Abstract
Atherosclerotic cardiovascular disease is a major problem despite the availability of drugs that influence major risk factors. New treatments are needed, and there is growing interest in therapies that may have multiple actions. Thyroid hormone modulates several cardiovascular risk factors and delays atherosclerosis progression in humans. However, use of thyroid hormone is limited by side effects, especially in the heart. To overcome this limitation, pharmacologically selective thyromimetics that mimic metabolic effects of thyroid hormone and bypass side effects are under development. In animal models, such thyromimetics have been shown to stimulate cholesterol elimination through LDL and HDL pathways and decrease body weight without eliciting side effects. We report here studies on a selective thyromimetic [KB2115; (3-[[3,5-dibromo-4-[4-hydroxy-3-(1-methylethyl)-phenoxy]-phenyl]-amino]-3-oxopropanoic acid)] in humans. In moderately overweight and hypercholesterolemic subjects KB2115 was found to be safe and well tolerated and elicited up to a 40% lowering of total and LDL cholesterol after 14 days of treatment. Bile acid synthesis was stimulated without evidence of increased cholesterol production, indicating that KB2115 induced net cholesterol excretion. KB2115 did not provoke detectable effects on the heart, suggesting that the pharmacological selectivity observed in animal models translates to humans. Thus, selective thyromimetics deserve further study as agents to treat dyslipidemia and other risk factors for atherosclerosis.
Consequences of atherosclerotic cardiovascular disease such as heart attack and stroke are major medical problems (1), and there is a need to develop improved means to treat these disorders and the risk factors that cause them, including hyperlipidemia and obesity (2). Thyroid hormones promote favorable effects on plasma cholesterol levels and weight loss (3–6), but also when administered in high doses can have deleterious effects on the heart (tachycardia, arrhythmia, and heart failure), bone, and muscle (7–9). Accordingly, synthetic thyroid hormone mimetic compounds that elicit preferentially the favorable metabolic effects of thyroid hormone without eliciting unfavorable effects of the hormone have received recent attention as potential treatment modalities for lowering cholesterol and promoting fat loss (10–12). In studies in several rodent models and monkeys, such compounds have been found to have effects on plasma lipid levels that appear to be favorable and to promote fat loss without eliciting unwanted effects on heart, bone, and muscle. These compounds exploit several mechanisms to derive selective actions, including hepatic first pass uptake, tissue selective uptake, and selective actions through the β-isoform of the thyroid hormone receptor known to regulate cholesterol metabolism and have minimal actions on heart (10, 12–14). However, there are species differences in the actions of hormones and in metabolism, especially lipid and lipoprotein metabolism, such that there is no certainty that animal data will be applicable to humans (15, 16). To our knowledge, there are no studies documenting that these compounds are effective in humans. The current study describes effects on one of these compounds, KB2115 (3-[[3,5-dibromo-4-[4-hydroxy-3-(1-methylethyl)-phenoxy]-phenyl]-amino]-3-oxopropanoic acid), administered to humans for 2 weeks. The results suggest that the compound can promote LDL lowering and bile acid synthesis, a potential reflection of net cholesterol loss, without eliciting favorable deleterious effects on the heart.
Results
Pharmacokinetics, Safety, and Tolerability.
The two studies reported (see Materials and Methods) were randomized, double-blinded, and placebo-controlled with subjects with moderately elevated total plasma cholesterol levels (mean total cholesterol 6.6 mmol/liter or 255 mg/dl) and body weights (mean body mass index of 31 kg/m2). In the first study, single escalating doses of KB2115 were administered up to 2,000 μg. In the second study, placebo or KB2115 at 50, 100, and 200 μg was administered orally once daily for 2 weeks. No effects were observed at any dose on body weight or oxygen consumption.
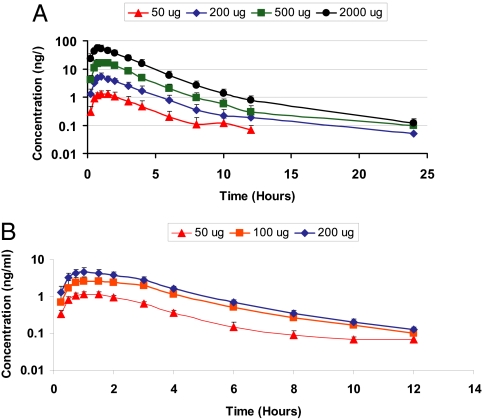
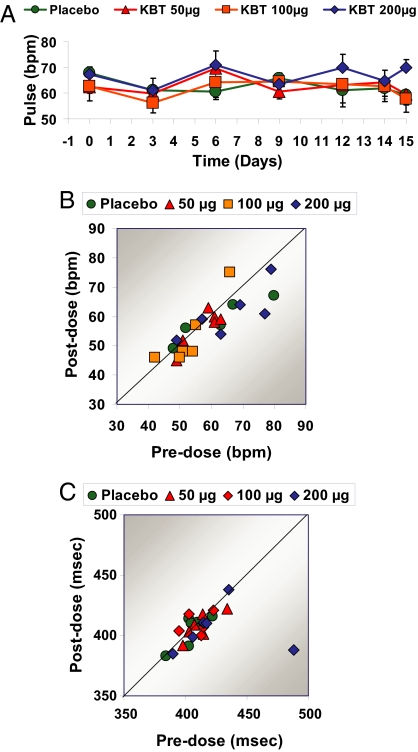
The pharmacokinetics of KB2115 were linear (Fig. 1). The drug was rapidly absorbed with a half-life of ≈2 h, and no accumulation of KB2115 was observed. KB2115 was well tolerated with no serious adverse events reported (data not shown), and there were no withdrawals from the study. The most frequent drug-related adverse events observed were mild increases in transaminase [alanine aminotranferease (ALT) and aspartate aminotransferase] levels, and one subject, who had elevated ALT before dosing with KB2115 developed a transient increase in ALT of ≈3-fold the upper level for normal. No symptoms or laboratory signs of musculoskeletal side effects were seen. No drug-related effects on the cardiovascular system were detected during the 2-week study, including heart rate (Fig. 2 A and B), electrocardiograms, corrected QT interval (QTc) (Fig. 2C), and blood pressure.
Fig. 1.
Pharmacokinetic properties of KB2115 after single doses (A) or multiple doses (B).
Fig. 2.
Effects of KB2115 (KBT) on the cardiovascular system after once-daily dosing for 2 weeks. (A and B) Effects on heart rate as measured by sequential measures during the treatment period (A) or by comparing heart rate before treatment versus after the last dose (B). (C) Effects on the QTc.
Pharmacodynamic Effects on Thyroid Hormone Homeostasis and Lipid Metabolism.
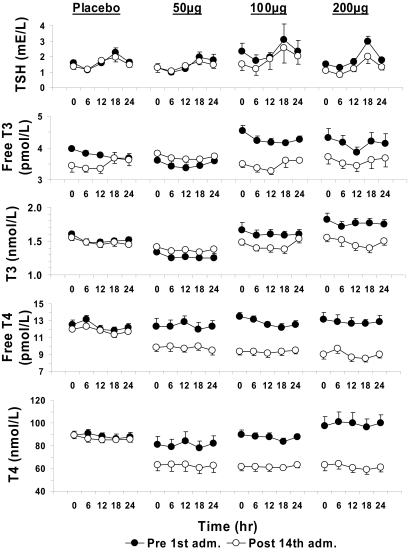
The hypothalamic-pituitary-thyroid axis (HPT) is under circadian regulation in humans (17). Potential effects of KB2115 on biomarkers of thyroid hormone homeostasis were therefore measured at 1, 6, 12, 18, and 24 h before treatment and at the same time points after the last dose (Fig. 3). There was a significant difference between the groups in free T4 and total T4 and the values decreased in all active groups when compared with placebo (Kruskal-Wallis, P < 0.001). Changes in total and free T3 and thyroid-stimulating hormone (TSH) were small and not statistically significant.
Fig. 3.
Effects of KB2115 on the hypothalamic-pituitary axis and thyroid hormone homeostasis after once-daily dosing for 2 weeks. Effects on (from top to bottom) TSH, free T3, total T3, free T4, and total T4 are shown. Significant difference in AUC (0–24 h) before and after 14 days of treatment with KB2115 was found for free T4 and total T4 (P = 0.002 and 0.001, respectively), and all active groups differed from placebo.
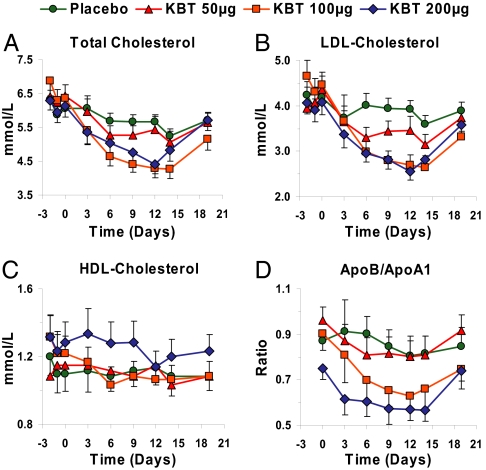
Serum total cholesterol (Fig. 4A), LDL cholesterol (Fig. 4B), and apolipoprotein (apo) B levels were reduced. LDL cholesterol were reduced up to 40%, from 4.5 mmol/liter (175 mg/dl) to 2.7 mmol/liter (105 mg/dl), whereas an 11% reduction was seen in the placebo group. Reduction at the two highest doses was observed after ≈1 week. Total and LDL cholesterol levels gradually returned to control values by 5 days after cessation of therapy. Changes in HDL cholesterol levels were not observed (Fig. 4C), and the apoB to apoA-I ratio decreased (Fig. 4D). We did not observe any statistically significant effects on serum triglyceride levels or lipoprotein(a) levels (data not shown) as reported in animal models with other thyromimetics (10, 12). However, in subjects with high baseline levels of triglycerides there was a trend toward the lowering of triglyceride levels (data not shown).
Fig. 4.
Effects of KB2115 on lipids and lipoproteins after once-daily dosing for 2 weeks. Effect on total cholesterol (A; P = 0.005, 100-μg group differs from placebo), LDL cholesterol (B; P = 0,01, 100-μg group differs from placebo), HDL cholesterol (C; P = 0.68), and apoB/apoA-I ratio (D; P = 0.04, 100-μg group differs from placebo).
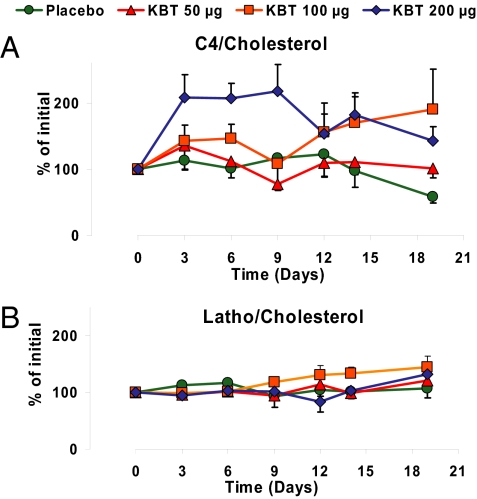
We also evaluated whether KB2115 treatment altered the synthesis of cholesterol and/or its catabolism to bile acids. For this purpose, serum levels of lathosterol were determined as a marker for total body cholesterol synthesis (18) and of 7α-hydroxy-4-cholesten-3-one (C4) as a marker for hepatic bile acid synthesis (18, 19). As shown in Fig. 5B, the plasma lathosterol/cholesterol ratio was not significantly altered in any of the dose groups, which indicates that KB2115 did not have a major effect on whole body cholesterol synthesis. As shown in Fig. 5A, the C4/cholesterol ratio was unchanged in the 50-μg dose group, but ≈50% and 100% increases were observed at the 100- and 200-μg doses, respectively. These data suggest that there is an increased synthesis of bile acids that is not secondary to an increase in cholesterol synthesis.
Fig. 5.
Effects of KB2115 on biomarkers for cholesterol synthesis and degradation after once-daily dosing for 2 weeks displayed as percent change from baseline for ratios between C4 and cholesterol (A; P = 0.01, 200-μg group differs from placebo) and ratios between lathosterol and cholesterol (B).
Discussion
These studies report on the pharmacological effects of a thyromimetic in humans where significant effects of the compound on clinically important endpoints were observed under conditions where there were no detectable effects on the cardiovascular system. The two clinically desired results were a lowering of serum LDL cholesterol levels, and based on measurements of established bio-markers for bile-acid and cholesterol synthesis (18, 19), an increase in bile acid synthesis in the absence of an effect on cholesterol synthesis. In the 2-week study, KB2115 lowered serum cholesterol levels to a maximal level of 40% reduction. Similar effects on total cholesterol levels and apoB were also observed. At high dose, KB2115 increased bile acid synthesis as reflected by increases of the C4/cholesterol ratio, under conditions where there was no apparent increase in cholesterol synthesis as reflected by the plasma lathosterol/cholesterol ratio. These data imply mechanistic differences from the statins that do not increase bile acid production but instead decrease cholesterol synthesis, thereby stimulating hepatic LDL receptor expression (20). The exact mechanism of action of KB2115 in humans cannot be determined from the present study. Given the major differences between humans and rodents, where many of the mechanistic studies of this class of thyromimetics have been performed (10–13), translation of mechanisms to humans should be done with caution.
Several mechanisms observed in rodents are possible (13); the effects of KB2115 could be caused by those on either the LDL or HDL pathways, or both, and on expression of cholesterol 7α-hydroxylase (CYP7A1), the rate-limiting enzyme in cholesterol breakdown to bile acids. Whereas the CYP7A1 gene is regulated by thyroid hormone in rodents (13, 21–23), it is less clear if this also occurs in humans (24). In various models, the compounds can increase liver LDL receptor levels. Thyromimetic compounds have been shown to increase hepatic RNA levels of both apoA-I and the SR-B1 HDL receptor (13). If HDL production and elimination were both stimulated, this could explain the lack of changes in serum HDL or apoA-I levels. Nevertheless, the observations that KB2115 both lowered plasma cholesterol and appeared to increase bile acid production suggest that this compound differs mechanistically from the statins. Further, the design of KB2115 to have both TRβ-selective actions and tissue-selective uptake actions is consistent with the observation that the effects of KB2115 on cholesterol and bile acids occur in the absence of detectable effects on the heart. These results contrast with the increases in heart rate in thyrotoxic patients (9) and subjects given exogenous L-T3 (4). These issues obviously need to be addressed in larger long-term studies.
The observation that these thyromimetics may act differently from the statins also suggests that these compounds might complement actions of the statins. Thus, it is possible that these compounds might be effective in lowering the dose requirement of statins in subjects intolerant to these drugs. We noted effects of KB2115 on parameters of the HPT axis and thyroid hormone homeostasis, including discrete reductions in plasma TSH and more evident reductions in free and total T4. These data indicate that KB2115 is recognized as a thyromimetic in the pituitary and that KB2115 acts together with the endogenous levels of T3 and T4 in the regulation of the HPT axis feedback control of peripheral thyroid hormone homeostasis. However, effects of KB2115 on the HPT axis need to be further evaluated in long-term studies. It is noteworthy, however, that in a study in rodents with another selective thyromimetic called GC-1, the plasma TSH, T3,and T4 levels were suppressed but the tissue levels of T3 were unchanged (25). We also noted mild increases in hepatic enzymes. Whether these results are related to the mechanism of action of KB211 or they are caused by off-target related liver effects is not yet clear. However, whereas no liver toxicity has been found in animal studies to date (data not shown), there have been associations of severe hyperthyroidism with hepatic abnormalities. Clearly, this aspect also needs careful monitoring in future studies. Although thyromimetics have been shown in rodents and monkeys to promote fat loss at high doses without muscle or bone loss (10, 12), we did not observe changes in metabolic rate or weight loss in our study. Overall, plasma cholesterol levels have been shown to be more sensitive to thyromimetics than effects on metabolic rate and body weight reduction (10, 12). Thus, it is possible that a lack of changes in metabolic rate and weight loss in humans as observed in this study may be because higher doses were not administered and the study was of short duration.
In conclusion, our data obtained in a small group of subjects treated with KB2115 show that a potent and rapid LDL lowering can be achieved together with a possible increased bile acid synthesis that, in turn, should promote net excretion of cholesterol from the body. Importantly, these effects of KB2115 on lipid metabolism were all observed at doses where effects on the heart could not be detected. Thus, at least for this compound, the pharmacological selectivity between effects on blood lipids and effects on heart rate, previously observed with thyromimetic compounds in animal models (10–13, 25, 26), also translates to humans. These findings in humans are an important step in evaluating thyromimetics as a strategy for treatment of dyslipidemia, as reported in this study, and potentially also for obesity as shown in animal models with other thyromimetics (10–12, 26).
Materials and Methods
Subjects.
Inclusion criteria for both studies were males or females (not of childbearing potential) between 18 and 60 years of age with a body mass index between 25 and 35 and total cholesterol >5.0 mmol/liter.
Study Design.
The studies were randomized, double-blind, placebo-controlled, single-dose, and repeated-dose studies performed in compliance with good clinical practice. In the single-dose study 24 subjects were enrolled in four groups of six subjects each. Subjects were then randomized to receive either KB2115 or placebo. Single doses of 50, 200, 500, and 2,000 μg were given sequentially, one dose per group of five fasting subjects. One subject on placebo was included in each dose group. Assessments were made for 2 days before and 5 days after administration. In the multiple-dose study, KB2115 was used at doses of 50, 100, and 200 μg or placebo and administered daily for 2 weeks. Twenty-four subjects were enrolled into three groups of eight subjects each. The subjects were then randomized to receive either KB2115 or placebo. Multiple doses (once daily) at the three dose levels (50, 100, and 200 μg) of KB2115 were administered in six subjects each. Two subjects on placebo were included in each dose group.
Measurements.
Spontaneously reported adverse events were recorded up to 5 days after the last administered dose. Routine laboratory tests of safety included hematology, serum chemistry, and urinalysis. In the single-dose study, vital signs and 12-lead ECG were recorded within 1 h before drug administration, 1 h after dose, and 1 day after administration. A 12-lead ECG was also recorded 5 days after administration. Telemetry was used continuously for 24 h beginning at dose administration. For the multiple-dose study, vital signs were recorded every second or third day during the treatment period and 1 day after administration. A 12-lead ECG was recorded before start of dosing and 1 h after the first, third, ninth, and last dose. Effects of KB2115 on the hypothalamic-pituitary axis were determined by measuring TSH, free and total T3, and free and total T4. This measurement was done in the single-dose study before and at 1, 6, 12, 18, 24, 30, 36, 48, 72, 96, and 120 h after administration of KB211 and in the multiple-dose study at 1, 6, 12, 18, and 24 h after the first and last dose of KB2115. An indirect RIA assay was used to estimate levels of total and free T4 and total and free T3. Serum lipids including cholesterol (total, LDL, HDL), triglycerides, free fatty acids, apoA-I, apoB, and lipoprotein a were measured in fasting samples. Also changes in bile acid and cholesterol synthesis rates were assessed by measurements of C4/cholesterol and lathosterol/cholesterol as described (18, 19).
Statistical Analysis.
All data were analyzed by Kruskal-Wallis test, followed by post hoc pairwise comparison of active treatment groups with placebo, when appropriate. For biomarkers of thyroid hormone homeostasis areas under the curve (AUCs) from 0–24 h was analyzed as described above, and AUCs before treatment were compared with those after 14 days of treatment. For C4/cholesterol and lathosterol/cholesterol ratios the AUCs from day 0 to day 15 were compared. For apoB/apoA-I ratio, total cholesterol, and HDL and LDL cholesterol, the data of the differences between the groups before treatment and after 14 days of treatment were tested.
ACKNOWLEDGMENTS.
We thank Drs. Paul W. Ladenson, Chester E. Ridgway, Irwin Klein, and John Kane for critical and constructive discussions on study design and Dr. Anders Tamsen and SedocPm (Solna, Sweden) for help with the clinical trial application to the Swedish Medical Product Agency. This study was supported by grants from the National Institutes of Health (to J.D.B.), the Swedish Industrial Fund (to Karo Bio AB), and the Swedish Research Council and the Swedish Heart-Lung Foundation (to B.A.).
Footnotes
Conflict of interest statement: A.B., J.K., K.M., B.C., J.M., S.R., N.G., and C.M.A. are employees of Karo Bio AB. B.A. and J.D.B. have proprietary interests in and serve as consultants to Karo Bio AB. F.S. is employed by Berzelius Clinical Research Center AB.
See Commentary on page 409.
References
- 1.Tenenbaum A, Fisman EZ, Motro M, Adler Y. Cardiovasc Diabetol. 2006;5:20. doi: 10.1186/1475-2840-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grundy SM. Nat Rev Drug Discov. 2006;5:295–309. doi: 10.1038/nrd2005. [DOI] [PubMed] [Google Scholar]
- 3.Cappola AR, Ladenson PW. J Clin Endocrinol Metab. 2003;88:2438–2444. doi: 10.1210/jc.2003-030398. [DOI] [PubMed] [Google Scholar]
- 4.Gwinup G, Poucher R. Am J Med Sci. 1967;254:416–420. doi: 10.1097/00000441-196710000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Monzani F, Caraccio N, Kozakowa M, Dardano A, Vittone F, Virdis A, Taddei S, Palombo C, Ferrannini E. J Clin Endocrinol Metab. 2004;89:2099–2106. doi: 10.1210/jc.2003-031669. [DOI] [PubMed] [Google Scholar]
- 6.Yen PM. Physiol Rev. 2001;81:1097–1142. doi: 10.1152/physrev.2001.81.3.1097. [DOI] [PubMed] [Google Scholar]
- 7.Bassett JH, Williams GR. Trends Endocrinol Metab. 2003;14:356–364. doi: 10.1016/s1043-2760(03)00144-9. [DOI] [PubMed] [Google Scholar]
- 8.Simonides WS, Thelen MH, van der Linden CG, Muller A, van Hardeveld C. Biosci Rep. 2001;21:139–154. doi: 10.1023/a:1013692023449. [DOI] [PubMed] [Google Scholar]
- 9.Kahaly GJ, Dillmann WH. Endocr Rev. 2005;26:704–728. doi: 10.1210/er.2003-0033. [DOI] [PubMed] [Google Scholar]
- 10.Grover GJ, Mellstrom K, Ye L, Malm J, Li YL, Bladh LG, Sleph PG, Smith MA, George R, Vennstrom B, et al. Proc Natl Acad Sci USA. 2003;100:10067–10072. doi: 10.1073/pnas.1633737100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Webb P. Exp Opin Invest Drugs. 2004;13:489–500. doi: 10.1517/13543784.13.5.489. [DOI] [PubMed] [Google Scholar]
- 12.Grover GJ, Egan DM, Sleph PG, Beehler BC, Chiellini G, Nguyen NH, Baxter JD, Scanlan TS. Endocrinology. 2004;145:1656–1661. doi: 10.1210/en.2003-0973. [DOI] [PubMed] [Google Scholar]
- 13.Johansson L, Rudling M, Scanlan TS, Lundasen T, Webb P, Baxter J, Angelin B, Parini P. Proc Natl Acad Sci USA. 2005;102:10297–10302. doi: 10.1073/pnas.0504379102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morkin E, Pennock GD, Spooner PH, Bahl JJ, Goldman S. Thyroid. 2002;12:527–533. doi: 10.1089/105072502760143935. [DOI] [PubMed] [Google Scholar]
- 15.Shulman AI, Mangelsdorf DJ. N Engl J Med. 2005;353:604–615. doi: 10.1056/NEJMra043590. [DOI] [PubMed] [Google Scholar]
- 16.Tailleux A, Torpier G, Mezdour H, Fruchart JC, Staels B, Fievet C. Trends Pharmacol Sci. 2003;24:530–534. doi: 10.1016/j.tips.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Greenspan SL, Klibanski A, Schoenfeld D, Ridgway EC. J Clin Endocrinol Metab. 1986;63:661–668. doi: 10.1210/jcem-63-3-661. [DOI] [PubMed] [Google Scholar]
- 18.Galman C, Angelin B, Rudling M. Gastroenterology. 2005;129:1445–1453. doi: 10.1053/j.gastro.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Galman C, Arvidsson I, Angelin B, Rudling M. J Lipid Res. 2003;44:859–866. doi: 10.1194/jlr.D200043-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Reihner E, Rudling M, Stahlberg D, Berglund L, Ewerth S, Bjorkhem I, Einarsson K, Angelin B. N Engl J Med. 1990;323:224–228. doi: 10.1056/NEJM199007263230403. [DOI] [PubMed] [Google Scholar]
- 21.Gullberg H, Rudling M, Forrest D, Angelin B, Vennstrom B. Mol Endocrinol. 2000;14:1739–1749. doi: 10.1210/mend.14.11.0548. [DOI] [PubMed] [Google Scholar]
- 22.Gullberg H, Rudling M, Salto C, Forrest D, Angelin B, Vennstrom B. Mol Endocrinol. 2002;16:1767–1777. doi: 10.1210/me.2002-0009. [DOI] [PubMed] [Google Scholar]
- 23.Shin DJ, Plateroti M, Samarut J, Osborne TF. Nucleic Acids Res. 2006;34:3853–3861. doi: 10.1093/nar/gkl506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drover VA, Agellon LB. Endocrinology. 2004;145:574–581. doi: 10.1210/en.2003-0993. [DOI] [PubMed] [Google Scholar]
- 25.Trost SU, Swanson E, Gloss B, Wang-Iverson DB, Zhang H, Volodarsky T, Grover GJ, Baxter JD, Chiellini G, Scanlan TS, Dillmann WH. Endocrinology. 2000;141:3057–3064. doi: 10.1210/endo.141.9.7681. [DOI] [PubMed] [Google Scholar]
- 26.Grover GJ, Mellstrom K, Malm J. Cardiovasc Drug Rev. 2005;23:133–148. doi: 10.1111/j.1527-3466.2005.tb00161.x. [DOI] [PubMed] [Google Scholar]