Abstract
Here we report the effects of loss of the Toll-like receptor-associated signaling adaptor myeloid-differentiation factor 88 (MyD88) on tumor induction in two distinct mouse models of carcinogenesis. The 7,12-dimethylbenz[a]anthracene (DMBA)/12-O-tetradecanoylphorbol 13-acetate (TPA)-induced skin papilloma model depends on proinflammatory processes, whereas the 3′-methylcholanthrene (MCA) induction of fibrosarcoma has been used by tumor immunologists to illustrate innate and adaptive immune surveillance of cancer. When exposed to a combination of DMBA/TPA, mice lacking MyD88 formed fewer skin papillomas than genetically matched WT controls treated in a similar manner. Unexpectedly, however, fewer MyD88−/− mice formed sarcomas than WT controls when exposed to MCA. In contrast, MyD88-deficient mice did not show a defective ability to reject highly immunogenic transplanted tumors, including MCA sarcomas. Despite the reported role of TNF in chronic inflammation, TNF-deficient mice were significantly more susceptible to MCA-induced sarcoma than WT mice. Overall, these data not only confirm the key role that MyD88 plays in promoting tumor development but also demonstrate that inflammation-induced carcinogenesis and cancer immunoediting can indeed occur in the same mouse tumor model.
Keywords: immunity, surveillance
Recent reports demonstrated that the immune system can facilitate cellular transformation or profoundly affect tumor outgrowth either quantitatively or qualitatively. In some studies, chronic infection leading to unresolved inflammation has been considered an important factor contributing to cellular transformation, tumorigenesis, and tumor progression (1–7). However, in other studies, the integrated effects of innate and adaptive immunity have been shown to be capable of either eliminating primary chemically induced or spontaneous tumors or promoting tumor outgrowth by mechanisms involving the dampening of tumor immunogenicity or attenuation of the protective antitumor response, a collective process called “cancer immunoediting” (8–10).
The apparently disparate effects of inflammation/immunity have led to a polarization of views about the cost/benefit ratios that result from immune system–tumor interactions. However, these conflicting observations may be more a result of the use of particular tumor models than actual all-or-none differences in the actions of inflammation/immunity. In fact, the possibility remains unexplored whether inflammation-induced cancer can occur together with cancer immunoediting in the same host. It also is unclear whether the specific inflammatory and/or immune components involved in tumorigenesis versus cancer immunoediting are identical or different or whether the eventual functional outcome of tumor growth depends on acute versus chronic inflammation. Nevertheless, despite the current polarization in views, there is generalized agreement that components of innate immunity are essential for either functional outcome.
The innate immune system detects the presence of pathogens or tumors through a variety of different mechanisms involving both cell surface receptors and intracellular sensors (11). Toll-like receptors (TLRs) represent one particularly well studied receptor family that has been shown to play essential roles in the development of innate responses to infectious agents by binding to pathogen-associated molecular patterns expressed by bacteria and viruses. Myeloid-differentiation factor 88 (MyD88) plays a critical role in mediating signaling by the majority of TLR family members, as well as the receptors for the proinflammatory cytokines of innate immunity IL-1 and IL-18 (12). Binding of ligands to different TLR receptors or IL-1/IL-18 receptors induces the association of MyD88 with the intracellular TIR domain of these receptors, resulting in the eventual activation of the NF-κB and AP-1 signaling pathways, which leads to initiation of the proinflammatory response (12). Very recently, two reports noted that mice lacking MyD88 formed fewer tumors than WT mice when exposed to specific carcinogens (i.e., diethylnitrosamine and azoxymethane) or when bred into a genetic background expressing a mutated form of the APC tumor suppressor gene (APCMIN) (13, 14). These data suggest that, in addition to inducing proinflammatory responses to infectious agents, MyD88 also may play a causative role in promoting inflammation-induced carcinogenesis.
Here we report the effects of loss of host MyD88 on tumor induction in two distinct mouse models of carcinogenesis: one [7,12-dimethylbenz[a]anthracene (DMBA)/12-O-tetradecanoylphorbol 13-acetate (TPA)] that is known to depend on proinflammatory processes and another [3′-methylcholanthrene (MCA)] that has been used as the central model to document the existence of the cancer immunoediting process. Not surprisingly, when exposed to a combination of carcinogenic (DMBA) and proinflammatory (TPA) stimuli, mice lacking MyD88 formed fewer tumors than genetically matched WT controls treated in a similar manner. Unexpectedly, MyD88−/− mice also formed fewer tumors than WT controls when exposed to MCA. This result was unexpected because, in the MCA model, components of both innate and adaptive immunity play critical roles in protecting the host against MCA-induced sarcoma formation. In contrast, MyD88−/− mice did not show a deficit in their ability to reject highly immunogenic transplanted tumors, including MCA sarcomas. Taken together, these data not only confirm the key role that MyD88 plays in promoting cellular transformation and tumor development but also demonstrate that inflammation-induced carcinogenesis and cancer immunoediting can indeed occur in the same mouse tumor model.
Results
DMBA/TPA Induction of Skin Papillomas Is MyD88-Dependent.
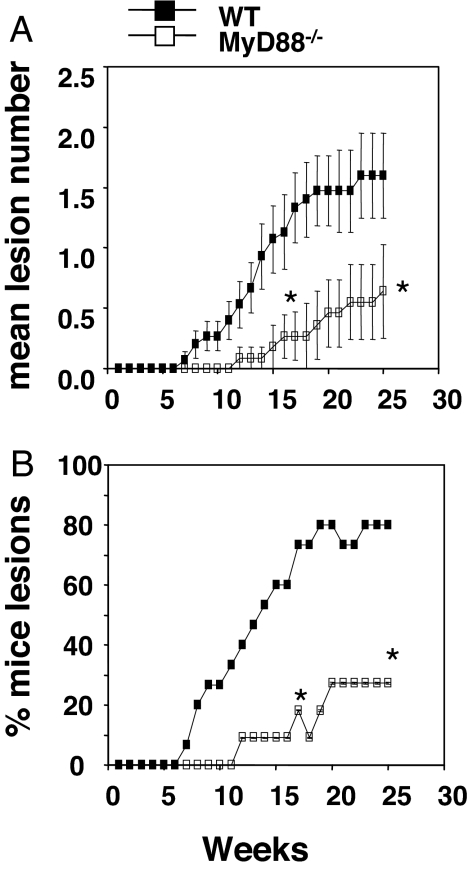
We first assessed the importance of MyD88 in the DMBA/TPA tumor-induction model, which is a well established model of inflammation-induced cancer. Others have used this model to document the importance of the proinflammatory cytokines TNFα, IL-23, and IL-1 in tumor development (15–17). Because MyD88 is the signaling adaptor molecule for the TLR-dependent induction of TNFα and IL-1, we expected MyD88-deficient mice to be relatively resistant to skin papilloma formation. Indeed, we found that MyD88−/− mice were more resistant to skin papilloma formation, compared with WT mice. Specifically, individual MyD88−/− mice developed fewer lesions per mouse than WT mice, and the percentage of MyD88−/− mice developing one or more lesions was lower than WT controls (Fig. 1 A and B). These data are consistent with the reports of IL-1 production after DMBA/TPA application (18), the resistance of TNFα- and IL-β-deficient mice to DMBA/TPA skin papilloma formation (15, 19), and the relationship among IL-1 signaling, MyD88, and TNF production (12, 20). Thus, the DMBA/TPA model shows the same dependency on MyD88 as other models of inflammation-induced tumors, such as the development of carcinogen-induced liver and colon adenocarcinomas (13, 14).
Fig. 1.
MyD88 promotes the formation of DMBA/TPA-induced skin papillomas. WT and MyD88−/− mice were injected with 25 μg of DMBA and 1 week later twice weekly with 4 μg of TPA for 20 weeks as described in Materials and Methods. Mice were subsequently monitored for skin papilloma development for 25 weeks (n = 11–15 mice per group). (A) Proportion of mice in the group with papillomas over time. (B) Mean lesion number per mouse over time. Significant differences in mean lesion number per mouse were observed by the unpaired Mann–Whitney U test (*, P < 0.05). Significant differences in proportion with papilloma at any one time point were determined by Fisher's exact test (*, P < 0.05). Differences were observed at all points between the asterisks (weeks 17–25).
MCA-Dependent Sarcoma Induction Is MyD88-Dependent.
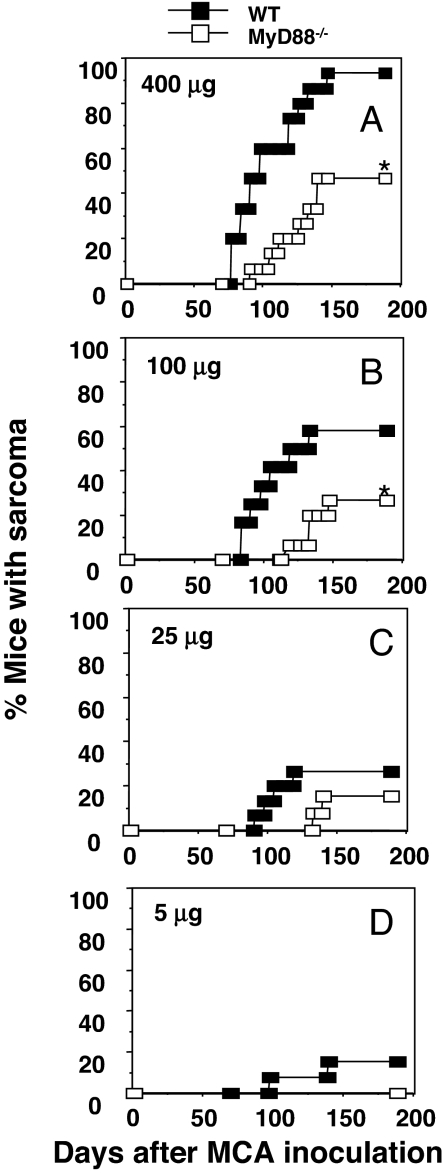
In contrast to DMBA/TPA, a single injection of MCA serves as an initiator and promoter of carcinogenesis, with fibrosarcomas developing over a 10- to 30-week period in a dose-dependent manner without a known large inflammatory component. Indeed, this model was previously used to demonstrate the importance of NK, NKT, γδ-T cells, αβ-T cells, NKG2D, perforin, TRAIL, IFN-γ, and IFN-α/β in host immune protection from MCA-induced fibrosarcoma (8, 21). In contrast, there are relatively few reports of molecular pathways that promote MCA-induced sarcoma formation, and to date no assessment has been made of a role for TLR signaling in regulating tumor initiation by MCA. Given the potential role of MyD88 in promoting antigen-presenting cell and lymphocyte function, the expectation was that MyD88-deficient mice would be more susceptible to MCA than WT mice. However, much to our surprise, fibrosarcoma formation was significantly depressed in MyD88−/− mice, compared with WT mice, especially when mice were treated with 400 and 100 μg of MCA, where a significant proportion of WT mice developed progressively growing sarcomas (Fig. 2). Tumor development also was delayed in MyD88−/−, compared with WT, mice. The growth behavior of these sarcomas, once established, was similar between MyD88−/− and WT mice (data not shown). Thus, in the model used to unequivocally document the occurrence of cancer immunosurveillance and immunoediting, clear evidence was obtained for the occurrence of inflammation-dependent cancer induction.
Fig. 2.
MyD88 promotes the formation of MCA-induced fibrosarcomas. WT and MyD88−/− mice were injected with 400 μg (A), 100 μg (B), 25 μg (C), or 5 μg (D) of MCA as described in Materials and Methods and subsequently monitored for tumor development over 200 days (n = 12–15 mice per group). Results are shown as the percentage of mice with sarcoma at each time point after MCA inoculation. Significant differences in tumor incidence were determined by Fisher's exact test (*, P < 0.05).
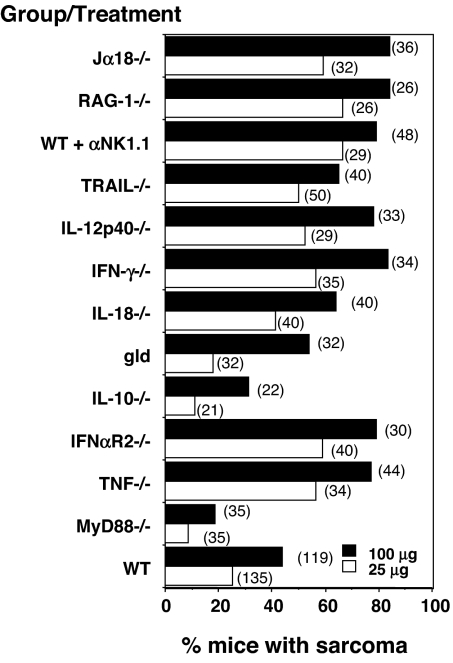
To explore the potential proinflammatory pathway contributing to MCA-induced carcinogenesis, we performed a large series of MCA experiments over several years comparing in each instance WT mice with cohorts of gene-targeted mice lacking specific cytokines or their receptors, which may either be modulated by or modulate MyD88 signaling [Fig. 3 and supporting information (SI) Fig. 7]. For reference, some previously published data from male mice have been included and pooled with the new data by using male mice to put all these experiments into context (SI Fig. 7) (21–25). There was considerable consistency in the penetrance of sarcoma in any one strain at any one dose across experiments (SI Fig. 7). We were particularly interested in assessing a causative role for TNFα in the MCA model because this cytokine has been implicated in many other models of inflammation-dependent cancer induction, including the DMBA/TPA model (15). Surprisingly, TNFα-deficient mice were more susceptible to MCA-induced sarcoma than WT mice (Fig. 3 and SI Fig. 7). Notably, TNFα-deficient mice were comparable to mice lacking TRAIL in their enhanced sensitivity to MCA, whereas mice with mutations in genes of other TNF superfamily members, such as gld mice (which have a mutated Fas Ligand gene), displayed WT mouse-like MCA sensitivity. Thus, despite the universally held view that TNFα is a major mediator of both acute and chronic inflammation, its role in the MCA model is host-protective. We therefore investigated whether other proinflammatory cytokines that participated in MyD88 signaling might be responsible for inducing MCA sarcomas. However, gene-targeted mice lacking the IL-18 cytokine or the Type I IFN receptor (IFNAR2) were either similarly or more sensitive to MCA, respectively, compared with WT controls (Fig. 3 and SI Fig. 7). Thus, although MyD88 promoted MCA-induced sarcomas, relevant cytokines, such as TNFα, IL-18, IL-10, and Type I IFN, did not account for this effect. Indeed, some of these inflammatory cytokines, such as TNF and Type I IFN, actually play a host-protective, tumor-suppressive role in this experimental system. Many possible mechanisms exist that we have not tested. Perhaps most interesting, IL-1β, but not IL-1α, was shown to promote the development and invasiveness of MCA-induced tumors (19). In addition, at least some forms of IL-1 have been shown to be up-regulated in mouse skin after DMBA exposure (18). These results may be particularly relevant because MyD88 is a critical component of IL-1 receptor signaling. Future work will need to test whether the IL-1 receptor/MyD88 signaling pathway is the effector mechanism leading to tumor formation by MCA.
Fig. 3.
TNFα functions to protect the host against MCA sarcoma induction. WT and various gene-targeted mice as indicated were injected with either 100 μg (filled bars) or 25 μg (open bars) of MCA as described in Materials and Methods and subsequently monitored for tumor development over 200 days (number of mice in each group shown in parentheses). Some WT mice were depleted of NK1.1+ cells weekly from the time of MCA inoculation to day 56. Results are shown as the percentage of mice with sarcoma in each group after MCA inoculation. Results are a collective of 3–12 experiments for each genotype/treatment performed over a 9-year period (all shown in SI Fig. 7), compared with WT controls within each experiment.
MyD88 Deficiency Does Not Compromise Immune Rejection of Transplanted Immunogenic Tumor Cells or Suppression of Experimental Metastases.
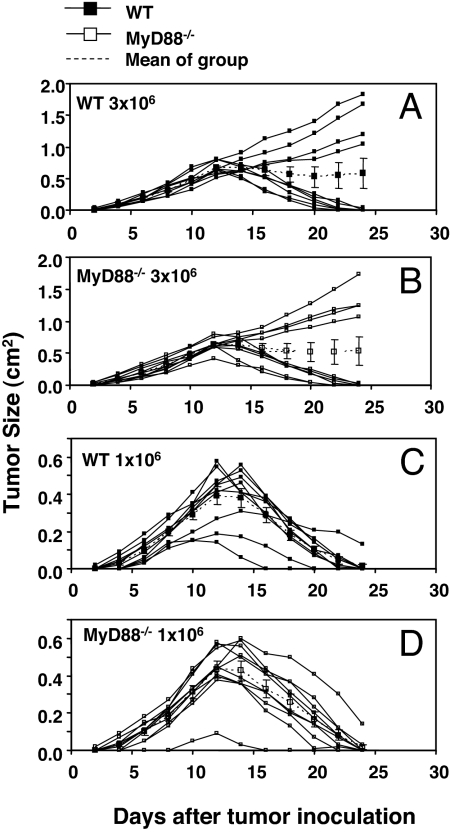
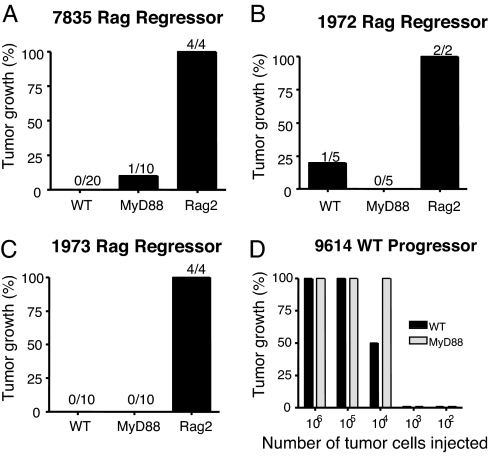
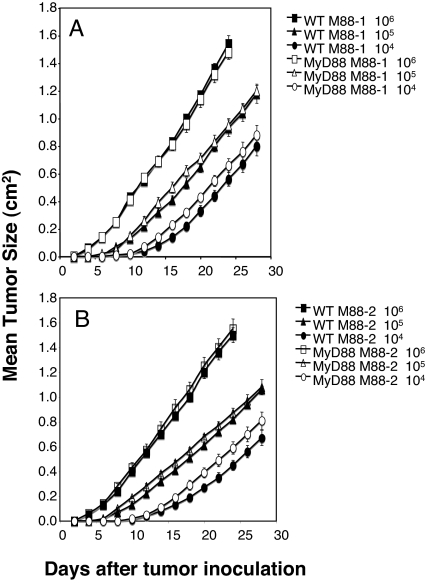
The use of tumor transplantation approaches bypasses the cellular transformation phase of carcinogenesis, allowing one to study, in isolation, the effects of antitumor immunity on preexisting tumor cells. We therefore examined whether NK cell- and T cell-mediated rejection of s.c. tumors or established experimental metastases were affected by host MyD88 expression. Subcutaneous EL4 engineered to express the model foreign antigen, ovalbumin (OVA), was previously shown to be controlled by WT mice in a CD8+ T cell-dependent manner (26). No differences were observed in the abilities of WT or MyD88−/− mice to control the growth of EL4-OVA lymphoma cells (Fig. 4). Some MCA-induced sarcomas derived from RAG-deficient mice were previously reported to be unedited and rejected by a combination of CD8+ and CD4+ T cells in WT mice (27). MyD88−/− mice were able to reject these highly immunogenic regressor MCA sarcomas (27) as efficiently as WT mice (Fig. 5). Furthermore, no differences were found in the growth of progressor MCA sarcomas in either WT or MyD88−/− mice (Fig. 5). Similarly, there was no difference in the ability of WT or MyD88−/− mice to control the growth of MHC class I-positive MC38 colon adenocarcinoma cells (data not shown). Thus, MyD88 does not play an important role in either promoting rejection of highly immunogenic tumors or facilitating the growth of tumors that develop the capacity to grow in a progressive manner in immunocompetent hosts. Finally, we determined whether the absence of MyD88 in the original tumor-bearing host had an effect on the quality (i.e., immunogenicity or tumorigenicity) of the tumor that developed. When transplanted s.c. into WT or MyD88−/− mice, fibrosarcomas derived from MCA-treated MyD88−/− mice grew in an equivalent manner (Fig. 6). These two sarcomas are representative of seven derived from MyD88 gene-targeted mice. Although in most cases their growth was marginally greater in B6 RAG-1−/− mice, tumors with a clear regressor and an unedited phenotype were not evident (data not shown). This result showed that the MyD88 environment from which a tumor is derived does not have a lasting effect on the intrinsic capacity of the tumor cells to grow in vivo.
Fig. 4.
MyD88 is not required for T cell-mediated rejection of a transplanted immunogenic tumor. Groups of 10 WT and MyD88−/− mice were challenged s.c. with either 3 × 106 (A and B) or 1 × 106 (C and D) EG7 tumor cells as indicated and subsequently monitored for tumor growth. Solid lines indicate tumor growth of individual mice; the dotted lines indicate the mean tumor size of the group.
Fig. 5.
MyD88 is not required for the rejection of transplanted immunogenic tumors or the growth of transplanted nonimmunogenic tumors. (A–C) WT, MyD88−/−, and Rag2−/− mice were challenged with 1 × 106 cells from MCA-induced immunogenic fibrosarcoma cell lines 7835 (A), 1972 (B), and 1973 (C). (D) The nonimmunogenic WT progressor tumor, 9614, was injected into MyD88 and WT mice at the indicated doses. Tumor cells were injected s.c. in the right flank of each mouse.
Fig. 6.
MyD88 does not control MCA-induced sarcomas that emerge from MyD88-deficient mice. Groups of five WT (filled symbols) and MyD88−/− (open symbols) mice were challenged s.c. with increasing tumor cell numbers (circles, 104; triangles, 105; squares, 106) of M88–1 sarcoma (A) or M88–2 sarcoma (B), both derived from MyD88−/− mice. Mice were subsequently monitored for tumor growth, and data are presented as the mean tumor area ± SEM (n = 5 mice per group).
We subsequently investigated whether MyD88 affected the ability of the host to control metastases. It is known that the suppression of B16F10 lung and 3LL liver metastases is mediated by NK cells (28). When WT and MyD88−/− mice were challenged with either high or low doses of B16F10 or 3LL cells, the numbers of lung and liver metastases that formed in WT and MyD88−/− mice were equivalent (SI Fig. 8 A and B). These data are consistent with the finding that the NK, iNKT, and T cell compartments in the spleen (SI Fig. 9 A–C), liver, and thymus (data not shown) of MyD88−/− mice were intact and that NK cell cytotoxicity in MyD88−/− mice was comparable to that in WT mice (SI Fig. 9D). In summary, we did not observe any differences between B16 melanoma cell invasiveness in MyD88−/− versus WT mice. In contrast, IL-1β−/− mice display a significant difference in metastasis control because they fail to develop B16 melanoma tumors (29).
Discussion
Herein we have shown the importance of MyD88 in promoting carcinogenesis in two distinct mouse models of cancer. In agreement with recent reports (13, 14), MyD88 was found to play a critical tumor-inducing role in a carcinogenesis model (DMBA/TPA) known to have a large inflammatory component. However, to our surprise, MyD88 also promoted the development of MCA-induced sarcomas, a model that has not been classically defined as having a significant inflammatory origin. Thus, in the model of carcinogen-induced tumor formation (MCA) that has been used extensively to document the existence of cancer immunoediting, there also exists a component of inflammation-dependent tumor formation. This result shows that inflammation-dependent tumor formation and cancer immunoediting can coexist in the same tumor model, although they may occur in a temporally distinct manner. In contrast, MyD88 does not play a detectable role in regulating growth and metastases of established tumor cell lines. It remains unclear whether MyD88 acts intrinsically to facilitate fibroblast and skin epithelial cell transformation (e.g., as part of a DNA damage or unfolded protein response) or extrinsically within the host stromal tissue or hematopoietic system to establish the protumorigenic environment.
Our findings suggest that the term “inflammation-induced cancer” is too general because it does not underscore the possible diverse effects that inflammatory cytokines can have in the tumor microenvironment, nor does it distinguish between inflammatory components, which, when left unchecked, promote tumor development, and those components that are needed acutely to induce a state of tumor-specific adaptive immunity. For example, in the primary MCA tumor model TNFα functions to protect the host, whereas in certain other models it appears to be a major effector of inflammation-dependent tumor induction (15). Thus, the use of distinct mouse models of cancer may reveal pleiotropic and even opposing effects of proinflammatory cytokines in inflammation-dependent tumor induction or induction of host-protective immunity. Nevertheless, as documented in this study, the MCA model of chemical carcinogenesis demonstrates the coexistence of inflammation-dependent tumor induction by MyD88, as well as the occurrence of the process of cancer immunosurveillance/immunoediting driven by cytokines known to be regulated by MyD88 (TNFα and IFN-α/β). Therefore, the complex interaction of the immune system and a developing tumor has multiple outputs, resulting in nonmutually exclusive events, whereby immunity can both promote and eliminate developing tumors and sculpt tumor immunogenicity, thereby ultimately facilitating tumor escape from immunologic control.
Materials and Methods
Mice.
Inbred WT C57BL/6 (WT) and C57BL/6 MyD88-deficient (MyD88−/−) mice (backcrossed nine generations to C57BL/6) were obtained from the Walter and Eliza Hall Institute of Medical Research [(WEHI) Melbourne, Australia]. Skin grafts between these mice and the C57BL/6 strain from WEHI to which they were backcrossed were maintained in both directions. Other C57BL/6 gene-targeted mice were all backcrossed at least 10 generations to C57BL/6, except C57BL/6 TNF-deficient mice, which were derived by using C57BL/6 ES cells. All mice were bred and maintained at the Peter MacCallum Cancer Centre. All experiments were performed according to animal experimental ethics committee guidelines.
Transplanted Tumors.
To examine primary tumor growth, male WT or MyD88−/− mice were inoculated s.c. with increasing doses of MC38 mouse colon adenocarcinoma, EL4-OVA (EG7) mouse lymphoma cells, or MCA-induced sarcomas derived from WT, RAG-2−/−, or MyD88−/− mice as previously described (27, 30). Primary tumors were measured every 2 days after tumor inoculation over the course of 30 days with a caliper square as the product of two perpendicular diameters (cm2) and represented as the mean ± SE of five mice in each group.
Tumor Initiation.
Groups of 12–15 male WT, MyD88−/−, and other gene-targeted mice were inoculated s.c. in the hind flank with 5, 25, 100, or 400 μg of MCA (Sigma–Aldrich) in 0.1 ml of corn oil. Some WT mice received weekly depletion of NK1.1+ cells from the time of MCA inoculation to day 56 (100 μg, PK136). Development of fibrosarcomas was monitored periodically over the course of 80–180 days. Tumors >2 mm in diameter and demonstrating progressive growth were recorded as positive. Sarcoma tumor cell lines derived from WT (9614), RAG−/− (7835, 1973, and 1972), and MyD88−/− (M88–1 and M88–2) mice were established and s.c. transplanted into WT, MyD88−/−, or RAG-1−/− mice as previously described (30). Two-stage chemical carcinogenesis by DMBA/TPA was as reported (31). Initiation was accomplished by pipette application of 25 μg of DMBA (Sigma–Aldrich) in acetone onto the shaved back skin of groups of 11–15 8-week-old female mice. Promotion was performed twice weekly with 4 μg of TPA (Sigma–Aldrich) from week 1 to week 20. Mice were assessed weekly for papilloma development for up to 25 weeks, and lesions were counted and measured.
Statistical Analysis.
Significant differences in tumor incidence at one time point were determined by Fisher's exact test. Values of P < 0.05 were considered significant.
Supplementary Material
ACKNOWLEDGMENTS.
We thank Michelle Stirling for maintaining the mice at the Peter MacCallum Cancer Centre and Nadeen Zerafa and Konstantinos Kyparissoudis for technical assistance. This work was supported by a National Health and Medical Research Council of Australia Fellowship and Program Grant (to M.J.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0708594105/DC1.
References
- 1.Beachy PA, Karhadkar SS, Berman DM. Nature. 2004;431:402. doi: 10.1038/431402a. [DOI] [PubMed] [Google Scholar]
- 2.Balkwill F, Charles KA, Mantovani A. Cancer Cell. 2005;7:211–217. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Clevers H. Cell. 2004;118:671–674. doi: 10.1016/j.cell.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Coussens LM, Werb Z. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karin M, Lawrence T, Nizet V. Cell. 2006;124:823–835. doi: 10.1016/j.cell.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 6.Robinson SC, Coussens LM. Adv Cancer Res. 2005;93:159–187. doi: 10.1016/S0065-230X(05)93005-4. [DOI] [PubMed] [Google Scholar]
- 7.Enzler T, Gillessen S, Manis JP, Ferguson D, Fleming J, Alt FW, Mihm M, Dranoff G. J Exp Med. 2003;197:1213–1219. doi: 10.1084/jem.20021258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smyth MJ, Dunn GP, Schreiber RD. Adv Immunol. 2006;90:1–50. doi: 10.1016/S0065-2776(06)90001-7. [DOI] [PubMed] [Google Scholar]
- 9.Dunn GP, Old LJ, Schreiber RD. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 10.Dunn GP, Old LJ, Schreiber RD. Annu Rev Immunol. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 11.Trinchieri G, Sher A. Nat Rev Immunol. 2007;7:179–190. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 12.Akira S, Takeda K. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 13.Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Science. 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 14.Rakoff-Nahoum S, Medzhitov R. Science. 2007;317:124–127. doi: 10.1126/science.1140488. [DOI] [PubMed] [Google Scholar]
- 15.Moore RJ, Owens DM, Stamp G, Arnott C, Burke F, East N, Holdsworth H, Turner L, Rollins B, Pasparakis M, et al. Nat Med. 1999;5:828–831. doi: 10.1038/10552. [DOI] [PubMed] [Google Scholar]
- 16.Langowski JL, Zhang X, Wu L, Mattson JD, Chen T, Smith K, Basham B, McClanahan T, Kastelein RA, Oft M. Nature. 2006;442:461–465. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- 17.Mueller MM. Eur J Cancer. 2006;42:735–744. doi: 10.1016/j.ejca.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 18.Li X, Eckard J, Shah R, Malluck C, Frenkel K. Cancer Res. 2002;62:417–423. [PubMed] [Google Scholar]
- 19.Krelin Y, Voronov E, Dotan S, Elkabets M, Reich E, Fogel M, Huszar M, Iwakura Y, Segal S, Dinarello CA, Apte RN. Cancer Res. 2007;67:1062–1071. doi: 10.1158/0008-5472.CAN-06-2956. [DOI] [PubMed] [Google Scholar]
- 20.Takeda K, Akira S. Semin Immunol. 2004;16:3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Swann JB, Hayakawa Y, Zerafa N, Sheehan KC, Scott B, Schreiber RD, Hertzog P, Smyth MJ. J Immunol. 2007;178:7540–7549. doi: 10.4049/jimmunol.178.12.7540. [DOI] [PubMed] [Google Scholar]
- 22.Smyth MJ, Thia KY, Street SE, Cretney E, Trapani JA, Taniguchi M, Kawano T, Pelikan SB, Crowe NY, Godfrey DI. J Exp Med. 2000;191:661–668. doi: 10.1084/jem.191.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smyth MJ, Crowe NY, Godfrey DI. Int Immunol. 2001;13:459–463. doi: 10.1093/intimm/13.4.459. [DOI] [PubMed] [Google Scholar]
- 24.Cretney E, Takeda K, Yagita H, Glaccum M, Peschon JJ, Smyth MJ. J Immunol. 2002;168:1356–1361. doi: 10.4049/jimmunol.168.3.1356. [DOI] [PubMed] [Google Scholar]
- 25.Street SE, Cretney E, Smyth MJ. Blood. 2001;97:192–197. doi: 10.1182/blood.v97.1.192. [DOI] [PubMed] [Google Scholar]
- 26.Hollenbaugh JA, Dutton RW. J Immunol. 2006;177:3004–3011. doi: 10.4049/jimmunol.177.5.3004. [DOI] [PubMed] [Google Scholar]
- 27.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 28.Smyth MJ, Taniguchi M, Street SE. J Immunol. 2000;165:2665–2670. doi: 10.4049/jimmunol.165.5.2665. [DOI] [PubMed] [Google Scholar]
- 29.Voronov E, Shouval DS, Krelin Y, Cagnano E, Benharroch D, Iwakura Y, Dinarello CA, Apte RN. Proc Natl Acad Sci USA. 2003;100:2645–2650. doi: 10.1073/pnas.0437939100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smyth MJ, Swann J, Cretney E, Zerafa N, Yokoyama WM, Hayakawa Y. J Exp Med. 2005;202:583–588. doi: 10.1084/jem.20050994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Girardi M, Oppenheim DE, Steele CR, Lewis JM, Glusac E, Filler R, Hobby P, Sutton B, Tigelaar RE, Hayday AC. Science. 2001;294:605–609. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.