Abstract
We found that mice infected with different isolates of lymphocytic choriomeningitis virus (LCMV) develop a mild hemorrhagic anemia, which becomes severe and eventually lethal in animals depleted of platelets or lacking integrin β3. Lethal hemorrhagic anemia is mediated by virus-induced IFN-α/β that causes platelet dysfunction, mucocutaneous blood loss and suppression of erythropoiesis. In addition to the life-threatening hemorrhagic anemia, platelet-depleted mice fail to mount an efficient cytotoxic T lymphocyte (CTL) response and cannot clear LCMV. Transfusion of functional platelets into these animals reduces hemorrhage, prevents death and restores CTL-induced viral clearance in a manner partially dependent on CD40 ligand (CD40L). These results indicate that, upon activation, platelets expressing integrin β3 and CD40L are required for protecting the host against the induction of an IFN-α/β-dependent lethal hemorrhagic diathesis and for clearing LCMV infection through CTLs.
Lymphocytic choriomeningitis virus (LCMV) is a natural mouse pathogen of the Arenaviridae family (1) that can infect humans and, in most instances, causes either subclinical symptoms or a self-limited febrile syndrome (2). In a few reported severe cases, infection by LCMV has been similar to that by Junin (a human arenavirus responsible for Argentine hemorrhagic fever), in which disease severity and poor prognosis have been linked to marked thrombocytopenia, platelet dysfunction, sustained viremia, mucocutaneous hemorrhage, and impaired cellular immunity (2, 3). Similar symptoms characterized the fatal outcome of LCMV transmission by organ transplantation (4).
Besides being the main cellular effectors of hemostasis, platelets modulate immune responses by promoting the trafficking of leukocytes [including virus-specific cytotoxic T lymphocytes (CTLs)] at sites of infection or inflammation (5–7) and by producing cytokines, chemokines and other inflammatory mediators [such as CD40 ligand (CD40L)] that may enhance adaptive immunity (8, 9). Thus, we reasoned that thrombocytopenia might compromise LCMV clearance in mice, a process mediated by virus-specific CTLs and the antiviral factors they produce (1). We show here that platelets not only are essential for a normal viral clearance mediated through LCMV-specific CTL responses but also protect the animals from an IFN-α/β-dependent lethal hemorrhagic diathesis.
Results and Discussion
LCMV Infection Causes Thrombocytopenia, Platelet Dysfunction, and a Hemorrhagic Anemia That Becomes Lethal in Platelet-Depleted Mice and Is Independent of CTL-Induced Pathology.
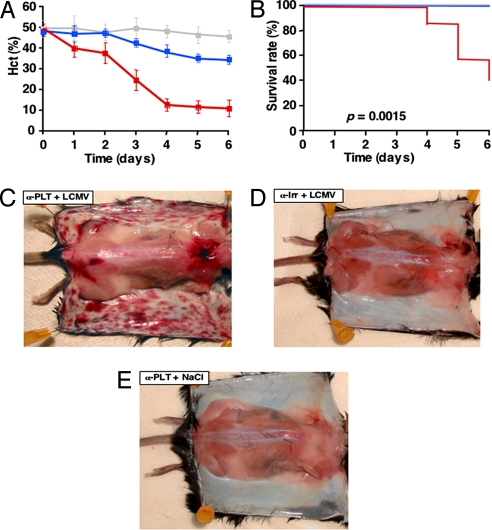
In all experiments described here, >98% platelet depletion (7), when indicated, was induced by injection of a platelet-depleting antibody (α-PLT) specific for mouse glycoprotein (GP) Ibα (10); control mice received an irrelevant isotype control antibody (α-Irr). In agreement with the studies described in ref. 11, B10D2 or C57BL/6J mice treated with α-Irr and infected with LCMV Arm (Table 1) or LCMV WE [supporting information (SI) Table 7] at the high dose of 106 pfu per animal, as opposed to mice receiving NaCl instead of virus, showed a reduction of ≈80% (P < 0.01) in platelet counts by days 4–6 after infection. Platelets isolated 2 or 4 days after infection, but not control platelets, showed an impaired aggregation in response to ADP (Fig. 1) or collagen (data not shown). Despite the significant thrombocytopenia with platelet dysfunction, LCMV-infected mice treated with α-Irr had signs of only mild hemorrhagic anemia [defined by a <20% reduction of hematocrit (Hct)] at day 5–6 with a positive test for fecal occult blood (FOB) and normal survival (Fig. 2, Table 2, and SI Table 7). By comparison, mice treated with α-PLT before infection became extremely thrombocytopenic (platelet count ≤20,000 per microliter, Table 1 and SI Table 7) and showed severe anemia with Hct values reduced by ≈50% (P < 0.01) and ≈75% (P < 0.01) at day 3 and days 4–6, respectively (Fig. 2A), a positive FOB test and decreased survival (Fig. 2, Table 2, and SI Table 7). There was no increase of serum unconjugated bilirubin (UCB) or significant decrease of fibrinogen (FG) (Table 2), which ruled out the occurrence of hemolysis or disseminated intravascular coagulation (DIC), respectively. At autopsy, the skin of LCMV-infected mice treated with α-PLT showed extensive hemorrhagic lesions that were always absent in control mice, whether treated with α-Irr before infection or treated with α-PLT but not infected (Fig. 2 C–E). Of note, there was no inflammatory infiltrate, no detectable viral RNA and no detectable LCMV nucleoprotein in the hemorrhagic skin, although both viral products were readily measurable in nonhemorrhagic spleens and livers (SI Figs. 7 and 8). Despite the hemorrhagic anemia, LCMV-infected mice (depleted or not of platelets) had no compensatory reticulocyte (RC) increase, but rather displayed a reduction of ≈40% in RC counts compared with baseline (P < 0.01). This was accompanied by a strong up-regulation (stronger at day 2 and still present at day 6 after infection) of the IFN-α/β inducible gene 2′–5′ oligoadenylate synthetase (OAS) in bone marrow (BM) (Table 1 and SI Table 7), liver, and spleen (data not shown), and high peak levels (≥ 400 ng/ml at day 2 after infection) of circulating IFN-α (Table 7). Hemorrhagic anemia, although less severe (≈50% reduction in Hct, P < 0.01 compared with α-Irr) and never lethal (SI Table 7), also occurred in mice treated with α-PLT and infected with a low dose of LCMV Arm (102 pfu per mouse). Mice injected with α-PLT and NaCl instead of virus maintained a normal Hct, had no bleeding symptoms, survived normally and displayed no skin hemorrhage (Fig. 2, Table 2, and SI Table 7). Similar to normal inbred mice, C57BL/6J mice lacking CD8+ cells (CD8−/−) and injected with either α-Irr or α-PLT before infection with LCMV Arm (106 pfu per mouse) developed mild or severe hemorrhagic anemia, respectively (SI Table 7), indicating that LCMV promotes bleeding independently of CTL-induced pathology. Along with the evidence that cutaneous hemorrhage was independent of both virus- and CTL-induced pathology, our results suggest that the bleeding propensity at this site likely reflects a distinct sensitivity of the skin to the protective function of platelets on vascular permeability.
Table 1.
Thrombocytopenia and suppressed erythropoiesis in LCMV-infected mice
| Treatment | n | Days p.i. | Platelets, 103/μl | RC, 103/μl | OAS BM | IFN-α, ng/ml |
|---|---|---|---|---|---|---|
| NaCl | 6 | 1 | 1,380 ± 68 | nd | nd | und |
| 6 | 2 | 1,289 ± 35 | nd | 1 | und | |
| 6 | 3 | 1,308 ± 41 | 106 ± 5 | nd | nd | |
| 6 | 4 | 1,209 ± 98 | 105 ± 7 | nd | und | |
| 6 | 5 | 1,321 ± 87 | 110 ± 4 | nd | nd | |
| 6 | 6 | 1,298 ± 91 | 108 ± 8 | 1 | nd | |
| α-Irr + LCMV, 106 PFU | 6 | 1 | 1,305 ± 48 | nd | nd | 110 ± 20 |
| 6 | 2 | 1,275 ± 66 | nd | 13.8 ± 0.9 | 470 ± 30 | |
| 6 | 3 | 954 ± 78 | 38 ± 6 | nd | nd | |
| 6 | 4 | 234 ± 52 | 38 ± 7 | nd | 50± 10 | |
| 6 | 5 | 218 ± 37 | 45 ± 4 | nd | nd | |
| 6 | 6 | 224 ± 49 | 46 ± 9 | 8.9 ± 1.1 | nd | |
| α-PLT + LCMV, 106 PFU | 6 | 1 | 7 ± 2 | nd | nd | 100 ± 10 |
| 6 | 2 | 8 ± 3 | nd | 13.5 ± 0.8 | 510 ± 40 | |
| 6 | 3 | 6 ± 1 | 39 ± 7 | nd | nd | |
| 6 | 4 | 6 ± 4 | 40 ± 8 | nd | 70 ± 20 | |
| 6 | 5 | 4 ± 2 | 43 ± 7 | nd | nd | |
| 6 | 6 | 18 ± 9 | 41 ± 5 | 7.8 ± 1.3 | nd | |
| α-PLT + NaCl | 6 | 1 | 9 ± 5 | nd | nd | und |
| 6 | 2 | 7 ± 2 | nd | 1 | und | |
| 6 | 3 | 7 ± 4 | 104 ± 9 | nd | nd | |
| 6 | 4 | 8 ± 1 | 102 ± 7 | nd | und | |
| 6 | 5 | 11 ± 2 | 109 ± 8 | nd | nd | |
| 6 | 6 | 13 ± 5 | 109 ± 7 | 1 | nd |
Platelet and RC counts (mean ± SD) in blood, OAS expression (fold-induction over NaCl-injected mice) assessed by RPA in total BM RNA, and serum circulating levels of IFN-α (mean ± SD) were assessed individually after the indicated treatment. und, undetectable; nd, not done; p.i., postinfection.
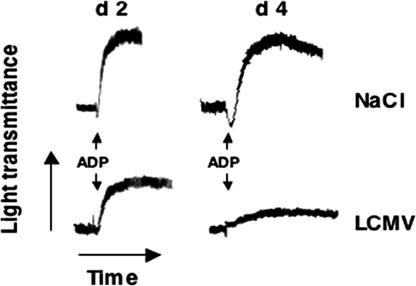
Fig. 1.
Platelet dysfunction in LCMV-infected mice. ADP-induced aggregation of platelet-rich plasma (PRP) from C57BL/6J mice injected 2 or 4 days earlier with either NaCl or LCMV Arm (106 pfu per mouse). The difference in the two control aggregation curves can be explained by the lower platelet count in the PRP used at day 4. Data are representative of four independent experiments.
Fig. 2.
LCMV infection causes lethal hemorrhagic anemia in platelet-depleted mice. (A) Hct values (mean ± SD) of B10D2 mice (20 per group) injected with α-PLT (red line) or α-Irr (blue line) before infection with LCMV Arm or injected with α-PLT followed by NaCl instead of LCMV (gray line). (B) Kaplan–Meier survival curves of mice described in A and identified by lines of the same colors. (C–E) Skin photographs of representative mice described in A and killed 6 days after injection.
Table 2.
LCMV infection causes mucosal hemorrhage
| Treatment | FOB | UCB | FG |
|---|---|---|---|
| NaCl | − | 0.1 | 240 |
| α-Irr + LCMV | + | 0.1 | 190 |
| α-PLT + LCMV | + | 0.1 | 180 |
| α-PLT + NaCl | − | 0.1 | 226 |
FOB, serum UCB, and plasma FG assessed in either fecal pellets or blood samples pooled from mice (n = 4) 6 days after the indicated treatment. Data are representative of three independent experiments. The changes in UCB and FG values are not statistically significant.
Influence of IFN-α/β on Platelet Function and Hemorrhagic Anemia.
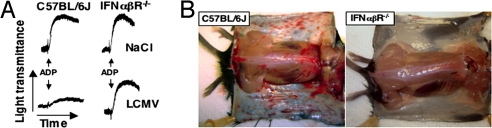
Compared with wild-type controls treated similarly, LCMV-infected C57BL/6J mice lacking the IFN-α/β receptor (IFN-α/βR−/−) and injected with either α-Irr or α-PLT developed a less severe anemia, with 7% (P < 0.01) and 22% (P < 0.01) Hct reduction, respectively, and no mortality (Table 3). In agreement with their unresponsiveness to IFN-α/β, documented by the lack of BM OAS induction, by day 6 after infection the two groups of IFN-α/βR−/− mice showed ≈1.5- and ≈6-fold increased RC counts, respectively, compared with uninfected littermates (Table 3). Of note, platelets of IFN-α/βR−/− mice isolated 4 days after LCMV infection had normal aggregation capacity, as opposed to those isolated from similarly infected C57BL/6J mice at the same time point (Fig. 3A). Accordingly, despite viral titers that were much higher than those in C57BL/6J controls (SI Fig. 8), IFN-α/βR−/− mice exhibited only mild hemorrhagic signs even after platelet depletion, as indicated by the lack of FOB (data not shown) and the absence of extensive bleeding in the skin (Fig. 3B). Thus, LCMV-infected IFN-α/βR−/− mice not only maintained a normal platelet aggregation response, but they were also protected from bleeding and death with a platelet count ≤20,000 per microliter. Two conclusions are supported by these results: (i) IFN-α/β is the necessary, if not sole, mediator of platelet dysfunction during the course of LCMV infection; and (ii) exceedingly few platelets, if functionally intact, protect infected mice from death, indicating that platelet dysfunction, more than thrombocytopenia per se, is the ultimate cause of bleeding in these animals.
Table 3.
Hemorrhagic anemia in LCMV-infected mice requires IFN-α/β signaling
| Treatment | Mouse strain | Platelets, 103/μl | Survival, % | Hct, % | RC, 103/μl | OAS BM |
|---|---|---|---|---|---|---|
| NaCl | C57BL/6J | 1,275 ± 53 | 100 | 47.7 ± 5.1 | 108 ± 9 | 1 |
| α-Irr + LCMV | C57BL/6J | 265 ± 45 | 100 | 35.5 ± 2.2 | 46 ± 8 | 12.2 ± 1 |
| α-PLT + LCMV | C57BL/6J | 12 ± 6 | 40 | 11.3 ± 4.2 | 41 ± 9 | 12.8 ± 1 |
| NaCl | IFN-α/βR−/− | 1,218 ± 48 | 100 | 43.0 ± 2.6 | 197 ± 4 | und |
| α-Irr + LCMV | IFN-α/βR−/− | 712 ± 39 | 100 | 40.2 ± 3.3 | 298 ± 2 | und |
| α-PLT + LCMV | IFN-α/βR−/− | 19 ± 7 | 100 | 33.2 ± 2.8 | 1,171 ± 6 | und |
Platelet counts, survival rate, and Hct and RC values (mean ± SD) were assessed individually in groups of C57BL/6J or IFN-α /βR−/− mice (10 mice per group) at 6 days after injection of NaCl, α-Irr + LCMV, or α-PLT + LCMV (LCMV Arm given at 106 pfu per mouse). OAS expression was measured as Table 1.
Fig. 3.
Influence of IFN-α/β on platelet function and hemorrhagic anemia. (A) ADP-induced platelet aggregation in PRP from C57BL/6J or IFN-α/βR−/− mice injected 4 days earlier with NaCl or LCMV Arm (106 pfu per mouse). Data are representative of four independent experiments. (B) Skin photographs of representative platelet-depleted C57BL/6J or IFN-α/βR−/− mice killed 6 days after LCMV infection.
In keeping with the hypothesis that IFN-α/β is the cause of hemorrhage in a setting of extreme thrombocytopenia, platelet-depleted uninfected mice treated with the IFN-α/β inducer, polyinosinic polycytidylic acid [poly (I:C)], exhibited defective ADP-induced aggregation (data not shown), high levels of circulating IFN-α—which peaked at ≥400 ng/ml by 12 h after injection (SI Table 8) and remained similarly elevated by day 2 after injection—and elevated OAS in BM (SI Table 8) and other organs (i.e., liver and spleen). Moreover, these mice exhibited an often lethal hemorrhagic anemia with ≈60% Hct reduction (P < 0.01 compared with the corresponding non-platelet-depleted mice) not unlike that seen in LCMV-infected mice (SI Table 8). A similar but less severe hemorrhage (and outcome) was observed when platelet-depleted mice were infected with high doses of vescicular stomatitis virus (VSV), another pathogen that induced high levels of BM OAS and circulating IFN-α, albeit more transiently. In fact, the levels of circulating IFN-α in VSV-infected mice were already <50 ng/ml 12 h following their peak value (≈400 ng/ml by 12 h after infection; SI Table 2). Conversely, little or no hemorrhagic anemia was detected in highly thrombocytopenic animals that were infected with high doses of vaccinia virus (VV) or mouse cytomegalovirus (MCMV), two pathogens that induced lower peak levels of circulating IFN-α (≤ 60 ng/ml at day 2 after infection) and a much less strong IFN-α/β response—i.e., OAS induction in BM (SI Table 2), liver, and spleen (data not shown).
Together, these results indicate that IFN-α/β is required and sufficient to cause the platelet dysfunction seen in the course of infection by LCMV and other selected viruses. The mechanism of this action does not appear to be direct, because we found that in vitro incubation with recombinant IFN-β has no consequence on the aggregation of C57BL/6J mouse platelets (data not shown). Of note, the onset of platelet dysfunction appears to be time-dependent, because IFN-α/β levels peaked at day 2 after infection and platelets isolated at this time had a better aggregation response in vitro than those isolated 2 days later when hemorrhagic anemia started to become more severe. This suggests that it may be necessary for even the small number of remaining normally functioning platelets to be removed from the circulation (the lifespan of mouse platelets in blood is ≈4 days) before the aggregation defect becomes apparent. At the same time, IFN-α/β may act on parent megakaryocytes, leading to the production of altered platelets and, thus, making the functional defect of circulating platelets apparent. Such an effect may account for the kinetics of platelet dysfunction after LCMV infection and the protective effect of normal platelet transfusion against severe hemorrhage and death (see below). In addition, or in alternative, IFN-α/β is known to up-regulate endothelial cell-derived platelet inhibitors, such as nitric oxide (12) and prostacyclin (13), which could contribute to the observed platelet dysfunction in vivo.
Transfusion of Platelets Capable of Becoming Activated Prevents Death and Ameliorates Hemorrhagic Anemia.
Platelet-depleted C57BL/6J mice were infected with LCMV and injected 3 days after infection with 6 × 108 mouse platelets lacking mouse but expressing human GPIbα (defined as TKK-PLT). These platelets are functionally normal (14) and cannot be depleted by α-PLT because of a lack of cross-reactivity with human GPIbα (7). Compared with platelet-depleted nontransfused animals also infected with LCMV, mice that received TKK-PLT transfusion exhibited 100% survival and much less decreased Hct (Table 4). No such improvement was observed when TKK-PLT were treated before transfusion with the activation inhibitor prostaglandin (PG) E1 (Table 4). PG E1-treated TKK-PLT transfused into uninfected mice depleted with α-PLT exhibited no ADP-induced aggregation 1 day after transfusion (data not shown). Because fully functional platelets, as shown here, can prevent death in LCMV-infected mice even at a count well below normal, transfusion of normal platelets should be considered, along with the neutralization of IFN-α/β activity, in the treatment of life-threatening arenavirus infections in humans. Pertinent to this, it is noteworthy that exceptionally high levels of circulating IFN-α are found in humans infected by Junin (15), also an arenavirus, and patients who progress to a fatal outcome often exhibit marked thrombocytopenia—possibly similar to that of LCMV-infected mice treated with α-PLT—associated with platelet dysfunction and mucocutaneous hemorrhage (2, 3).
Table 4.
Transfusion of platelets capable of becoming activated prevents death and ameliorates hemorrhagic anemia
| Treatment | n | Platelets, 103/μl | Survival, % | Hct, % |
|---|---|---|---|---|
| NaCl | 5 | 1,009 ± 45 | 100 | 46.6 ± 4.2 |
| α-Irr + LCMV | 8 | 121 ± 36 | 100 | 33.9 ± 3.9 |
| α-PLT + LCMV | 8 | 3 ± 1 | 37.5 | 9.9 ± 3.4 |
| α-PLT + LCMV + TKK PLT | 8 | 54 ± 12 | 100 | 24.6 ± 2.6 |
| α-PLT + LCMV + TKK PLT PGE1 | 5 | 271 ± 13 | 40 | 11.6 ± 1.7 |
| α-PLT + TKK PLT | 5 | 263 ± 36 | 100 | 46.4 ± 3.2 |
B10D2 mice were injected with α-Irr or α-PLT 3 h before LCMV Arm infection (106 pfu per mouse). Three days after infection, one group of α-PLT-treated mice received 6 × 108 TKK-PLT, and another group received 6 × 108 TKK-PLT pretreated with PGE1. Mice receiving NaCl or α-PLT and 6 × 108 TKK-PLT but no LCMV served as controls. Platelet counts (mean ± SD) were assessed individually 1 day after transfusion. Survival and Hct (mean ± SD) were assessed individually 6 days after infection.
Severe Hemorrhagic Anemia in Integrin β3−/− Mice.
In line with the notion that PGE1-treated platelets cannot activate the integrin αIIbβ3 required for normal aggregation (16), integrin β3−/− mice developed a severe hemorrhagic anemia after LCMV infection, even without platelet depletion (Table 5). In contrast, LCMV-infected mice lacking either GPIbα or P-selectin, two receptors also involved in platelet adhesive interactions, developed a mild hemorrhagic anemia similar to that of LCMV-infected C57BL/6J controls (SI Table 9). Hemorrhagic anemia remained also mild in LCMV-infected mice treated with the anticoagulant warfarin at a dose that caused a sevenfold increase in the prothrombin time (PT) (SI Table 9), implying that platelets can control blood loss independently of their procoagulant function. The mechanism by which platelets protect from bleeding, therefore, is not a mere reflection of the overall integrity of the hemostatic system.
Table 5.
Severe hemorrhagic anemia in integrin β3-deficient mice
| Treatment | Mouse strain | n | Days p.i. | Platelets, 103/μl | Hct, % |
|---|---|---|---|---|---|
| NaCl | C57BL/6J | 3 | 6 | 1,305 ± 72 | 46.7 ± 3.2 |
| LCMV | C57BL/6J | 6 | 6 | 291 ± 52 | 34.4 ± 2.9 |
| NaCl | Integrin β3−/− | 3 | 6 | 1,309 ± 61 | 46.1 ± 4.0 |
| LCMV | Integrin β3−/− | 6 | 6 | 189 ± 38 | 18.3 ± 3.6 |
Platelets and Hct (mean ± SD) were assessed individually at the indicated time after injection. p.i., postinfection.
Depletion of Platelets Results in Poor Viral Clearance and Reduced Virus-Specific CTL Responses.
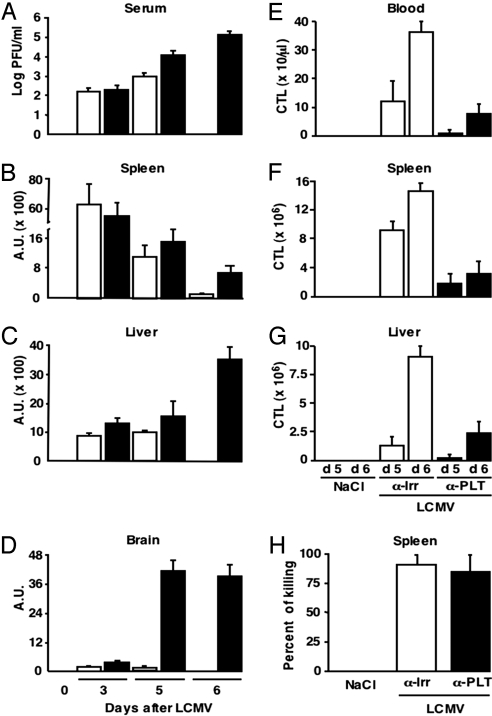
When compared with LCMV-infected controls, platelet-depleted mice killed 3 days after infection displayed similar viral titers in the serum and similar viral RNA in the spleen and liver (Fig. 4 A–C), indicating that platelet depletion did not affect the capacity of LCMV to infect these organs. LCMV was cleared from the serum, spleen and liver of infected controls by day 6 after infection (Fig. 4 A–C), and viral RNA was almost never detectable in the brain (Fig. 4D). In contrast, platelet-depleted mice that survived up to the same time points failed to show viral clearance and exhibited LCMV RNA, albeit at low levels, in the brain (Fig. 4 A–D). Consistent with the fact that CTLs are necessary to clear LCMV (1), there was a marked expansion of LCMV-specific CTLs in blood, spleen, and liver of infected control B10D2 mice (assessed by intracellular IFN-γ staining of the absolute number of CD8+ T cells specific for the H2d-restricted immunodominant peptide epitope NP118; Fig. 4 E–G). Similar results were obtained when tetramers were used to estimate the absolute number of CD8+ T cells specific for the H2b-restricted immunodominant peptide epitope GP33 (SI Fig. 9) or NP396 (data not shown) in the blood or spleen of infected C57BL/6J mice. Using both techniques, platelet-depleted infected mice showed ≈80% (P < 0.01) fewer LCMV-specific CTLs at these sites (Fig. 4 E–G and SI Fig. 9) and displayed a much reduced hepatic inflammatory infiltrate (SI Fig. 10). To confirm independently that platelet depletion impairs CTL expansion, Thy-1.1+ naïve P14 TCR transgenic CD8+ T cells (specific for GP33) were transferred into Thy-1.2+ C57BL/6J mice that were injected 2 days later with either α-PLT or α-Irr before LCMV infection. Splenic Thy-1.1+ cells isolated from platelet-depleted animals at day 6 after infection expanded ≈4-fold less (P < 0.01) than those recovered from platelet-undepleted controls (SI Fig. 10).
Fig. 4.
Effect of platelet depletion on viral clearance and virus-specific CTL responses. (A) Quantification by plaque assay of the viral titers in the serum of groups of B10D2 mice (n = 6) injected with α-Irr (white bars) or α-PLT (black bars) before LCMV Arm infection (106 pfu per mouse) and killed at the indicated time points after infection. Results (mean ± SD) are expressed as log pfu/ml. (B–D) Content of LCMV-specific transcripts in the total RNA isolated from the spleen (B), liver (C), and brain (D) of the animals described in A. Analysis was performed by Northern blot, and quantification was obtained by measuring band intensities after phosphor imaging; results in arbitrary units (A.U.) were normalized to the content of the housekeeping gene GAPDH. (E–G) Assessment by intracellular IFN-γ staining of the absolute number of NP118-specific CD8+ T cells recovered from blood (E), spleens (F), or livers (G) of the animals described in A and killed at the indicated time points after infection. Results are expressed as mean ± SD. (H) In vivo cytotoxicity assay in mice infected with LCMV Arm (106 pfu per mouse) and injected at day 8 after infection with α-Irr or α-PLT 3 h before the injection of target cells. The animals were killed 45 min later.
To monitor whether platelet depletion affected not only the number but also the function of LCMV-specific CTLs, the capacity of splenic CTLs to kill in vivo target cells (CFSE-labeled spleen cells pulsed with an LCMV-specific peptide) was assessed in mice that were maintained under α-PLT treatment since before infection. By day 6 after infection, fewer target cells were killed in the spleen of these animals compared with those isolated from the spleen of undepleted control mice (data not shown). Notably, the difference in the rejection of CFSE-labeled cells between the two groups of mice paralleled that of CTL numbers recovered from their spleen (data not shown), suggesting that the CTL killing function is not affected (on a per-cell basis) by platelet depletion. To confirm these findings under conditions of similar CTL numbers, target cells were injected at day 8 after infection into mice that were treated 3 h earlier with either α-PLT or α-Irr. At this time, most infection-related antigen was cleared, and the endogenous LCMV-specific CTL response was maximal and quantitatively comparable (data not shown). Target cells were killed with similar efficiency in both groups of mice (Fig. 4H), which reiterates the notion that CTLs killing function is intact in platelet-depleted mice.
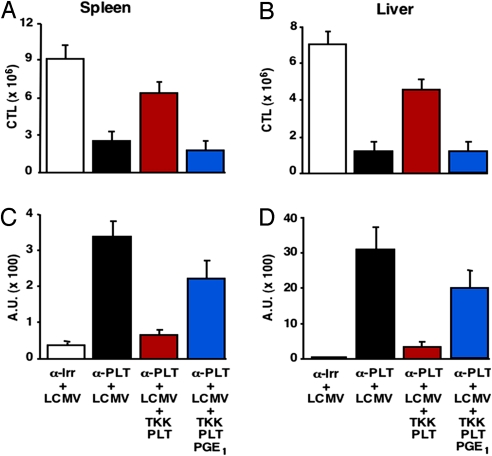
Transfusion of Normal but Not Activation-Blocked Platelets Restores LCMV-Specific CTL Responses and Viral Clearance.
Transfusion of TKK-PLT, in addition to preventing lethal hemorrhagic anemia (see Table 4), enhanced LCMV-specific CTL responses (Fig. 5 A and B) and viral RNA clearance from spleen and liver (Fig. 5 C and D), with concurrent accumulation of inflammatory cells in these organs (SI Fig. 9 and data not shown). In contrast, none of these events occurred after transfusion of PGE1-treated TKK-PLT (Fig. 5 and SI Fig. 9), indicating that platelet activation is required for viral clearance. Of note, blood platelet counts in platelet-depleted LCMV-infected mice transfused with TKK PLT were fivefold lower than either those of mice receiving PGE1-treated TKK-PLT (P < 0.01) or those of uninfected platelet-depleted mice receiving TKK PLT (P < 0.01) (Table 4). This indicates that a significant percentage of transfused platelets may become activated and consumed in the process of controlling hemorrhage and promoting CTL-induced viral clearance.
Fig. 5.
Transfusion of normal but not activation-blocked platelets restores LCMV-specific CTL responses and viral clearance. (A and B) Assessment by intracellular IFN-γ staining of the absolute number of NP118-specific CD8+ T cells recovered 6 days after infection from spleen (A) and liver (B) of the same mice described in Table 4. Results are expressed as mean ± SD. (C and D) Content of LCMV RNA [expressed in arbitrary units (A.U.); seeSI Materials and Methods] in spleen (C) and liver (D) of mice shown in Table 4.
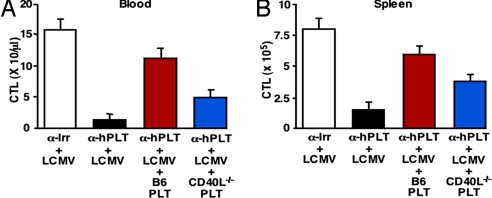
Amelioration of Hemorrhage and Restoration of LCMV-Specific CTL Responses Partially Depends on Platelet Expression of CD40L.
TKK mice, expressing human GPIbα, were depleted of platelets, using an anti-human GPIbα mAb (α-hPLT) (17) that fails to cross-react with platelets expressing mouse GPIbα (data not shown). Platelet-depleted TKK mice infected with LCMV and transfused with platelets from CD40L-deficient mice (CD40L−/− PLT) showed only a partial improvement of Hct (Table 6), which was ≈58% (P < 0.01) of that seen with infusion of platelets from control C57BL/6J mice (B6PLT). Moreover, LCMV-specific CTL responses in blood and spleen of these mice (Fig. 6 A and B) were also ≈50% lower (P < 0.01), and their viral titers were higher (data not shown) than in mice transfused with B6PLT. The notion that CTL accumulation leading to LCMV clearance was only partially restored in platelet-depleted mice transfused with CD40L−/− platelets agrees with the concept that platelet CD40L modulates adaptive immunity (18). Because wild type platelets were relatively efficient at restoring splenic CTL responses once transfused into platelet-depleted mice 3 days after infection [at which time most CTLs should not require further stimulation by antigen presenting cells (19)], the results indicate that activated platelets (in part through CD40L) contribute to the expansion phase of CTLs resulting in their accumulation at sites of infection. In agreement with this hypothesis, we found (data not shown here) that depletion of platelets 3 days after LCMV infection results in a decreased CTL response at day 6 p.i., compatible with a platelet involvement not in CTL priming but more in the subsequent expansion phase. It is also noteworthy that CD40L−/− mice, in which the defect occurs not only in platelets but also CD4+, CD8+, and γδ T cells (18, 20), mount a primary LCMV-specific CTL response numerically comparable to that of wild type controls [despite altered LCMV-specific T cell memory responses (21)], suggesting that alternative immune-stimulatory mechanisms can compensate for the congenital absence of CD40L.
Table 6.
Amelioration of hemorrhage and restoration of LCMV-specific CTL responses involves platelet CD40
| Treatment | Mouse strain | n | Platelets, 103/μl | Survival, % | Hct, % |
|---|---|---|---|---|---|
| NaCl | TKK | 3 | 1,009 ± 45 | 100 | 46.3 ± 3.8 |
| α-Irr + LCMV | TKK | 5 | 213 ± 38 | 100 | 34.9 ± 3.1 |
| α-hPLT + LCMV | TKK | 5 | 9 ± 4 | 40 | 12.0 ± 3.2 |
| α-hPLT + LCMV + B6 PLT | TKK | 5 | 156 ± 23 | 100 | 21.7 ± 1.8 |
| α-hPLT + LCMV + CD40L−/− PLT | TKK | 5 | 188 ± 33 | 80 | 17.6 ± 2.9 |
Groups of TKK mice were injected with α-hPLT 3 h before LCMV Arm infection (106 pfu per mouse). After 3 days, one group received 6 × 108 B6 PLT, and another group received 6 × 108 CD40L−/− PLT. Mice receiving NaCl alone or α-Irr before LCMV infection served as controls. Platelet counts (mean ± SD) were assessed individually 1 day after transfusion. Survival and Hct (mean ± SD) were assessed individually 5 days after infection.
Fig. 6.
Restoration of LCMV-specific CTL responses involves platelet CD40L. LCMV-specific CTL responses in blood (A) and spleens (B) of the same mice described in Table 6 were measured 5 days after infection as indicated in the legend to Fig. 5.
In summary, our results indicate that LCMV-infected mice exhibit an IFN-α/β-dependent platelet dysfunction that, if associated with thrombocytopenia below a critical threshold, results in severe bleeding and acute anemia. Moreover, the decreased platelet count and function is linked to a reduction in the virus-specific CTL response measured in blood and infected organs, such that viral clearance cannot occur. Platelets, therefore, play a key role in the progression and severity of LCMV infection, because they are essential to control the propagation of the pathogen and the consequences of its presence. Ultimately, the death of LCMV-infected mice is the result of uncompensated anemia consequent to mucocutaneous bleeding caused by a defect of platelet number and function. IFN-α/β, through its receptor, directly or indirectly influences all steps of this pathogenetic mechanism, in which platelets play a dual and center-stage role of host defense by controlling hemorrhage and supporting viral clearance.
Materials and Methods
Mice, Viruses, and Reagents.
CD8−/−, TNFR1−/−, IFNα/βR−/−, GPIbα−/−, and TKK mice are described in refs. 14 and 22. Integrin β3−/− mice (16) were provided by Mark Ginsberg (University of California at San Diego, La Jolla, CA). P-selectin−/− (23), CD40L−/− (24), and P14 TCR-transgenic mice (25) were purchased from; The Jackson Laboratory. In all experiments, mice were matched for age (8 weeks); sex (males); and, in the case of integrin β3−/− and GPIbα−/− mice, for Hct values before experimental manipulation. The experiments described in this report were approved by the Animal Research Committee of The Scripps Research Institute. LCMV strains Armstrong (Arm) and clone 2.2 of the WE isolate (WE) (26, 27) were used. Mice were also infected i.v. with (i) 2 × 106 pfu of VSV; (ii) 107 pfu of a recombinant VV, and (iii) 105 pfu of MCMV, as described in refs. 28–30. Poly(I:C) was purchased from Sigma–Aldrich and injected (200 μg/mouse) i.v. once a day for 3 d. Infectious LCMV in serum and tissues was quantified by plaque assay on Vero cell monolayers as described in ref. 26. Serum IFN-α titers were measured by using a commercially available ELISA kit (R&D Systems), according to the manufacturer's instructions.
Depletion, Transfusion, and Aggregation of Plateles.
Platelet depletion in C57BL/6J mice was performed as described in ref. 7. For experiments with TKK mice, animals were injected i.v. with 150 μg of a purified mAb against human GPIbα (17) (α-hPLT) or the corresponding isotype controls (17) (α-Irr) 3 h before and 3 days after virus infection. Platelets, white blood cells, red blood cells, hemoglobin, and hematocrit values were counted with an automated cell counter (System 9000; Serono–Baker Diagnostics). For RC count, whole blood was mixed 1:1 with new methylene blue and incubated at room temperature for 10 min. A drop of the mixture was then used to prepare a conventional air-dried blood film, counterstained with Wright's stain and examined at the light microscope. FOB test (Hemoccult SENSA; Beckman Coulter), was performed according to the manufacturer's instruction. Platelet transfusion was performed as described in ref. 7. Each transfused mouse received a single i.v. injection of 6 × 108 platelets. Platelet aggregation was performed as described in ref. 7. Warfarin treatment and PT (INR) determination were performed as described in ref. 7. Single cell suspensions were prepared from peripheral blood, spleen, and liver as described in ref. 7. Details can be found in SI Materials and Methods.
CTL Analyses.
Intracellular IFN-γ staining or tetramer staining was performed in the presence or absence of NP118, GP33 or NP396 stimulation as described in ref. 29. The in vivo cytotoxicity assay was carried out as described in ref. 31. Details can be found in SI Materials and Methods.
Histological Analyses.
For histological analysis, livers and skins were fixed and stained with hematoxylin/eosin as described in ref. 7. Immunohistochemical staining for LCMV was carried out as described in ref. 27.
Statistical Methods.
Data are expressed as mean ± SD. Statistical significance was assessed by using the Student t test. P < 0.05 was considered statistically significant.
Supplementary Material
ACKNOWLEDGMENTS.
We thank S. Wieland, J. C. de la Torre, and J. L. Whitton for helpful discussions and A. Althage, B. Boyd, M. Chadwell, and S. Medrano for excellent technical assistance. This work was supported by National Institutes of Health Grants HL31950, HL42846, and HL78784 (to Z.M.R.); CA40489 (to F.V.C.); and AI40696 (to L.G.G.). This is manuscript number 18211-MEM from the Scripps Research Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0711200105/DC1.
References
- 1.Oldstone MB. Biology and pathogenesis of lymphocytic choriomeningitis virus infection. Curr Top Microbiol Immunol. 2002;263:83–117. doi: 10.1007/978-3-642-56055-2_6. [DOI] [PubMed] [Google Scholar]
- 2.Peters CJ. Lymphocytic choriomeningitis virus—an old enemy up to new tricks. N Engl J Med. 2006;354:2208–2211. doi: 10.1056/NEJMp068021. [DOI] [PubMed] [Google Scholar]
- 3.Cummins D. Arenaviral haemorrhagic fevers. Blood Rev. 1991;5:129–137. doi: 10.1016/0268-960x(91)90029-c. [DOI] [PubMed] [Google Scholar]
- 4.Fischer SA, et al. Transmission of lymphocytic choriomeningitis virus by organ transplantation. N Engl J Med. 2006;354:2235–2249. doi: 10.1056/NEJMoa053240. [DOI] [PubMed] [Google Scholar]
- 5.Diacovo TG, Puri KD, Warnock RA, Springer TA, von Andrian UH. Platelet-mediated lymphocyte delivery to high endothelial venules. Science. 1996;273:252–255. doi: 10.1126/science.273.5272.252. [DOI] [PubMed] [Google Scholar]
- 6.Wagner DD, Burger PC. Platelets in inflammation and thrombosis. Arterioscler Thromb Vasc Biol. 2003;23:2131–2137. doi: 10.1161/01.ATV.0000095974.95122.EC. [DOI] [PubMed] [Google Scholar]
- 7.Iannacone M, et al. Platelets mediate cytotoxic T lymphocyte-induced liver damage. Nat Med. 2005;11:1167–1169. doi: 10.1038/nm1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weyrich AS, Zimmerman GA. Platelets: signaling cells in the immune continuum. Trends Immunol. 2004;25:489–495. doi: 10.1016/j.it.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Elzey BD, et al. Platelet-mediated modulation of adaptive immunity. A communication link between innate and adaptive immune compartments. Immunity. 2003;19:9–19. doi: 10.1016/s1074-7613(03)00177-8. [DOI] [PubMed] [Google Scholar]
- 10.Bergmeier W, Rackebrandt K, Schroder W, Zirngibl H, Nieswandt B. Structural and functional characterization of the mouse von Willebrand factor receptor GPIb-IX with novel monoclonal antibodies. Blood. 2000;95:886–893. [PubMed] [Google Scholar]
- 11.Binder D, Fehr J, Hengartner H, Zinkernagel RM. Virus-induced transient BM aplasia: major role of interferon-alpha/beta during acute infection with the noncytopathic lymphocytic choriomeningitis virus. J Exp Med. 1997;185:517–530. doi: 10.1084/jem.185.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faruqi TR, Erzurum SC, Kaneko FT, DiCorleto PE. Role of nitric oxide in poly(I-C)-induced endothelial cell expression of leukocyte adhesion molecules. Am J Physiol. 1997;273:H2490–H2497. doi: 10.1152/ajpheart.1997.273.5.H2490. [DOI] [PubMed] [Google Scholar]
- 13.Eldor A, et al. Interferon enhances prostacyclin production by cultured vascular endothelial cells. J Clin Invest. 1984;73:251–257. doi: 10.1172/JCI111198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ware J, Russell S, Ruggeri ZM. Generation and rescue of a murine model of platelet dysfunction: the Bernard–Soulier syndrome. Proc Natl Acad Sci USA. 2000;97:2803–2808. doi: 10.1073/pnas.050582097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levis SC, et al. Correlation between endogenous interferon and the clinical evolution of patients with Argentine hemorrhagic fever. J Interferon Res. 1985;5:383–389. doi: 10.1089/jir.1985.5.383. [DOI] [PubMed] [Google Scholar]
- 16.Hodivala-Dilke KM, et al. Beta3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J Clin Invest. 1999;103:229–238. doi: 10.1172/JCI5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanaji T, et al. Megakaryocyte proliferation and ploidy regulated by the cytoplasmic tail of glycoprotein Ibalpha. Blood. 2004;104:3161–3168. doi: 10.1182/blood-2004-03-0893. [DOI] [PubMed] [Google Scholar]
- 18.Elzey BD, Sprague DL, Ratliff TL. The emerging role of platelets in adaptive immunity. Cell Immunol. 2005;238:1–9. doi: 10.1016/j.cellimm.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hermann P, Van-Kooten C, Gaillard C, Banchereau J, Blanchard D. CD40 ligand-positive CD8+ T cell clones allow B cell growth and differentiation. Eur J Immunol. 1995;25:2972–2977. doi: 10.1002/eji.1830251039. [DOI] [PubMed] [Google Scholar]
- 21.Whitmire JK, Slifka MK, Grewal IS, Flavell RA, Ahmed R. CD40 ligand-deficient mice generate a normal primary cytotoxic T-lymphocyte response but a defective humoral response to a viral infection. J Virol. 1996;70:8375–8381. doi: 10.1128/jvi.70.12.8375-8381.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bachmann MF, Oxenius A, Mak TW, Zinkernagel RM. Thymic positive selection is biased toward the helper phenotype. J Immunol. 1995;155:3727–3733. [PubMed] [Google Scholar]
- 23.Mayadas TN, Johnson RC, Rayburn H, Hynes RO, Wagner DD. Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice. Cell. 1993;74:541–554. doi: 10.1016/0092-8674(93)80055-j. [DOI] [PubMed] [Google Scholar]
- 24.Renshaw BR, et al. Humoral immune responses in CD40 ligand-deficient mice. J Exp Med. 1994;180:1889–1900. doi: 10.1084/jem.180.5.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pircher H, Burki K, Lang R, Hengartner H, Zinkernagel RM. Tolerance induction in double specific T cell receptor transgenic mice varies with antigen. Nature. 1989;342:559–561. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]
- 26.Guidotti LG, et al. Noncytopathic clearance of lymphocytic choriomeningitis virus from the hepatocyte. J Exp Med. 1999;189:1555–1564. doi: 10.1084/jem.189.10.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guidotti LG, et al. Viral cross talk: intracellular inactivation of the hepatitis B virus during an unrelated viral infection of the liver. Proc Natl Acad Sci USA. 1996;93:4589–4594. doi: 10.1073/pnas.93.10.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steinhoff U, et al. Antiviral protection by vesicular stomatitis virus-specific antibodies in alpha/beta interferon receptor-deficient mice. J Virol. 1995;69:2153–2158. doi: 10.1128/jvi.69.4.2153-2158.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kakimi K, Isogawa M, Chung J, Sette A, Chisari FV. Immunogenicity and tolerogenicity of hepatitis B virus structural and nonstructural proteins: implications for immunotherapy of persistent viral infections. J Virol. 2002;76:8609–8620. doi: 10.1128/JVI.76.17.8609-8620.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cavanaugh VJ, Guidotti LG, Chisari FV. Inhibition of hepatitis B virus replication during adenovirus and cytomegalovirus infections in transgenic mice. J Virol. 1998;72:2630–2637. doi: 10.1128/jvi.72.4.2630-2637.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barber DL, Wherry EJ, Ahmed R. Cutting edge: rapid in vivo killing by memory CD8 T cells. J Immunol. 2003;171:27–31. doi: 10.4049/jimmunol.171.1.27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.