across a locality after it is no longer recognized as a distinct type. Diffusion of improved local seed across localities is less likely to occur because of agronomic and cultural heterogeneity (20), but regional differences should be expected inasmuch as introduced criollos are rare in the southeast and central regions but common in western Mexico.
Conclusions
Seed replacement and exchange across households can result in the spread of some local seed lines and the extinction of others, in some cases independently of any visible difference or agronomic advantage. Saving and selecting seed within the household also can lead to loss of diversity through continuous population bottlenecks. Because no collective action is undertaken to prevent bottlenecks or loss of vulnerable seed types, introduced seed might be necessary to maintain maize's phenotypic and genotypic diversity. Current accounts nevertheless tend to misrepresent the scope and nature of seed diffusion. The high rate of introduction in Cuzalapa is not representative of maize's center of diversity in southeast and central Mexico. Moreover, seed introduced into a locality does not necessarily become part of local seed stocks. Most introduced seed is discarded immediately, perhaps because it is not suitable for local preferences and agro-ecological conditions. A small fraction of introduced seed is nevertheless saved and diffused across a locality, contributing variation in the form of type diversity or, in the case of híbrido types, through introgression into local seed. Able to persist long after it disappears as a distinct type, this germplasm is bound to influence the evolution of seed stocks in the locality.
The evolution of maize is often described as the result of individual farmers painstakingly improving and maintaining diverse seed stocks. According to this perspective, seed diffusion often is a random force, akin to genetic drift, capable of preventing local adaptation through selection. In reality, seed diffusion and improvement are the result of a complex social process whereby new and old technologies are continuously assessed and appropriated. A narrower view of farmer-led evolution risks confusing breeders' and farmers' goals. Farmers' main goal is appropriating value, whether economic, cultural, or ritual. Whereas some might achieve this through improvement of local seed stocks, others might prefer to keep these stocks unchanged, defying our conceptions of improvement. Others may find it optimal to replace those stocks. It does not follow that seed improvement and conservation traditionally have been performed by farmers specialized as seed curators. Unlike modern maize farmers and breeders who specialize in distinct tasks, most Mexican farmers engage in seed improvement, diffusion, and farming simultaneously. Although individual management decisions have a specific intent (i.e., to preserve or replace seed), it is the sum of farmers' actions that drives changes in maize populations. These actions can have unintentional albeit predictable effects on the metapopulation dynamics of maize. Seed demography can shed light on the implications of farmer actions for the evolution of maize. Seed lots might seem arbitrary or inconsequential to scientists focusing on gene flows, which one might think would seem to compromise the integrity of seed lots and their importance as a unit of analysis. Yet seed lots are undeniably the object of farmer practices.
We have proposed a framework to formulate hypotheses on crop metapopulation dynamics and gather the data needed to rigorously test them. This article deals only cursorily with the social and environmental processes determining rates of change in seed populations. It offers a snapshot of maize seed dynamics across Mexico at a single point in time. Informal seed systems can be disrupted at times by catastrophic weather. Government or relief agencies might substitute temporarily for these systems, but normal dynamics are bound to return after seed diffusion through institutional channels ceases (25). Maize dynamics may have changed profoundly in the past decade as a result of social and economic forces, including trade (26). Although it has been suggested that the abandonment of traditional farming and habitat fragmentation may have altered maize population structure, the possible effects of these forces are still being sorted out (15, 16).
Methods and Data
The Demographic Unit.
The analysis of seed dynamics requires understanding the purpose and object of farmer practices. Farmers often seek to generate and appropriate value in crops through their decisions involving seed, not individual seeds but specific groups or types of seed. For instance, when farmers select maize seed, they cull ears matching an ideotype out of a harvest pile; when they choose seed, they decide which of different named types to grow on a plot of land; when they replace seed, it is a particular batch of seed they discard for another (4, 6). Thus, every decision involving type diversity has a specific (although possibly unintended) effect on the composition of local seed stocks. Maize farmers recognize an assortment of seed types that often cut across phenotypic and genotypic variability, such as color types. They also recognize a criollo type, i.e., a landrace, as distinct from an híbrido, a generic term for IVs and their advanced generations. IVs include true hybrids and open pollinated varieties, but not all farmers recognize this. Farmer classifications do not necessarily reflect a seed's common ancestry or genetic similarity, and rarely are they objective. Some types nest neatly, others overlap. Yet definitions of types undoubtedly endow maize populations with a particular demographic structure; e.g., seed often is replaced by the same color type, and one wide-kernel type can displace another (6).
Farmers also recognize specific varieties, i.e., modern cultivars and landraces, as distinct types. A maize landrace is a group of seed lots that share the same name and are considered by farmers as belonging to the same type (4). Implicit in this definition is the role of farmer subjectivity in naming and thus defining landraces, but this is clearly of the essence in crop diversity, inasmuch as only characteristics of value are named and preserved. Also implicit is the fact that landraces nest lesser types of seed, namely seed lots; i.e., the set of kernels of a specific type selected by a farmer and sown during a cropping season to reproduce that particular type (4). Seed lots are perhaps the most basic and tangible type of seed, and unlike varieties, they are defined objectively. A farmer can diffuse a seed lot by selecting more than one batch of seed from the same harvest pile and giving it to another farmer. This copy becomes a different seed lot, i.e., a distinct type. The set of copies of a single seed lot constitutes yet another more inclusive type that can be called a seed line. Although undeniably important, seed lines, varieties, and races are not the most appropriate units to describe crop population structure and dynamics. Their subjectivity is a drawback. More importantly, farmers manage these types of seed by acting on individual seed lots; e.g., replacing one variety for another entails discarding one seed lot and taking up another. This makes seed lots a natural unit of analysis to study seed population dynamics.
Management practices can be construed as events in the life history of seed lots. An event's probability, i.e., the probability that a farmer will manage seed in a given way, is associated with specific lot attributes (e.g., age or color) and defines a transition in the lot's life history. A seed lot “survives” across cycles if the farmer saves seed; it “reproduces” if the farmer diffuses seed among fellow farmers, and it “migrates” if it is introduced into a locality. Adopted seed becomes a “new” lot that can age and diffuse indefinitely. These events can be organized into an age-structured life graph, and a complete schedule of probabilities can be built into a life table (8). Articulating events within a demographic model can shed light on the ways in which management practices combine to shape seed population dynamics.
Model.
Consider a closed population N consisting of Nt seed lots at time t. At the end of the period, seed lots are saved with probability p and diffused with probability q from one farmer to C others. These new seed lots become part of the t + 1 population along surviving lots, such that Nt+1 = (p + qC) Nt. More generally, assuming constant survival and diffusion probabilities over time, population size at t is given by:
where λ is the population's expected growth rate. The population grows (i.e., λ > 1) if seed diffusion offsets seed loss or replacement. If survival and diffusion probabilities are the same for seed lots in N, λ is the growth rate of both N and every seed line in it. But even if λ > 1, specific seed lots or seed lines can become extinct unless there is a perfect negative correlation between seed survival and diffusion.
If there is a one-time introduction of nonlocal seed into the population at t = τ, such that N incorporates rNτ-1 introduced seed lots along with saved and locally diffused seed, then Nτ = (p + qC + r)Nτ-1. The number and proportion of introduced seed lots are, respectively, NI,τ = rNτ-1 and nI,τ = NI,τ/Nτ = r/(p + qC + r). Assuming that introduced lots are saved and diffused at the same rate as the local lots, the population grows at a rate of λ = p + qC after τ, so that Nt = λt−τ Nτ. Thus, the population at t consists of surviving lines (i.e., original lots plus copies) of the mixed-origin τ population, and the proportion of nonlocal seed (i.e., introduced lots plus copies) is constant. If introductions are continuous, the rate of introduction (r) becomes part of the population growth rate: Nt = (p + qC + r)t N0 = λtN0. Because the local subpopulation grows at the rate of λL = p + qC < λ, the proportion of local lots in the population decreases continuously:
At carrying capacity, λ = 1 and λL < 1, so the number of local lots drops exponentially until they are completely replaced by introduced seed.
The dynamics of distinct seed types can be analyzed by letting NI and NJ represent separate subpopulations of N. If all rates are homogeneous across subpopulations, then both NI and NJ grow at the rate of λ = p + qC + r. If rates differ, NI,t = (pI + qIC + rI)t NI,0 (and likewise for NJ,t). Interactions between subpopulations can be made explicit by decomposing diffusion and introduction rates: qI,0 = qII + qIJ nJI,0 and rI,0 = rII + rIJ nJI,0, where qII and qIJ are, respectively, diffusion rates of seed lots in NI with respect to itself and with respect to NJ (and likewise for rII and rIJ), and nJI,t = NJ,t/NI,t represents relative abundance at time t. Substituting and regrouping terms,
where λII = pI + qIIC + rII and sIJ = qIJC + rIJ represent subpopulation NI's intrinsic growth and its interaction with subpopulation NJ, e.g., seed replacement within NI and replacement of variety NJ by NI. Growth of NI is thus a function of nJI,t, whose rate of change is itself the ratio of NIs and NJs growth rates:
Inspection of Eq. 4 reveals two possible stable equilibria nJI: either growth rates balance out and subpopulations coexist, or one subpopulation prevails and the other becomes extinct; i.e., nJI = 0 or ∞. When λJJ = λII and sJI = sIJ, nJI converges to 1. If rates differ across types, subpopulations coexist as long as there is a strictly positive solution for nJI in λJJ − λII = sIJ nJI − sJI nJI−1; that is, as long as intrinsic growth differences are offset by replacement across populations. When differences are restricted to interaction terms (i.e., λJJ = λII and sIJ ≠ sJI), there is an analytical solution:
Subpopulations coexist whenever there is either some cross-replacement (sIJ > sJI > 0) or none at all (sIJ = sJI = 0) but not when replacement is one-sided (sIJ > sJI = 0).
Data.
Seed lot survival, diffusion, and introduction rates were estimated for select maize populations based on the 2002 ENRHUM. The survey was undertaken in January and February 2003 by the Programa de Estudios del Cambio Económico y la Sustentabilidad del Agro Mexicano, El Colegio de México, and the University of California at Davis, in collaboration with Mexico's census bureau, Instituto Nacional de Estadística, Geografía e Informática (INEGI). The ENHRUM sample consists of 1,769 households in 80 localities across 14 states; it is representative of 80% of the rural population nationwide and in each of Mexico's five census regions. INEGI defines as “rural” the population living in communities with <2,500 inhabitants.
ENRHUM provides detailed information on the activities and assets of the rural population. It is also the source of nationwide data on maize management presented here. The survey form is available online (http://precesam.colmex.mx/ENHRUM/PAG%20PRIN_ENHRUM_.htm). Information on every type of maize managed by a household at the time of the survey was gathered, including detailed data for 2002 and retrospective data on seed diffusion for the previous 5 years. Annual data were used to estimate rates of seed replacement, diffusion, introduction, and mixing. Rate differences were determined through the analysis of three-way tables based on log-linear models (7). The determinants of the diffusion process were estimated by using a zero-inflated-Poisson regression model and retrospective data (9). The model corrects for the presence of excessive no-diffusion events in a two-stage process by modeling the probability of diffusion with a binary logit (with parameters β) and the number of events with a Poisson count (with parameters γ) regressions. The date of seed acquisition was used to estimate the age of seed lots. Survival curves were adjusted to observed age distributions for different seed categories by using Weibull functions (8): Nt = N0 e−a, where a = (t/ωev/c)c.
Supplementary Material
ACKNOWLEDGMENTS.
We thank Paul Gepts for comments. This work was supported by grants from the University of California Institute for Mexico and the United States, Consejo Nacional de Ciencia y Tecnología, and the William and Flora Hewlett Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0706321105/DC1.
References
- 1.Smith BD. Proc Natl Acad Sci USA. 2001;98:1324–1326. doi: 10.1073/pnas.98.4.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fedoroff NV. Science. 2003;302:1158–1159. doi: 10.1126/science.1092042. [DOI] [PubMed] [Google Scholar]
- 3.Doebley J. Annu Rev Genet. 2004;38:37–59. doi: 10.1146/annurev.genet.38.072902.092425. [DOI] [PubMed] [Google Scholar]
- 4.Louette D, Charrier A, Berthaud J. Econ Bot. 1997;51:20–38. [Google Scholar]
- 5.Pressoir G, Berthaud J. Heredity. 2004;92:88–94. doi: 10.1038/sj.hdy.6800387. [DOI] [PubMed] [Google Scholar]
- 6.Perales RH, Brush SB, Qualset CO. Econ Bot. 2003;57:21–34. [Google Scholar]
- 7.Sokal RR, Rohlf FJ. Biometry. 3rd Ed. San Francisco: Freeman; 1995. [Google Scholar]
- 8.Ebert TA. Plant and Animal Populations: Methods in Demography. San Diego: Academic; 1999. [Google Scholar]
- 9.Lambert D. Technometrics. 1992;34:1–14. [Google Scholar]
- 10.Zeven AC. Euphytica. 1999;110:181–191. [Google Scholar]
- 11.Feder G, Just RE, Zilberman D. Econ Dev Cult Change. 1985;33:255–298. [Google Scholar]
- 12.Brush S, Taylor JE, Bellon MR. J Dev Econ. 1992;39:365–387. [Google Scholar]
- 13.Bellon MR. Hum Ecol. 1996;50:26–39. [Google Scholar]
- 14.Smale M. In: Valuing Crop Biodiversity: On-Farm Genetic Resources and Economic Change. Smale M, editor. Wallingford, UK: CABI; 2005. pp. 1–16. [Google Scholar]
- 15.Dyer GA, Yúnez A. NAFTA and Conservation of Maize Diversity in Mexico. Montreal: Commission for Environmental Cooperation; 2003. [Google Scholar]
- 16.Dyer GA. In: Valuing Crop Biodiversity: On-Farm Genetic Resources and Economic Change. Smale M, editor. Wallingford, UK: CABI; 2005. pp. 17–32. [Google Scholar]
- 17.Servicio Nacional de Inspección y Certificación de Semillas. Comportamiento de la Producción de Semillas Certificadas. Mexico City, Mexico: Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación; 2006. [Google Scholar]
- 18.Badstue LB, Bellon MR, Berthaud J, Juárez X, Rosas IM, Solano AM, Ramírez A. Hum Ecol. 2006;34:249–273. [Google Scholar]
- 19.Morris ML, Risopoulos J, Beck D. Genetic Change in Farmer-Recycled Maize Seed: A Review of the Evidence. Mexico, D.F., Mexico: Centro Internacional de Mejoramiento de Maíz y Trigo; 1999. [Google Scholar]
- 20.Perales RH, Benz BF, Brush SB. Proc Natl Acad Sci USA. 2005;102:949–954. doi: 10.1073/pnas.0408701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rice E, Smale M, Blanco JL. World Dev. 1998;26:1625–1640. [Google Scholar]
- 22.Sánchez JJ, Goodman MM, Stuber CW. Econ Bot. 2000;54:43–59. [Google Scholar]
- 23.Frankel OH. Biol Conserv. 1970;2:162–169. [Google Scholar]
- 24.Harlan JR. Science. 1975;188:618–621. doi: 10.1126/science.188.4188.617. [DOI] [PubMed] [Google Scholar]
- 25.Hintze LH, Renkow M, Sain G. Agric Econ. 2003;29:307–317. [Google Scholar]
- 26.Berthaud J, Clément JC, Emperaire L, Louette D, Pinton F, Sanou J, Second S. In: Broadening the Genetic Base of Crop Production. Cooper HD, Spillane C, Hodgkin T, editors. Wallingford, UK: CABI; 2001. pp. 81–103. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.