Abstract
The ecological impacts of the indirect effects of predators are well established, but the evolutionary consequences are unknown. Predators often decrease prey density, which indirectly increases the resources available to surviving prey. This ecological effect could provide a link to evolution because it is generally assumed that resource availability influences life history evolution. Yet, predictions from theory that consider food availability are inconsistent, and evidence for an important role of resources in shaping life history evolution is absent. We compared life history traits in a Trinidadian killifish, Rivulus hartii, from fish communities that differ in predation intensity; predators are associated with lower population density and faster growth rates. To determine whether the indirect effects of predators influence evolutionary change, we reared second-generation-born fish under two food levels that approximated natural differences in resources between communities. Rivulus from sites with predators are younger and smaller at maturity. They have increased reproductive investment and produce many small eggs and smaller hatchlings. Such divergence is predicted as a direct effect of predation. We also found significant interactions between predator community and food level for age and size at maturity, fecundity, and egg size. These interactions, whereby the differences between communities were more pronounced at high-food levels, argue that evolution in Rivulus has been influenced by the indirect effects of predators mediated through resource availability. Rivulus from sites with predators better exploit the higher resources in those habitats. Therefore, both direct and indirect effects of predators have evolutionary consequences.
Keywords: density, guppy, resource availability, trophic cascade
Resource availability has historically been considered an important selective pressure on the evolution of life histories. For instance, Lack (1, 2) proposed that clutch size variation is driven by food limitation, although differences in resource availability were implicitly assumed in r- and K-selection (3, 4). Despite this interest, evidence demonstrating how resource availability molds the evolution of general life history strategies is severely lacking. One reason is that life history theory has failed to yield consistent predictions as to how evolution should proceed when resources are limited (5–7). Furthermore, differences in resource availability often covary with additional ecological selective pressures such as population density and predators. For example, although predators have the direct effect of increasing mortality rates, they also have the indirect effect of reducing the population density of prey and possibly increasing resource availability to surviving prey (8–10). Therefore, the indirect effects of predators, which are a prevalent feature of ecological systems (8), potentially have evolutionary consequences that are mediated through resource availability. However, theory suggests that evolutionary responses to resource availability may be similar to (or opposite to) responses caused by the direct effects of predator-induced mortality (5–7, 11). For these reasons, independently evaluating the direct and indirect effects of predators and elucidating associated influences of resource availability on evolutionary change in nature are daunting.
An effective way to reveal the influence of resource availability on evolutionary change is to measure interactions between food levels and fitness (12–14). Falconer and Latyszewski (12) selected for increased growth rates in mice reared at either high or low levels of food availability. They were successful in selecting for higher growth rates in both environments. However, when they compared selected lines, they found significant interactions between ration level and growth rate; mice selected for high growth rates on high rations grew faster than the mice selected for high growth rate on low rations, but only when both were reared on high food rations. When they were reared on low food rations, these differences in growth rate disappeared. Analogous interactions between resource quality and fitness were observed in selection experiments on Pseudomonas fluorescens in which selected lines were reared over multiple generations on different nutrient concentrations (14). These selection experiments are important because they clearly demonstrate that resource availability can shape how organisms evolve, and the examination of interactions between multiple food treatments and fitness best illuminates the responses to resource-based selection.
Fish communities on the island of Trinidad provide a forum for evaluating the life history consequences of the direct and indirect effects of predation. A killifish, Rivulus hartii, exhibits a greater dispersal capability than all other species, which allows them to colonize aquatic environments that are not accessible to other species. As a result, Rivulus are repeatedly found in localities in which they are the only species present (hereafter “Rivulus-only” sites) but also in “high-predation” sites that contain the highly piscivorous Crenicichla alta and Hoplias malabaricus. These differences in community composition are important because Rivulus suffer increased mortality rates in sites with predators; their probability of survival per 60 days is 20% higher in Rivulus-only compared with high-predation localities (J.F. Gilliam and D.F. Fraser, personal communication). Theory predicts that high-predation Rivulus should evolve earlier maturation and increased reproductive investment in response to higher rates of predation (5, 15, 16). However, population densities dramatically decrease (seven times), and rates of growth increase (double) in sites with predators (17, 18). These large differences in density and growth are apparently caused by a much lower abundance of Rivulus when they co-occur with predators. Because these sites do not differ in water temperature, which can affect growth rates, and because Rivulus from these two types of localities do not differ in growth rate when compared in a laboratory environment with controlled levels of food (see Results), we interpret these differences in nature as most likely being caused by higher food availability in high-predation sites that are indirect consequences of predation (19). This confounding effect of predation and resource availability means that both factors can play a role in shaping Rivulus life history evolution (11).
Here, we quantify the differences in life histories of Rivulus from Rivulus-only and high-predation communities after two generations of common-garden rearing. We assume that differences that persist for two generations in a common environment have a genetic basis. Because Rivulus suffer higher mortality rates in sites with predators, we predict that Rivulus from high-predation communities will attain sexual maturity at an earlier age and have higher levels of reproductive effort than their counterparts from Rivulus-only communities. More importantly, to evaluate the extent to which the indirect effects of predators mediated through resource availability have influenced trait evolution, we rear Rivulus under multiple food levels that approximate natural differences in resource availability and assess interactions between food level and predator community. If differences in resource availability are important factors in the evolution of this species, then the trait variation between Rivulus from Rivulus-only and those from high-predation communities will be a function of the food rations that they receive. For example, Rivulus-only fish may actually mature earlier than high-predation fish when reared on a ration that approximates their rate of growth in nature (11, 20).
Results
Rivulus exhibit clear life history differences that are correlated with predation intensity (Table 1). More importantly, significant interactions for most dependent variables show that the differences between Rivulus from high-predation versus Rivulus-only communities depend on food level (Table 1). Below, we first evaluate differences between fish communities resulting from the independent influence of each main effect, and subsequently we examine the significant statistical interactions among treatments.
Table 1.
Analyses for life history traits
| Effects | Trait, df | Male age at maturity, F | Male size at maturity, F | Female age at maturity, F | Female size at maturity, F | Fecundity, F | Egg size, F | Larval size, F | RA, F |
|---|---|---|---|---|---|---|---|---|---|
| Predator | 1 | 256.6*** | 164.59*** | 56.8*** | 7.13** | 10.61** | 20.85*** | 12.25*** | 9.46** |
| River | 1 | 26.4*** | 16.68*** | 1NS | 3.53NS | 0.2NS | 0.288NS | 0.055NS | 0.6NS |
| Food | 1 | 35.1*** | 28.16*** | 50.9*** | 43.86*** | 25.96*** | 3.35+ | 4.16* | 5.24* |
| Predator × Food | 1 | 6.9* | 8.22** | 4.6* | 5.84* | 3.82+ | 4.2* | 0.56NS | 0.05NS |
| River × Food | 1 | 0NS | 0.002NS | 0NS | 0.14NS | 0.556NS | 0.218NS | 0.443NS | 0.004NS |
| Predator × River | 1 | 1.8NS | 0.005NS | 2.1NS | 1.07NS | 0.038NS | 0.753NS | 2.43NS | 1.63NS |
| Predator × River × Food | 1 | 2.4NS | 0.45NS | 1.2NS | 0.579NS | 0.928NS | 1.22NS | 0.323NS | 1.5NS |
| RSS (df) | 0.093 (83) | 0.208 (83) | 0.116 (79) | 0.45 (79) | 15.9 (77) | 2e-7 (74) | 2e-7 (64) | 2.7 (74) |
Bold entries represent significant terms; RA, reproductive allotment; RSS(df), residual sums of squares (degrees of freedom); NS, not significant (P > 0.1); +, 0.05 < P < 0.1;
*, P < 0.05;
**, P < 0.01;
***, P < 0.001.
Predator Effects.
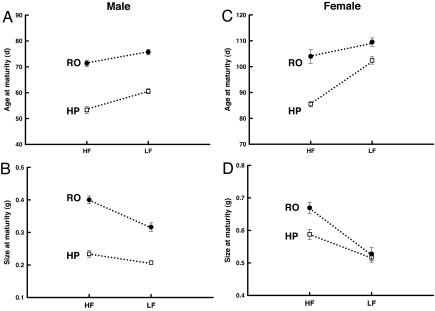
In both males and females, Rivulus from high-predation sites matured significantly earlier and at a smaller size than Rivulus-only sites (Fig. 1). The differences in maturation between these fish communities are dramatic. High-predation males and females matured on average 17.5 and 14.2 days faster than Rivulus-only fish, which results in a 15% reduction in the time required to begin reproducing. This rapid maturation by high-predation fish corresponded to sizes at maturity that were 35% and 10% smaller, by wet weight, than Rivulus-only males and females, respectively.
Fig. 1.
Differences in age and size at maturity between fish communities. (A) Male age at maturity. (B) Male size at maturity. (C) Female age at maturity. (D) Female size at maturity. □, high-predation; ●, Rivulus-only; HF, high food; LF, low food; HP, high predation; RO, Rivulus only. Error bars indicate ±1 SE. Significant predator and food effects (P < 0.05) were found for all four traits. Significant interactions between predator community and food level were also observed for each trait.
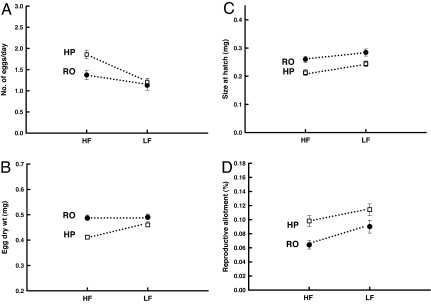
High-predation fish produced significantly more eggs in the 2-week period after maturation than Rivulus-only fish (Fig. 2A and Table 1). On average, high-predation females produced 25% more eggs daily. In addition, Rivulus from high-predation sites produced significantly smaller eggs (Fig. 2B and Table 1) that yielded larvae that were significantly smaller at hatching. Larval dry weight was ≈10% lighter in high-predation populations (Fig. 2C and Table 1). Finally, daily reproductive investment was significantly higher in high-predation females (Table 1). This variable takes into account the contrasting rates of egg production and egg sizes between communities and shows that at a given body size, high-predation females exhibit a 25% higher daily reproductive allotment (Fig. 2D). High-predation fish are able to maintain a 25% higher reproductive allotment even though their eggs are smaller because they initiate reproduction at a smaller body size.
Fig. 2.
Differences in fecundity (A), egg size (B), larval size at hatching (C), and reproductive allotment (D). □, high predation; ●, Rivulus only; HF, high food; LF, low food; HP, high predation; RO, Rivulus only. Error bars indicate ±1 SE. Significant predator and food effects (P < 0.05) were found for all traits, although the influence of food availability on egg size was marginally nonsignificant (0.05 < P < 0.1). Significant interactions between predator community and food level were observed for fecundity and egg size.
Food Effects.
All fish were measured for total length and wet weight every 20 days to quantify rates of growth on each ration level. High-predation and Rivulus-only fish grew at similar rates on each ration level because there were no significant differences between the slopes of the size-at-age curves (F1,129 = 0.727, P = 0.4). Therefore, the differences in growth observed in the field are phenotypic in origin and likely represent contrasting resource levels between Rivulus communities.
There was a highly significant effect of ration level on age and size at maturation in both males and females (Fig. 1 and Table 1). Limited food resulted in delayed maturation in both males and females by an average of 7 and 12 days, respectively. Size at maturity was ultimately smaller in the low-food treatments because both sexes were ≈20% smaller at maturation when reared at low food.
Low food resulted in the production of significantly fewer eggs in all populations (Fig. 2A). The effect of limited food was quite strong, as indicated by the 35% decline in daily egg production in low-food treatments. Additionally, there was a marginally nonsignificant (0.05 < P < 0.10) effect of resource availability on egg size that differs among populations (Fig. 2B). High-predation fish responded to low food by increasing egg mass, whereas Rivulus-only fish produce large eggs regardless of the ration level (Fig. 2B). Furthermore, both populations responded to less food by significantly increasing size at hatching. Larval size at hatching was 10% larger in low-food treatments (Fig. 2C and Table 1). Finally, daily reproductive allotment was significantly higher in the low-food treatments (Fig. 2D).
River Effects.
We observed a significant effect of river of origin only for male age and size at maturity; males from the Guanapo River matured earlier and at a smaller size than males from the Arima River.
Statistical Interactions.
We obtained a significant interaction between predator community and food level for all dependent variables except larval size and reproductive allocation (Table 1 and Figs. 1 and 2). For each of these traits, the magnitude of the trait differences between high-predation and Rivulus-only communities was smaller under low-food conditions, and in some cases (female size at maturity, fecundity) there were no differences between localities under low food levels.
Discussion
Our results revealed strong genetic differences in life history traits between fish communities that differ in predation intensity (Figs. 1 and 2). Rivulus from high-predation sites mature significantly earlier and at a smaller size than those from Rivulus-only communities (Fig. 1). In addition, Rivulus produce many small eggs that yield a smaller size at hatching in sites containing predators (Fig. 2). These changes generally concur with the predictions of theory that considers predator-induced extrinsic mortality (5, 15, 16). Our most salient findings, however, were the significant statistical interactions between predation and food availability for age and size at maturation in both sexes as well as fecundity and egg size. These interactions strongly suggest that the observed patterns of trait evolution are not solely direct responses to predation (Table 1). In all cases, differences in trait values between high-predation and Rivulus-only fish are less pronounced under low-food conditions than they are at high food levels (Figs. 1 and 2). For two of these traits (female size at maturation and fecundity) no differences between fish communities under low-food conditions were observed. Based on the known differences in mortality, population density, and growth rate between fish communities (17, 18), a likely explanation for the significant interactions is that Rivulus-only fish are adapted to chronically low levels of resource availability, whereas Rivulus from high-predation sites have evolved to exploit the consistently higher levels of food availability that they encounter in nature (20). These results are important because prior work on natural populations failed to observe a relationship between intraspecific density, resource availability, and life history traits (21). Therefore, here we provide evidence that the indirect effects of predators have evolutionary consequences, and more importantly, differences in resource availability influence life history evolution in nature.
The results of Drosophila selection experiments provide a clear example of the selective influence of population density and resource availability on the evolution of general life history strategies (22–29). Mueller et al. (22–25) reared populations of Drosophila at high or low densities and evaluated the evolution of population growth rates, which is a composite of the life history traits considered in the present work. This research showed that the high-density selected lines evolved higher population growth rates under high-density conditions, whereas the opposite was observed for low-density conditions (22, 24). Furthermore, significant interactions between selected line and population density were demonstrated for larval survivorship, larval-to-adult development time, and adult body size (26). Even though resource availability was not specifically evaluated, it was generally assumed that food was limiting in the high-density lines. However, a subsequent experiment whereby only the larval stage was subjected to high or low densities explicitly demonstrated that resource availability can influence resultant trait evolution. Examination of trait expression across multiple ration levels revealed significant interactions between food level and selected line for larval survivorship because the high-density larval lines had lower survival only at low levels of food availability (27). Similar interactions between selection regime and food treatment were also observed in Drosophila lines that were selected for increased and decreased weight at ecolsion under two contrasting food levels (28, 29).
As in the Drosophila selection experiments, the interactions between growing environment and trait values in Rivulus are important because they suggest that differences in the relative fitness between Rivulus communities are also context-specific. Rivulus-only fish never “outperformed” high-predation fish by maturing earlier or producing more eggs, as observed in the selected Drosophila lines (24), but the magnitude of the differences in life history traits was either reduced or disappeared entirely at low food availability. Similar environment-specific trait profiles between the Drosophila experiments and Rivulus further support the notion that resource availability is an important factor in the evolution of Rivulus. Also, these traits alone are an incomplete measure of fitness; Rivulus-only fish also produce larger offspring, which have been shown to have higher fitness in low-food environments in guppies found in these same streams (see below; ref. 30).
In Trinidad, guppies Poecilia reticulata are found in sites with and without the presence of large predators; and in agreement with age-specific mortality theory (5, 15, 16), high-predation guppies have evolved a smaller offspring size (31), earlier maturation, a smaller size at maturity, a greater frequency of reproduction, increased fecundity, and a higher reproductive effort (32, 33). Interestingly, guppies do not display the same interactions between predator community and food level as seen in Rivulus. Two possibilities, which are not mutually exclusive, may contribute to these contrasting patterns across species. First, population density or resource availability may exert a greater influence on evolutionary change in Rivulus. Rivulus densities are inversely related to stream size and can be many times that of guppies in small tributaries but several times lower in larger streams (17). For instance, the density of Rivulus is at least seven times greater in Rivulus-only sites than in high-predation sites, whereas the density of guppies differs by only 3-fold in the same river system (17). However, other facets of research on guppies have illustrated a potential role for density dependence and resource availability as an agent of selection. For instance, guppies experience uniformly higher rates of extrinsic mortality across all age classes in high-predation sites (34), and age-specific life history theory predicts no evolutionary change in this case (15, 16). Yet introduction experiments have clearly demonstrated life history evolution (35, 36). Theory that incorporates density regulation and the indirect effects of predators when mortality is uniformly distributed across age classes can provide a better fit to the results obtained for guppies (11, 16, 37). For example, higher reproductive effort may evolve when extrinsic mortality is not size-selective and the organism is density-regulated via juvenile-stage mortality (36). Similar effects of population density and resource availability have also been indicated in the evolution of senescence (38) and offspring size (39). Alternatively, subtle methodological and biological differences may explain the divergent results between Rivulus and guppies. Rivulus were started on quantified food at an earlier age (20 days vs. 25 days for guppies), yet they are older at maturation than guppies. The Rivulus food treatments also began at a slightly lower level than guppies. As a result, Rivulus are exposed to each food treatment for a longer period, which may increase the likelihood of detecting statistical interactions.
Adaptive Significance of Larval Traits.
The potential importance of resource availability is also reflected in the differences in larval trait plasticity in Rivulus from the two types of localities. Both fish communities produced fewer eggs under low-food conditions (Fig. 2A), but only high-predation fish responded to low food by increasing the size of eggs. Rivulus-only fish showed no such response (Fig. 2B). Furthermore, the eggs produced by females fed a limited ration generally yielded larvae that were larger at hatching (Fig. 2C). The production of larger eggs and/or larvae in the low-food treatments is important because a larger egg and offspring size can facilitate a higher larval survival (40), particularly in competitive environments (30). More importantly, Bashey (39) observed the same patterns of plasticity in offspring size in guppies. She compared the sizes of offspring produced by female guppies from high- and low-predation environments when reared on a limited food rations and found that guppies from high-predation environments produced larger offspring when they were reared on low-food availability. Guppies from low-predation environments instead equally provisioned offspring under high and low food rations. Because low-predation guppy and Rivulus-only sites are characterized by high densities and slow rates of growth (17–18), intraspecific competition is likely intense. Consequently, the fitness advantages of a larger egg size and/or offspring size may be favored at the expense of plasticity in these sites (see also 41). Additional mechanisms, such as the selection of different trait means in different environments and differential costs of plasticity, may also contribute to the observed patterns of plasticity (39).
Conclusions
The ecological importance of predator-induced indirect effects is widespread because such effects can influence community- and even ecosystem-level processes (8, 42). Here, we exploited known differences in individual growth rates between fish communities as a proxy for the differences in resource availability (18, 19), and we evaluated population × food interactions when these fish were reared in the laboratory on a ration that yielded approximately the same growth differences that are observed in nature. This method demonstrated that predator-induced differences in resource availability also have evolutionary consequences, which is important because the question of how population density and resource availability affect life history evolution, an area of intense research in the 1970s and 1980s (3, 4), remains unanswered (43–46). As a result, our approach, whereby trait expression is evaluated on specific ration levels that mimic differences in nature, provides a way to resolve this important question.
Materials and Methods
Rivulus were collected from Rivulus-only and high-predation populations from the Arima and Guanapo Rivers in July 2005. In each river, the Rivulus-only sites were located in tributaries connected to the main river but above barrier waterfalls that prevent the upstream migration of all other species found in the high-predation sites. Laboratory stocks were established from ≈10 wild-caught males and females from each locality. Each wild-caught female was placed in a 9-liter aquarium supplied with artificial spawning substrate and paired with a male from the same locality. Eggs were then collected daily and placed in Petri dishes. Upon hatching, larvae from each pairing were placed in aquaria at a maximum density of eight fish per tank and were reared on an ad libitum diet of liver paste and brine shrimp nauplii.
To generate the second generation, a mature female from each lineage in the first generation was mated to a mature male from the same locality but different lineage. Pairings were arranged so that all crosses were unique, all lineages were represented, and there were no full-sibling matings. As a result, the genetic diversity from the original wild-caught fish was maintained.
The offspring from these crosses were then reared at densities of eight fish per 9-liter aquarium and were fed ad libitum. At an age of 20 days, eight fish per pairing from the middle of the size distribution were selected to enter the life history assay. Each of these fish was individually placed in 9-liter aquaria and reared until maturity. At random, four of these fish were chosen to receive a high level of food availability, whereas the other four received a low ration level (see below).
The food levels used in this experiment are based on naturally occurring differences in growth across Rivulus-only and high-predation communities. Rivulus grow twice as fast in high-predation compared with Rivulus-only sites (18). Consequently, each day all fish were given quantified portions of liver paste in the morning and brine shrimp nauplii in the afternoon that resulted in growth trajectories that matched the growth exhibited by Rivulus in high-predation and Rivulus-only sites in nature. Food availability was increased in 2-week increments to accommodate growth. All fish received the same ration for each 2-week period regardless of differences in size.
Dependent variables that were estimated include age and size at maturity, fecundity, egg size, larval size at hatching, and reproductive allotment (defined below). Maturity in both males and females was quantified by mating each individual with a mature conspecific of the opposite sex as they approach maturity for a period of 12–16 h. As males approach maturity, white stripes form first on the bottom and then across the top of the caudal fin. Developing males were mated as soon as the bar began to form along the base of the caudal fin. Because there are no physical characteristics that indicate female maturity, assays for their maturity began at approximately the same time. An individual was classified as mature if an egg was found on the spawning substrate. Such eggs were subsequently monitored for 24–48 h to confirm that the embryo is viable and actively developing. Because males use a courtship dance to initiate reproduction, both individuals must be mature for the deposition of an egg to occur (M.R.W., unpublished data). When a mating trial failed to produce a fertilized egg, the individual was isolated and mated with a different conspecific approximately every 2–3 days thereafter. These assays began before maturation because all fish were mated at least once preceding maturation, and generally several matings were required before a fish was mature.
Upon maturation in females, eggs were collected for 14 days to quantify fecundity and egg size. An additional 10 eggs were collected from each female and allowed to develop until hatching, which allowed size at hatching to be measured. An estimate of the per-day allocation toward reproduction was subsequently calculated as [(mean per day egg production × mean egg size)/mean size of female during egg collecting period] × 100. This variable describes the total per day investment in reproduction as a function of body size.
Statistical Design and Analysis.
Each dependent variable was analyzed by using general linear models with predator community (high-predation or Rivulus-only), river (Arima or Guanapo), and ration level (high or low) treated as fixed effects. Our use of fixed effects is based on the definition given by Sokal and Rohlf (47). They state that a fixed-effects ANOVA tests for differences among group means because of an added treatment component, although the treatment does need to be understood or manipulated by the experimenter, as long as it is repeatable. The potential effects of rivers and predators fall under this latter scenario. Both the nature of the fish community and the sites from which our fish were sampled are repeatable features of our sampling design. Each dependent variable was analyzed separately, as were male and female age and size at maturation. We evaluated the interaction between food level and predator community to determine whether resource availability contributed to the differences between sites. The presence of normality and homogeneity of variance was evaluated for each variable, and transformations were performed when necessary. We log transformed age at maturity (male and female) to remove heteroscedascity.
We evaluated several potentially important covariates and included them in analyses where the assumptions of analysis of covariance were met and where the covariate accounted for a significant portion of variance. Female size at maturation was included as a covariate in the analysis of fecundity and egg size. The initial size of each fish upon entering the life history assay was included as a covariate in the analysis of age and size at maturity. Finally, the temperature of each tank was monitored weekly and was included as a covariate for age/size at maturation analyses. None of these potential covariates was significant, so none was reported in the analyses.
Missing Values and Outliers.
Some fish died during the course of the experiment. Reasons for mortality included suicidal jumps out of aquaria or being killed by a conspecific that was placed in the tank for the purposes of mating. If they died before maturation, they were not included in any analysis. Two fish produced eggs for only 1 day after maturing and died the next day. Because no eggs were measured from these fish and none hatched, these fish did not produce any data to be included in the larval-trait analyses. Five females died after >7 days of egg collection. Fecundity and egg size estimates were attained from these fish, although none of these fish produced eggs that hatched. Therefore, these fish did not yield any data for size at hatching. Also, three females survived the 2-week egg collection period and laid eggs that initiated development but failed to hatch. These individuals were also excluded from these latter analyses. These missing data were not biased toward one population because all were represented.
Three fish that produced eggs nearly one-third the size of their population means were found to be statistical outliers and were removed from analyses. Two of these females yielded larvae that were one-third the size of population means, which were therefore also excluded from the size-at-hatching analyses. The overall consequence of these missing data and outliers is that the analyses of traits measured later in life tended to have a lower number of degrees of freedom than the analyses for age/size at maturation.
ACKNOWLEDGMENTS.
We thank Jim Gilliam and Doug Fraser for continually sharing knowledge and data stemming from their work on Rivulus. We thank Doug Fraser for help with fish collection. The Fisheries Division of the Ministry of Agriculture, Land, and Marine Resources of Trinidad kindly granted collection and export permits. Comments by Doug Fraser, the D.N.R. laboratory group, and two reviewers improved the quality of this paper. This work was supported by a Department of Education Graduate Assistance in Areas of National Need Fellowship (to M.R.W.) and by National Science Foundation Grants DEB0416085 and EF0623632 (to D.N.R.). This is the first publication resulting from the National Science Foundation Frontiers in Integrative Biological Research grant.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Lack D. The Natural Regulation of Animal Numbers. Oxford, UK: Oxford Univ Press; 1954. [Google Scholar]
- 2.Lack D. Ecological Adaptations for Breeding in Birds. London: Methuen; 1968. [Google Scholar]
- 3.MacArthur RH, Wilson EO. The Theory of Island Biogeography. Princeton: Princeton Univ Press; 1967. [Google Scholar]
- 4.Pianka ER. Am Nat. 1970;104:592–597. [Google Scholar]
- 5.Gadgil M, Bossert PW. Am Nat. 1970;104:1–24. [Google Scholar]
- 6.Kozlowski J, Uchmanski J. Evol Ecol. 1987;1:214–231. [Google Scholar]
- 7.Kozlowski J, Wiegert RG. Evol Ecol. 1987;1:231–244. [Google Scholar]
- 8.Wootton JM. Am Nat. 1994;25:443–466. [Google Scholar]
- 9.Menge BA. Ecol Monogr. 1995;65:21–74. [Google Scholar]
- 10.Werner EE, Peacor SD. Ecology. 2003;84:1083–1100. [Google Scholar]
- 11.Abrams P, Rowe L. Evolution (Lawrence, Kans) 1996;50:1052–1061. doi: 10.1111/j.1558-5646.1996.tb02346.x. [DOI] [PubMed] [Google Scholar]
- 12.Falconer DS, Latyszewski M. J Genet. 1952;51:67–80. [Google Scholar]
- 13.Tessier AJ, Leibold MA, Tsao J. Ecology. 2000;81:826–841. [Google Scholar]
- 14.Barrett RDH, MacLean RC, Bell G. Am Nat. 2005;166:470–480. doi: 10.1086/444440. [DOI] [PubMed] [Google Scholar]
- 15.Law R. Am Nat. 1979;114:399–417. [Google Scholar]
- 16.Charlesworth B. Evolution in Age-Structured Populations. Cambridge, UK: Cambridge Univ Press; 1980. [Google Scholar]
- 17.Gilliam JF, Fraser DF, Alkins-Koo M. Ecology. 1993;74:1856–1870. [Google Scholar]
- 18.Fraser DF, Gilliam JF, MacGowan MP, Arcaro CM, Guillozet PH. Ecology. 1999;80:597–607. [Google Scholar]
- 19.Reznick D, Butler MJ, IV, Rodd FH. Am Nat. 2001;157:126–140. doi: 10.1086/318627. [DOI] [PubMed] [Google Scholar]
- 20.Stearns SC, Koella JC. Evolution (Lawrence, Kans) 1986;40:893–913. doi: 10.1111/j.1558-5646.1986.tb00560.x. [DOI] [PubMed] [Google Scholar]
- 21.Bradshaw WE, Holzapfel CM. Am Nat. 1989;133:869–887. [Google Scholar]
- 22.Mueller LD, Ayala FJ. Proc Natl Acad Sci USA. 1981;78:1303–1305. doi: 10.1073/pnas.78.2.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mueller LD. Proc Natl Acad Sci USA. 1988;85:4383–4386. doi: 10.1073/pnas.85.12.4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mueller LD, Guo P, Ayala FJ. Science. 1991;253:433–435. doi: 10.1126/science.1907401. [DOI] [PubMed] [Google Scholar]
- 25.Mueller LD. Annu Rev Ecol Evol Syst. 1997;28:269–288. [Google Scholar]
- 26.Bierbaum TJ, Mueller LD, Ayala FJ. Evolution (Lawrence, Kans) 1989;43:382–392. doi: 10.1111/j.1558-5646.1989.tb04234.x. [DOI] [PubMed] [Google Scholar]
- 27.Joshi A, Mueller LD. Evol Ecol. 1996;10:463–474. [Google Scholar]
- 28.Hillesheim E, Stearns SC. Evolution (Lawrence, Kans) 1991;45:1909–1923. doi: 10.1111/j.1558-5646.1991.tb02696.x. [DOI] [PubMed] [Google Scholar]
- 29.Hillesheim E, Stearns SC. Evolution (Lawrence, Kans) 1991;46:745–752. doi: 10.1111/j.1558-5646.1992.tb02080.x. [DOI] [PubMed] [Google Scholar]
- 30.Bashey F. Riverside: Univ of California; 2002. PhD dissertation. [Google Scholar]
- 31.Reznick DN. Evolution (Lawrence, Kans) 1982;36:1236–1250. doi: 10.1111/j.1558-5646.1982.tb05493.x. [DOI] [PubMed] [Google Scholar]
- 32.Reznick DN, Endler JA. Evolution (Lawrence, Kans) 1982;36:160–177. doi: 10.1111/j.1558-5646.1982.tb05021.x. [DOI] [PubMed] [Google Scholar]
- 33.Reznick DN, Bryga H. Am Nat. 1996;147:339–359. [Google Scholar]
- 34.Reznick DN, Butler MJ, Rodd FH, Ross P. Evolution (Lawrence, Kans) 1996;50:1651–1660. doi: 10.1111/j.1558-5646.1996.tb03937.x. [DOI] [PubMed] [Google Scholar]
- 35.Reznick D, Bryga H, Endler JA. Nature. 1990;346:357–359. [Google Scholar]
- 36.Reznick DN, Shaw FH, Rodd FH, Shaw RG. Science. 1997;275:1934–1937. doi: 10.1126/science.275.5308.1934. [DOI] [PubMed] [Google Scholar]
- 37.Michod RE. Am Nat. 1979;113:531–550. [Google Scholar]
- 38.Reznick DN, Bryant MJ, Roff D, Ghalambor CK, Ghalambor DE. Nature. 2004;431:1095–1099. doi: 10.1038/nature02936. [DOI] [PubMed] [Google Scholar]
- 39.Bashey F. Evolution (Lawrence, Kans) 2006;60:348–361. [PubMed] [Google Scholar]
- 40.Walsh MR, Munch SB, Chiba S, Conover DO. Ecol Lett. 2006;9:142–148. doi: 10.1111/j.1461-0248.2005.00858.x. [DOI] [PubMed] [Google Scholar]
- 41.Svensson E, Sinervo B. Evolution (Lawrence, Kans) 2000;54:1396–1403. doi: 10.1111/j.0014-3820.2000.tb00571.x. [DOI] [PubMed] [Google Scholar]
- 42.Schmitz OJ. Ecol Lett. 2003;6:156–163. [Google Scholar]
- 43.Stearns SC. Annu Rev Ecol Evol Syst. 1977;8:145–177. [Google Scholar]
- 44.Stearns SC. The Evolution of Life Histories. Oxford, UK: Oxford Univ Press; 1992. [Google Scholar]
- 45.Roff DA. Evolution of Life Histories: Theory and Analysis. New York: Chapman & Hall; 1992. [Google Scholar]
- 46.Reznick D, Bryant MJ, Bashey F. Ecology. 2002;83:1509–1520. [Google Scholar]
- 47.Sokal RR, Rohlf FJ. Biometry. New York: Freeman; 1995. pp. 202–203. [Google Scholar]