Abstract
American water shrews (Sorex palustris) are aggressive predators that feed on a variety of terrestrial and aquatic prey. They often forage at night, diving into streams and ponds in search of food. We investigated how shrews locate submerged prey using high-speed videography, infrared lighting, and stimuli designed to mimic prey. Shrews attacked brief water movements, indicating motion is an important cue used to detect active or escaping prey. They also bit, retrieved, and attempted to eat model fish made of silicone in preference to other silicone objects showing that tactile cues are important in the absence of movement. In addition, water shrews preferentially sniffed model prey fish and crickets underwater by exhaling and reinhaling air through the nostrils, suggesting olfaction plays an important role in aquatic foraging. The possibility of echolocation, sonar, or electroreception was investigated by testing for ultrasonic and audible calls above and below water and by presenting electric fields to foraging shrews. We found no evidence for these abilities. We conclude that water shrews detect motion, shape, and smell to find prey underwater. The short latency of attacks to water movements suggests shrews may use a flush-pursuit strategy to capture some prey.
Keywords: insectivore, olfaction, somatosensory, tactile, whiskers
The American water shrew (Sorex palustris) is the smallest mammalian diver, typically weighing only ≈15 grams. Yet this species is voracious and feeds on any prey it can overpower, including terrestrial, semiaquatic, and aquatic species. Water shrews most commonly prey on insects and other invertebrates (1), but they have repeatedly been observed diving and capturing fish (2–4). Historical observations of this sort occurred during daylight; however, water shrews have activity peaks during the hours of darkness (5). Consistent with this observation, we most often collect shrews along stream banks at night, when they can presumably forage with low risk of predation. How does this tiny mammal find prey, underwater, in near total darkness?
To address this question, we examined water-shrew hunting behavior in the laboratory using a high-speed video system and infrared lighting. We then examined their responses to water movements, shapes of imitation prey items, movement of stimuli, and electric fields. We also tested for the use of echolocation or sonar both above and below water by recording ultrasonic and audible calls. Finally, previous investigations have shown that water shrews can detect odorants while underwater with a specialized sniffing behavior (6). By observing this behavior, we were able to note the context in which shrews used underwater olfaction.
Our results indicate that water shrews can locate prey without eyesight using several different cues. We found no evidence for echolocation, sonar, or electroreception. However, water shrews vigorously attacked brief sudden water movements designed to simulate disturbances caused by escaping aquatic prey. They also sampled the shape of objects with vibrissae and sniffed stationary objects underwater. This seems to pose a conundrum for prey, which may be detected through touch or olfaction when immobile but may also reveal themselves by initiating an escape response.
Results
Water shrews have small eyes and a small optic nerve but prominent vibrissae in an organized geometric pattern typical of other semiaquatic small mammals (e.g., ref. 7). Under infrared lighting, shrews aggressively pursue crayfish and fish in water and are remarkably fast. Attacks were usually initiated in <25 ms after contact with the vibrissae or detection of water movements. Close pursuit of prey occurred with the mouth agape (Fig. 1), and this facilitated rapid grasping of prey that were overtaken. Although previous investigations have shown that seals can track fish based on wake turbulence (8), we found no evidence for this in water shrews. Rather, they seemed to probe in a haphazard manner and often lost track of prey that outpaced them. They were, however, quick to subsequently relocate many prey items through contact with the vibrissae, or when nearby prey moved. Movement seemed to be an important cue for prey detection, but first we addressed the use of eyesight during foraging by comparing capture latencies under lighted and infrared conditions (Fig. 2). Shrews were presented with minnows (Pimephales promelas) in a 13 × 17-cm rectangular Plexiglas enclosure set within a larger (50 × 25 × 16-cm) aquarium filled to a depth of 4.5 cm with water. Behavior was filmed with a high-speed video system.
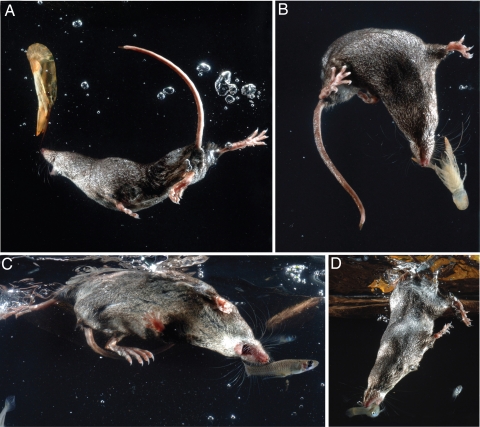
Fig. 1.
Water shrews in various stages of pursing and capturing prey. Shrews attacked fish and crayfish and often lunged with mouth open during pursuits. (Copyright for this figure is held by K.C.C.)
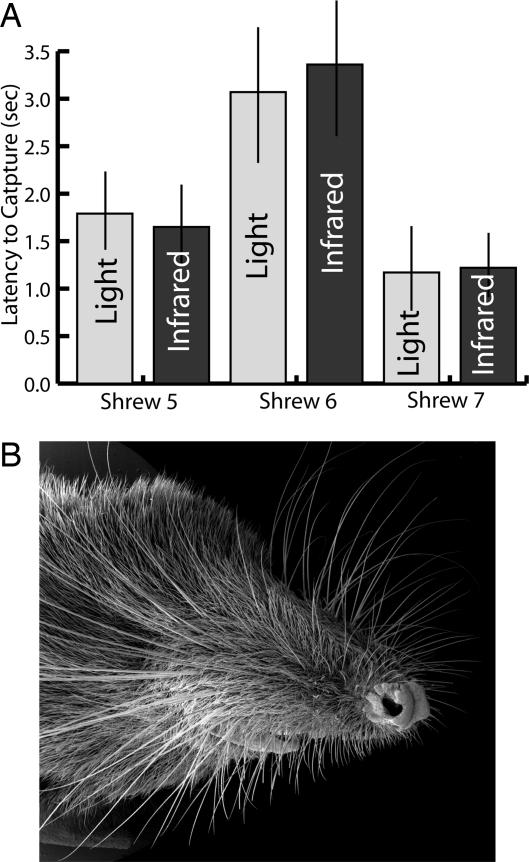
Fig. 2.
Evidence for water shrew sensory abilities. (A) Latency to capturing fish (time from entering water to grasping fish) was not different for three shrews under lighted or infrared conditions. Bars are SEM. Shrews can thus efficiently capture prey without eyesight. (B) Water shrew face under the SEM, illustrating the prominent sensory vibrissae. The eyes are small and in this case not visible behind the vibrissae.
In 10 trials for each condition for three shrews, there was no difference in latency to prey capture (time from entering water to capture) between lighted and infrared conditions (Fig. 2). Under both conditions, minnows were generally caught in 1–3 seconds, although in numerous trials, under both conditions, minnows were located, pursued, and captured in <1 second. Clearly, water shrews are capable of efficiently capturing prey without eyesight.
Responses to Water Movements.
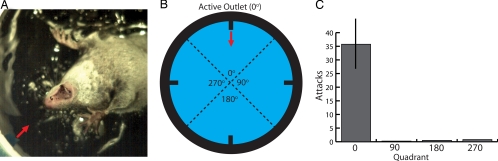
Observation of foraging shrews suggested water movement was an important potential cue, because prey often initiated C-start (fish) or tail-flick (crayfish) escape responses when the shrew was in close proximity [e.g., Fig. 1 and supporting information (SI) Movie 1]. To test this possibility, we generated water movements in a 10-cm-diameter circular chamber placed within a larger aquarium (as described above). Four 1.5-mm water outlets were connected by tubing to a pressurized water reservoir controlled through a programmable stimulator to provide a series of sudden and brief pulses of water (75-ms pulse of 0.5 cc every 150 ms). It was not possible to coordinate the water pulse with the shrew's position in the chamber. Instead, the repeated activation of the water pulse ensured that shrews randomly passed the outlet during one or more of the brief stimuli. Shrews were fed live fish between trials and were filmed under lighted conditions at 250 frames per second (fps) at high shutter speeds (<1 ms). Four animals were tested, and each vigorously attacked the water movements, as indicated by gaping lunges directed toward the outlet (Fig. 3a and SI Movie 2). Each animal was tested for 20 trials (20 entrances to chamber), and each consistently responded to the water movements in the randomly chosen active quadrant (Fig. 3 b and c). After gaping lunges at the water movement, shrews often performed an “underwater sniff” (SI Movie 2, end of clips 1–3), suggesting olfaction is used to explore the source of a water movement when no prey is detected.
Fig. 3.
Responses to brief water movements. (A) A frame from the high-speed video showing a shrew in midattack to a 75-ms water pulse (arrow) emanating from the outlet. (B) Schematic of the chamber used to test water shrews. Responses to water movements were scored for the four quadrants as indicated (0° corresponds to the active outlet). (C) Histogram showing the average number of attacks (open-mouthed lunges) in each quadrant for 20 trials for each of four shrews. Bars are standard error of the mean. See SI Movie 2 for behavior.
Responses to Prey Shape.
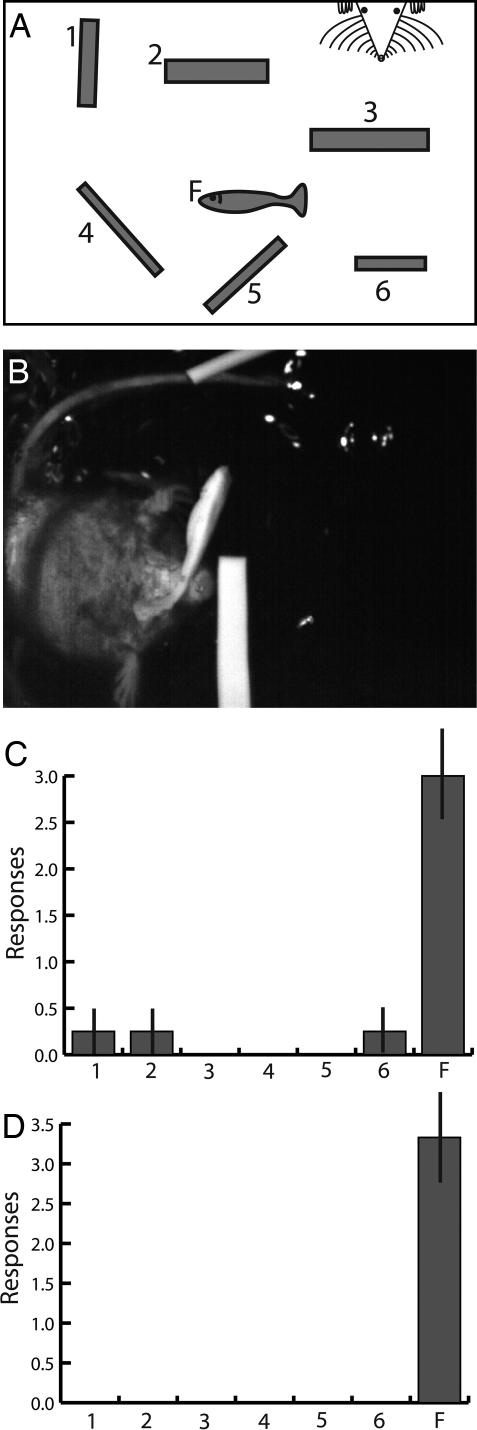
To test whether shrews learn the shapes and textures of nonmoving prey, silicone cast fish were made (see Materials and Methods) and presented along with six different cast silicone cylinders and rectangles (Fig. 4, SI Movie 3). Four shrews were tested in the previously described rectangular enclosure and filmed at 250 fps with infrared lighting. The shrews were fed dead fish before and between the trials. Shrews entered the chamber and explored the various shapes with vibrissae and occasional underwater sniffs. The first shrew (shrew 5) retrieved the model fish in 9 of 10 trials. This shrew was then presented with >50 other silicone cylinders and squares and retrieved the model fish (SI Movie 3, clip 5). The second shrew (shrew 6) retrieved the model fish three times, then chose a rectangle and stopped retrieving any silicone objects. The other two shrews (shrews 7 and 9) attacked or retrieved the model fish in two of four trials and four of four trials, respectively, then each stopped retrieving or attending to the silicone objects. Thus, three of four shrews showed a clear preference for the model fish (probability of randomly choosing the model fish, shrews 5 and 9, P < 0.001; shrew 6, P = 0.01), whereas for shrew 7, P = 0.10. Presumably our fish models were not perfect replicas (e.g., they had no lateral fins and were heavier than fish) and did not smell like fish (shrews often sniffed underwater), and thus shrews learned to discriminate them from real fish.
Fig. 4.
Paradigm for investigating responses to model fish. (A) Three silicone rectangles and three silicone cylinders were placed in the chamber (items 1–6) along with a silicone model fish (item F). (B) A single frame from the video showing a water shrew biting the model silicone fish (which was retrieved). (C) Histogram showing the average number of times the model fish was bitten (see SI Movie 3) compared with the other six items for the first four trials of the four shrews tested (after which three of four shrews stopped responding). (D) Histogram showing the average number of responses (responses included retrieving, biting, or lunging at the fish with open mouth) to the moving model fish for the first four trials of the three shrews that responded (see SI Movie 5). Bars are SEM. Contrast was enhanced in B.
We then examined responses to a moving nonprey object compared with the six nonmoving objects. A small piece of ferrous metal was placed into a silicone rectangle, and a spinning magnet below the aquarium generated movements. Shrew 5 investigated and retrieved the moving rectangle on the first trial and then closely investigated and sniffed it on the second trial before moving on (SI Movie 4). The other three shrews investigated the object and made aborted attacks as indicated by a brief open mouth lunge (e.g., SI Movie 4, clip 3) on the first (shrews 6 and 7) or first and second trials (shrew 7) and then no longer attended to the object. The number of responses (open mouth lunges or retrieval) for each shrew was small, and only one shrew actually retrieved the object from the enclosure. Thus, shrews were quick to discriminate the shape as inappropriate prey. In contrast, shrews usually retrieved the model silicone fish described above and then carried it to the home cage and often attempted to eat it or cached it with real food items.
As a final variation, a magnet was placed into a model silicone fish, and the model was moved in a circle by a motorized magnet below the aquarium. Six nonmoving nonprey shapes (rectangles and cylinders) were also placed in the enclosure. Shrews 5, 6, and 7 each responded to the moving model fish by attacking (biting) or lunging at the object with mouth open (SI Movie 5) in the first several trials (six times for shrew 5, three times for shrew 6, and twice for shrew 7), whereas shrew 9 ignored the moving model and all other objects. These results seem to demonstrate the greater stimulus value of movement combined with shape, because shrews 6 and 7 had stopped responding to the stationary model fish and moving rectangle (probability of randomly choosing the moving model; P < 0.0001 for shrew 5, P < 0.01 for shrew 6, and P = 0.02 for shrew 7).
Underwater Sniffing.
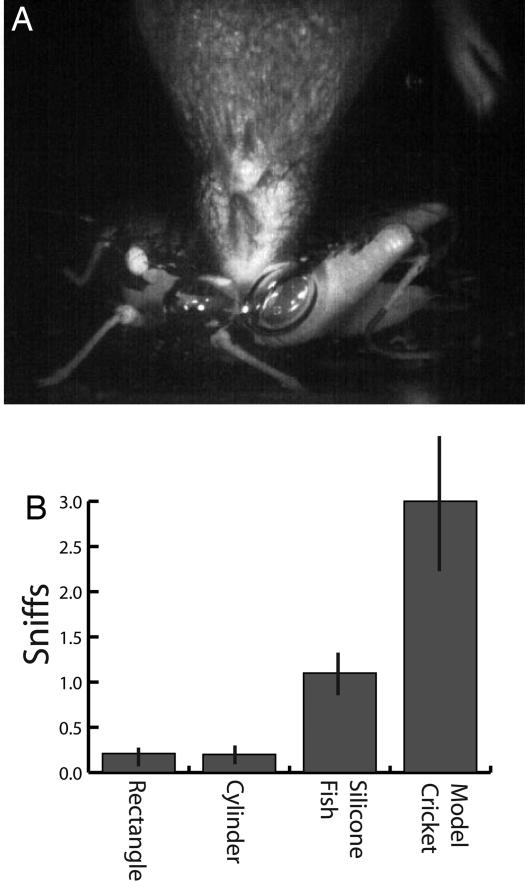
Throughout the behavior trials described above, water shrews exhibited an underwater sniffing behavior consisting of expiration and inspiration of air through the nostrils while submerged (6). A particularly interesting result was recorded when we presented a submerged model cricket to the shrews (Fig. 5A, SI Movie 6). In contrast to our silicone fish model, the model cricket was a commercially available clay (body) and metal (legs) simulation that had the gross features of an orthopteran insect but lacked fine structural detail (in contrast, tiny scales were reproduced on model fish). In response to this object, shrews extensively investigated the surface with microvibrissae and performed multiple underwater sniffs. Only one shrew bit the model (SI Movie 6, clip 1), but all shrews explored the model cricket in preference to the six other objects.
Fig. 5.
Underwater sniffing of four different objects. (A) Shrews attended particularly to the model cricket, which was extensively explored compared with other objects and repeatedly sniffed by each shrew as illustrated (see SI Movie 6). (B) The average number of sniffs made to different objects. Shrews occasionally sniffed the silicone cylinders and rectangles, usually the model fish, and the model cricket multiple times. Bars are SEM. Contrast was enhanced in A.
We determined the average number of sniffs made to different objects from the slow-motion video. Fig. 5b shows these data for the silicone distractors (rectangles and cylinders), the silicone fish, and the model cricket. Although shrews sniffed periodically throughout the trials, they most often sniffed when an object was encountered and explored with the microvibrissae. There was an ≈20% chance of sniffing a silicone distractor when encountered, whereas a cast fish was usually sniffed once, and the cricket was sniffed an average of three times when encountered.
Tests for Echolocation, Sonar, and Electroreception.
To examine whether water shrews were emitting ultrasonic pulses to aid in foraging, shrews were tested under several conditions both above and below water. These included an open field (both above and below water), an open field with an obstacle present (both above and below water), and an open field with a prey item present (earthworm) both above and below water. Recordings were made with a portable ultrasound processor connected to a U30 bat detector or hydrophone (see Materials and Methods). Behavior was simultaneously videotaped and an audible transducer was used for real-time monitoring of behavior and for synchronization of audio output with video recordings. No ultrasonic emissions were detected from five shrews tested under these conditions. However, as expected, some ultrasound was occasionally detected when claws were scraped to the substrate or during mastication.
To test for electrosensory abilities, three shrews were presented electrical stimuli in the circular chamber described. A battery-powered DC current source was controlled by a programmable stimulator and connected by stainless steel electrodes to the chamber through two 30-cm water bridges terminating in 1.5-mm openings in the edge of the chamber separated by 1 cm. Animals were filmed under lighted conditions at 250 fps and presented with current strengths of 2, 10, and 20 μA for both DC and square wave stimuli (five trials for each condition). No responses (bites, lunges, or open-mouth attack in electrode quadrant) were obtained. Finally, the skin surface of the snout and oral region of two water shrews was examined and surveyed under the scanning electron microscope to identify potential ampullary type (9) or duct gland electroreceptors (10). None were observed.
Discussion
Water shrews have predatory abilities that seem extraordinary given their small size and habit of diving in search of prey (11). Although a number of investigations have explored the sensory abilities of small mammals in terrestrial settings (e.g., refs. 12–16), we wondered how shrews might detect prey underwater where visual, olfactory, and tactile cues could be obscured or attenuated. Our results indicate that water shrews are efficient at capturing prey underwater and suggest they detect water movements, shape, and olfactory cues to identify prey. Our main strategy was to first film shrews with a high-speed video system and observe predatory behaviors for clues to how prey were detected. We then designed several experiments to test hypotheses resulting from these observations with a goal of eliciting predatory responses to stimuli simulating prey features. The results for each experiment are discussed below.
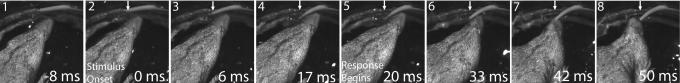
It is important to note that many of the behaviors cannot be observed without a high-speed video system. SI Movies 1–7 are supplied, and several clips show real-time behavior to provide a frame of reference for reaction times. This illustrates a general feature of water shrew behavior that contributes importantly to their predatory abilities: water shrews are fast. Attack latencies to underwater stimuli were on the order of 20 ms (to initiate movement toward stimulus) and in ≈50 ms from stimulus onset, shrews could move their open mouth 1–2 cm to the source (Fig. 6). These latencies are remarkably short, and the behaviors include accurate directional responses suggesting the shrew's nervous system might be specialized for rapid processing of sensory information. In addition to facilitating prey capture, moving and foraging quickly may be required, because small size dictates low O2 storage capacity and high mass-specific metabolism, limiting water shrews to short dives (11).
Fig. 6.
Latency to attack. To provide a detailed measure of reaction time, behavior was videotaped at 1 ms per frame as a shrew responded to a water movement (selected frames shown above). The water pulse came from an opening (arrow) covered with a flexible rubber tab, so stimulus onset could be visualized. The stimulus began at plate 2 (time 0), as indicated by the smallest initial movement of the rubber cover, and reached a maximum at 17 ms (plate 4). The first movement of the shrew toward the stimulus occurred by 20 ms (plate 5), and by 50 ms (plate 8), the shrew's open mouth was at the point of stimulation caused by the water (which was deflected leftward by the rubber tab).
Vision in Water Shrews.
Our behavioral trials revealed no difference in fish-capture latency between lighted and infrared conditions, demonstrating that water shrews can pursue and capture prey without eyesight. This is perhaps not surprising, given that they often hunt at night (5) and have small eyes (Figs. 1 and 2). However, water shrews are also active during the day, and it is possible they make use of eyesight for hunting, navigation, and predator avoidance in this context. Although it seems unlikely, it is also possible that the similar capture latencies illustrated in Fig. 2 reflect the balanced outcome of the combined use of eyesight for both predator and prey, i.e., the shrew's use of eyesight to pursue prey under lighted conditions could be offset by the fish's use of eyesight to evade the shrew. We are currently examining the number of fibers in the water shrew's optic nerve and the size of its visual cortex to further assess the role of vision in this species.
Responses to Movement and Shape.
Movement is a characteristic feature of most animals, and the salience of movement is well appreciated for sensory processing in the visual system (e.g., refs. 17–19). Thus, many predators and prey selectively attend to movement in a visual scene, for obvious reasons. Some predators, such as the painted red-start, capitalize on prey movement in a flush-pursuit strategy that triggers escape circuitry, and hence movement, to increase the visual salience of insect prey (20–22). Our results suggest water shrews could have a similar strategy using the somatosensory, rather than the visual, system.
Water shrews clearly attend to, and attack, water movements designed to simulate disturbances caused by escaping prey (Fig. 3, SI Movie 2). However, this was not a general response to any moving object. Minimal responses were obtained to constantly moving rectangles or cylinders. This is a telling contrast to the repeated responses obtained to brief pulses of water when no object was present. Two explanations for this difference can be offered. First, moving silicone rectangles do not have the characteristic shape or texture of prey. Second, water shrews often forage in flowing water where they encounter continuously moving objects (e.g., movement of vegetation in a stream and along its shore). In this context, the most important cue would presumably be a sudden movement associated with the shrew's approach. Our stimulus was designed to mimic this condition. Whether water shrews can be considered flush-pursuit predators that take advantage of escape responses requires further study of their microhabitat, corresponding prey distributions, and predator-prey interactions. However, as a final observation in the regard, it is interesting to consider clip 3 of SI Movie 1, during which a live fish and a dead fish were encountered simultaneously, and the live fish is pursued.
Tactile cues were apparently used for detecting prey in the absence of movement. Our model fish was a detailed reproduction down to the level of surface scales, although the lateral fins were lost in the casting process. Shrews preferentially retrieved the model fish and often spent considerable time trying to eat the model. Similar results have been obtained for Etruscan shrews (Suncus etruscus) hunting on land. Anjun et al. (16) used detailed plastic model crickets to elicit repeated attacks from Etruscan shrews in the absence of movement.
For the most part, water shrews tested in the present study learned to discriminate the fish model from real dead fish within approximately four trials. Their interest was briefly resurrected by moving the model with a magnet below the aquarium. This is perhaps expected, given that water shrews attended to shape and movement independently. As with the stationary model, shrews learned to subsequently discriminate the moving model from real fish. This is also not surprising, given the unnatural movement of the model fish caused by the rotating magnet (SI Movie 5), its consistent location at the bottom of the aquarium (in contrast to real fish), and the lack of olfactory cues.
In their study of Etruscan shrews, Anjun et al. (16) trimmed vibrissae to demonstrate their importance in guiding attacks. Although we did not trim water shrew whiskers, there is little doubt that vibrissae are likewise the major tactile sensory organs used for discriminating shapes and detecting water movements. This is evident from their behavior, the prominence of the whiskers (Fig. 2), their large representation in the somatosensory cortex (23), and the lack of other obvious mechanisms for such discriminations (see below). In addition, seals use whiskers to detect water movements (7) and to track fish (8).
Underwater Sniffing.
Studies of mammalian olfaction often require a mechanism for measuring sniffing, such as a thermistor in the nasal cavity, a chamber with pressure transducers, or thermal imaging equipment for detecting airflow (24, 25). In contrast, underwater sniffs can be observed using high-speed video (but see ref. 6 for olfactory discrimination task underwater). Although we did not test shrews on specific olfactory tasks in the present study, we were able to note when shrews sniffed underwater. Sniffs most often occurred as the shrew paused after contact of an object or wall, but shrews sometimes sniffed in open water. They also commonly sniffed after lunging at a brief water movement (e.g., SI Movie 2). Although not easily quantified, these behaviors are consistent with an important role for olfaction underwater. More quantifiable results come from comparing the number of sniffs made while exploring specific objects. Fig. 5 shows these data for the rectangle, cylinder, model fish, and model cricket. The number of sniffs was well correlated with the attentiveness of shrews to each object, as gauged by the time spent investigating with vibrissae (SI Movie 6). The reaction to the model cricket was surprising, because we expected either swift attacks, as in the case of the model fish, or brief contact and rejection, as in the case of rectangles and cylinders. Instead, the shrews extensively investigated the model cricket, yet only one shrew clearly bit the model. It was hard not to conclude that shrews were conflicted by the contradictory cues presented by the life-like general shape but inappropriate fine structure for an orthopteran insect. It is also unusual for a cricket to be submerged, and thus the location of the item was inappropriate. Finally, it is possible that the clay body, which was ultimately soluble in water, produced an odor detected by the shrews.
Echolocation and Electroreception.
Because water shrews forage underwater and can capture prey without eyesight, it seemed important to explore the possibility of additional mechanisms for detecting prey. A number of marine mammals use sonar (26), and the semiaquatic duck-billed platypus detects electric fields (27). In addition, some shrews produce ultrasound (28–30). In the present investigation, we found no evidence for these abilities. No ultrasonic emissions were detected while shrews foraged above or below water. Shrews also showed no response to DC or low-frequency (4-hz) fields producing currents of 2, 10, or 20 μA. Last, all passive electroreceptors that have been identified are visible as ducts or pits in the epidermis [e.g., ampullary organs on salamanders and fish (31) or duct-gland electroreceptors of the platypus (32, 33)]. No such receptors were found on the face of water shrews.
Taken together, our results suggest water shrews detect prey movement and form with their vibrissae while sampling odorants through underwater sniffing. These combined strategies provide an efficient foraging strategy that allows shrews to detect both stationary and escaping prey. Because water shrews are likely to be rare predators, they presumably exert little selective pressure on escape strategies for prey and thus could potentially take advantage of movement to aid in localization (34, 35). Alternatively, the shrews' ability to discriminate stationary objects through touch and olfaction underwater may make flight the best option for dexterous prey in any case. We have observed fish initiating escape responses just as shrews approached and sniffed, suggesting detection was imminent (SI Movie 7).
Materials and Methods
Ten water shrews were collected in PA or Manitoba, Canada, with Sherman live traps under permits COL00087 (U.S.) and WB06062 or WB06062 (Canada). Shrews were housed in Plexiglas cages (50 × 28 × 20 cm) containing peat moss, soil, and sphagnum moss with a water bowl and fed fish, mealworms, crickets, wax worms, and cat food. For behavior trials, shrew cages were connected to aquaria through 3.5-cm diameter flexible tubing. Shrews were filmed with an HS-3 or -4 camera (Redlake) with either fluorescent overhead lighting supplemented by fiber optic illuminators or with four 42-light-emitting diode compact infrared illuminators (Rainbow). Procedures met guidelines set by the National Institutes of Health, the Animal Welfare Act, and the Vanderbilt University Institutional Animal Care and Use Committee. Please note that expanded methods for some components are included as SI Text.
Responses to Water Movement.
To examine responses to water disturbances, a modified primate reward delivery system (Crist Instruments, model 5-RPD-M1) with a pressurized liquid reservoir was controlled by a Master 8 digital stimulator (A.M.P.I.) to provide water pulses to the previously described enclosure through one of four randomly chosen 1.5-mm outlets at a rate of one 0.5-cc pulse every 150 ms. Trials began with the shrew's voluntary entrance to the chamber and concluded with its exit. Shrews were fed live fish before and between trials. High-speed video was captured with MotionProX software (Redlake) on a Macintosh computer.
Model Prey.
Model fish were made by suspending dead Pimephales promelas in a container filled with AeroMarine Silicone RTV rubber (John Greer). After curing, the mold was split and washed with water and alcohol, and the cavity was lightly coated with petroleum jelly. The mold was reassembled and filled with AeroMarine Silicone, using a syringe, and cured. Various cylinders and rectangles were also cast. All castings were washed with water and alcohol before trials. To generate movements, a magnet or piece of iron was inserted. The motor and magnet from a stir plate were positioned below the aquarium to produce movements. The model cricket was an imitation made of clay and wire (Kathy's Critters).
Scanning EM.
Tissue was fixed in 4% paraformaldehyde, dehydrated through alcohols, critical point-dried using CO2 in an E3000 drier (Quorum Technologies), sputter-coated with gold, and viewed in a Tescan Vega II microscope (Tescan).
Echolocation Tests.
Five shrews were tested in 94 × 48 × 50-cm glass-enclosed terrestrial and aquatic settings (17.5 cm of 20°C tap water) with and without earthworm prey or obstacle (15 cm of 3.8 cm OD black PVC pipe) on the bottom, 50 cm from a 20.3-cm cinder block platform, illuminated by a 25-watt incandescent red bulb, and cleaned between trials. Behavior was videotaped with a Sony DCR-TRV120 camcorder (“NightShot” setting), and audible (frequency-divided) output from an Ultra Sound Advice U30 (Ultra Sound Advice) bat detector (20–200 kHz, terrestrial trials), or Sonotronics DH-3 (Sonotronics) hydrophone (100 Hz–75 kHz, aquatic trials) was recorded simultaneously to test whether sound production was associated with predation or navigation. Trials began with the shrew's introduction to the platform from a PVC tube and continued after the subject left the platform (open-field trials) or discovery of obstacle/prey item in object-based trials for 10- to 19-second sampling periods wherein ultrasound cycled through an Ultra Sound Advice Portable Ultrasound Processor (Ultra Sound Advice; 224-kc/sec sampling rate) and was archived at 1:5 expansion to MiniDisc (Sony MZ-N707S recorder).
Electric Fields.
Stimuli from a battery-powered DC source were controlled by a Master 8 programmable stimulator to successively provide 2-, 10-, and 20-μA currents. Two electrodes were connected through water bridges (3-mm i.d. tygon tubing) to two openings in the chamber (see Results). Water conductivity was 190 S/cm at 18°C. Each of three animals was tested for five trials each with square-wave stimuli (75 ms on, 150 ms off) and DC fields at each stimulus magnitude. Stimuli progressed from lower to higher currents (2, 10, and then 20 μA) in the successive five trial blocks. Electrode position was randomly chosen. Shrews were fed live fish before and between trials. All trials were videotaped at 250 fps under lighted conditions.
Supplementary Material
ACKNOWLEDGMENTS.
We thank Danielle Gauthier, Jon Ledford, Troy Apple, Ken Salleng, Karen Jackson, and the Vanderbilt Department of Animal Care for exceptional care of the water shrews. This work was supported by a National Science Foundation Career Award and a MacArthur Award (K.C.C.) and by Natural Sciences and Engineering Research Council Discovery grants (K.L.C. and J.F.H.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0709534104/DC1.
References
- 1.Conaway CH. Am Midland Nat. 1952;48:219–248. [Google Scholar]
- 2.Lanpman BH. J Mamm. 1947;28:1. [Google Scholar]
- 3.Nussbaum RA, Maser C. The Murrelet. 1969;50:23–24. [Google Scholar]
- 4.Buchner CH. The Blue Jay. 1970;28:171–172. [Google Scholar]
- 5.Sorenson MW. Am Nat. 1962;68:445–462. [Google Scholar]
- 6.Catania KC. Nature. 2006;444:1024–1025. doi: 10.1038/4441024a. [DOI] [PubMed] [Google Scholar]
- 7.Dehnhardt G, Hyvaerinen H, Palviainen A, Klauer G. J Comp Neurol. 1999;411:550–562. doi: 10.1002/(sici)1096-9861(19990906)411:4<550::aid-cne2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 8.Dehnhard G, Mauck B, Hanke W, Bleckmann H. Science. 2001;293:102–104. doi: 10.1126/science.1060514. [DOI] [PubMed] [Google Scholar]
- 9.Zakon HH. In: Electroreception. Bullock TH, Heiligenberg W, editors. New York: Wiley; 1986. pp. 103–156. [Google Scholar]
- 10.Gregory JE, Iggo A, McIntyre K, Proske U. J Physiol. 1988;400:349–366. doi: 10.1113/jphysiol.1988.sp017124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gusztak RW, MacArthur RA, Campbell KL. J Comp Physiol B. 2005;175:87–95. doi: 10.1007/s00360-004-0465-x. [DOI] [PubMed] [Google Scholar]
- 12.Carvell GE, Simons DJ. J Neurosci. 1990;10:2638–2648. doi: 10.1523/JNEUROSCI.10-08-02638.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartmann MJ. Auton Robots. 2001;11:249–254. [Google Scholar]
- 14.Salines E. Annu Rev Neurosci. 2001;24:107–137. doi: 10.1146/annurev.neuro.24.1.107. [DOI] [PubMed] [Google Scholar]
- 15.Crish SD, Rice FL, Park TJ, Comer CM. Brain Behav Evol. 2003;62:141–151. doi: 10.1159/000072723. [DOI] [PubMed] [Google Scholar]
- 16.Anjum F, Turni H, Mulder PGH, van der Burg J, Brecht M. Proc Natl Acad Sci USA. 2006;103:16544–16549. doi: 10.1073/pnas.0605573103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ewert JP, Arend B, Becker V, Borchers HW. Brain Behav Evol. 1979;16:38–51. doi: 10.1159/000121822. [DOI] [PubMed] [Google Scholar]
- 18.Ewert JP, Borchers WH, Weitersheim A. J Comp Physiol A. 1978;126:43–47. [Google Scholar]
- 19.Ewert JP, Buxbaum-Conradi H, Dreisvogt F, Glagow M, Merkel-Harff C, Rottgen A, Schurg-Pfeiffer E, Schwippert WW. Comp Biochem Physiol A. 2001;128:417–461. doi: 10.1016/s1095-6433(00)00333-0. [DOI] [PubMed] [Google Scholar]
- 20.Jablonski PG, Strausfeld NJ. Brain Behav Evol. 2000;56:94–106. doi: 10.1159/000006680. [DOI] [PubMed] [Google Scholar]
- 21.Jablonski PG. Proc R Soc London Ser B. 2001;268:1017–1022. doi: 10.1098/rspb.2001.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jablonski PG, McInerney C. Ethology. 2005;111:381–396. [Google Scholar]
- 23.Catania KC. J Comp Neurol. 1999;410:55–72. [PubMed] [Google Scholar]
- 24.Settles GS. J Fluids Eng. 2005;127:189–218. [Google Scholar]
- 25.Kepecs A, Uchida N, Mainen ZF. Chem Senses. 2006;31:167–179. doi: 10.1093/chemse/bjj016. [DOI] [PubMed] [Google Scholar]
- 26.Norris KS. In: The Biology of Marine Mammals. Anderson HT, editor. New York: Academic; 1969. pp. 391–424. [Google Scholar]
- 27.Pettigrew JD. J Exp Biol. 1999;202:1447–1454. doi: 10.1242/jeb.202.10.1447. [DOI] [PubMed] [Google Scholar]
- 28.Gould E, Negus NC, Novick A. J Exp Zool. 1964;156:19–38. doi: 10.1002/jez.1401560103. [DOI] [PubMed] [Google Scholar]
- 29.Tomasi TE. J Mamm. 1979;60:751–759. [Google Scholar]
- 30.Forsman KA, Malmquist MG. J Zool London. 1988;216:655–662. [Google Scholar]
- 31.Fritzsch B, Wahnschaffe U. Cell Tissue Res. 1983;229:483–503. doi: 10.1007/BF00207693. [DOI] [PubMed] [Google Scholar]
- 32.Andres KH, von During M, Iggo A, Proske U. Anat Embryol. 1990;184:371–393. doi: 10.1007/BF00957899. [DOI] [PubMed] [Google Scholar]
- 33.Bohringer RC. Aust Mamm. 1981;4:93–105. [Google Scholar]
- 34.Jablonski PG. Behav Ecol. 1999;10:7–14. [Google Scholar]
- 35.Dawkins R. The Extended Phenotype. Oxford, UK: Oxford Univ Press; 1982. pp. 64–67. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.