Abstract
Solar radiation is the main cause of skin cancers. However, it also is a main source of vitamin D for humans. Because the optimal status of vitamin D protects against internal cancers and a number of other diseases, a controversy exists: Will increased sun exposure lead to net health benefits or risks? We calculated the relative yield of vitamin D photosynthesis as a function of latitude with a radiative transfer model and cylinder geometry for the human skin surface. The annual yield of vitamin D is 3.4 and 4.8 times larger below the equator than in the U.K. and Scandinavia, respectively. In populations with similar skin types, there are clear latitude gradients of all major forms of skin cancer, indicating a north–south gradient in real sun exposure. Surprisingly, the incidence rates of major internal cancers also increase from north to south. However, the survival prognosis also improves significantly from north to south. Reasons for these findings are discussed in view of the role of vitamin D. In Norway, melanoma rates increased by a factor of 6 from 1960 to 1990, while the prognosis improved in the same period. After 1990, melanoma rates have remained constant or even decreased in age groups <50 years, whereas the prognosis has not improved further. These data, together with those for internal cancers and the beneficial effects of an optimal vitamin D status, indicate that increased sun exposure may lead to improved cancer prognosis and, possibly, give more positive than adverse health effects.
Keywords: body mass index, cutaneous malignant melanoma, squamous cell carcinoma, ultraviolet radiation
There is a controversy as to whether increased sun exposure to Western populations would prolong or shorten lifetime expectancy, result in fewer or more cancer deaths, and, in general, lead to health benefits or risks (1, 2). For years, emphasis has been placed on the increasing time trends of incidence and mortality rates of cutaneous malignant melanoma (CMM) (3, 4) and, in contrast, on the protective role of vitamin D regarding many types of internal cancer and other diseases (5–7). Too much sun exposure has been blamed for the high and increasing incidence rates of CMM. However, solar radiation is a major, if not the main, source of vitamin D in humans. Therefore, a population's increased sun exposure leads to improved vitamin D status. The observation that the incidence and mortality of several types of internal cancers decreases with decreasing latitude in the United States and other countries initiated the research on vitamin D–cancer relationships in the 1980s and 1990s. However, in some cases, there is an inverse gradient of the rates of internal cancer with latitude (1), with the rates being higher in regions with high annual UV fluences (New Zealand and Australia) than in countries with low annual UV fluences (Northern Europe, Scandinavia, and the U.K.), despite the fact that the populations of these regions are closely related genetically or, at least, have similar skin types, which is important for the photosynthesis of vitamin D.
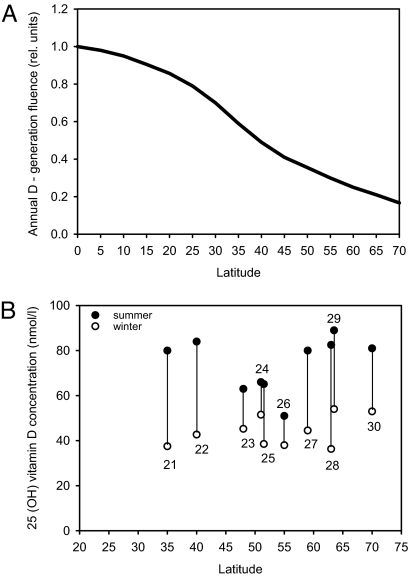
These issues have health consequences far beyond those of cancer because a number of diseases are associated with inadequate vitamin D levels or low sun exposure: neurological, cardiovascular, metabolic, immune, and bone diseases (2, 7). Evolutionary arguments involving skin color also should be taken into account. A dark skin color is found among Africans and, possibly, early hominids who lived close to the equator (8). This pigment may protect against skin cancer and folate photodegradation (8, 9). A white skin color developed later in our history, as humans left Africa and went north. Because dark skin needs about 6 times higher solar exposure for vitamin D photosynthesis than white skin (10, 11) and because the fluence rate of vitamin D-generating solar radiation decreases with increasing latitude (Fig. 1A), one can argue that skin whitening may be related to the need for vitamin D and the lack of sunshine at high latitudes.
Fig. 1.
Vitamin D as a function of latitude. (A) The dependency of annual vitamin D photosynthesis on latitude, calculated by using the in vivo action spectrum of pre-vitamin D synthesis (43) and known fluence rates of solar radiation as earlier described (37). (B) Summer (filled circle) and winter (empty circle) values for 25(OH)D levels in different populations living at different latitudes. The numbers in the graph indicate the citation number in the reference list.
Results and Discussion
Is CMM Caused by Solar Radiation?
Because the mortality rates of CMM are much higher than those of nonmelanoma skin cancer (in some populations, more than a factor of 10 higher), this problem is the most important one to solve regarding the negative consequences of sun exposure. The solution is by no means certain yet. A number of investigators disagree, as we reviewed earlier (12, 13). The main arguments against the concept that sun exposure causes CMM are that: (i) CMM is more common among persons with indoor work than among those people with outdoor work (14, 15); (ii) in younger generations, more CMMs arise per unit skin area on partly shielded areas (trunk and legs) than on face and neck (16); and (iii) CMMs sometimes arise on totally shielded areas (acral CMM and uveal melanomas). Although the connection between these melanoma types and sun exposure is controversial (17–19), their inclusion in the present discussion is justified because of the possible involvement of vitamin D.
However, in our opinion, a significant fraction of CMMs is related to sun exposure (16, 20). The main arguments for this relationship are: (i) the north–south gradients in CMM incidence between Scandinavia and Australia (16), (ii) before the advent of the “top-less” fashion, few women developed CMM on the breast area (13, 16), and (iii) in some animals (Sinclair swine, Monodelphis domestica, the fish Xiphophorus, white horses, angora goats, transgenic mice, etc.) UV exposure leads to CMM (16). The reason that CMM incidence rates decrease with decreasing latitude in Europe is likely because of differences in skin color from region to region.
Seasonal Variations of the Vitamin D Status.
As shown in Fig. 1B (21–30), a pronounced seasonal variation is evident in most of the published investigations on 25(OH)D (the serum marker of vitamin D status). Summer values can be >100% larger than winter values. In Tromsø, Norway, at 70°N, people have a higher intake of vitamin D (mainly from cod liver) in the cod season (January–March) than in the rest of the year (31, 32). The annual vitamin D photosynthesis is modest, compared with further south (Fig. 1A). In Bergen, Norway (61°N), there is no vitamin D photosynthesis from October to March (7). Despite this fact, the serum level of 25(OH)D in a population living in Tromsø is 30% higher in late summer than in late winter (30). Thus, we can conclude that, even at such high latitudes, the sun is an important source of vitamin D. This finding is supported by controlled sun bed experiments, which show that exposure to suberythemal doses gives 25(OH)D contributions of 10–50 nmol/liter in serum (33). Our recent investigations (A.C.P., Ø. S. Bruland, L. Aksnes, W. Grant, and J.M., unpublished work) support this notion and even show that a high sun bed-induced 25(OH)D level cannot be maintained by daily intakes of the recommended amount of vitamin D (200 units in the form of cod-liver oil pills).
Seasonal Variations of Cancer Prognosis.
Because our demonstration of the prognostic advantage of diagnosis in late summer and autumn [≈20% difference in relative risk of death in these seasons when the 25(OH)D status is optimal] (35), we conducted several more detailed studies showing similar trends. Many cancer forms are now on our list: prostate, breast, colon, and lung cancers, as well as lymphomas and even melanomas (36–40). Other investigators have found comparable results (41, 42). These data argue for a positive role of sun induced-vitamin D in cancer prognosis or that a good vitamin D status is advantageous when in combination with standard cancer therapies.
North–South Gradients of Vitamin D.
Our calculations, which are based on known ozone levels, cloud covers, and the in vivo action spectrum for photosynthesis of pre-vitamin D from 7-dehydrocholesterol (43), show that there is a pronounced north–south gradient in vitamin D-generating solar radiation (Fig. 1A). It should be emphasized that, in contrast to earlier investigations (16), we calculated the doses for a vertical cylinder, expecting such a geometry to represent the human body better than a horizontal, flat surface. With our approach, the annual, equatorial fluence of vitamin D-inducing radiation is ≈3.4 times larger than that in the U.K. and ≈4.8 times larger than that in Scandinavia (Fig. 1A). A crucial and as-yet-unanswered question is: Are there north–south gradients in sun exposure habits and in vitamin D intake? In Norway, we know that the vitamin D intake is 10–20% larger in the north than in the south. This finding is mainly related to the consumption of cod liver (32). However, the population's sun exposure is definitely larger in the south than in the north, as shown by calculations as well as skin cancer epidemiological investigations (see Fig. 1, ref. 13, and www.kreftregisteret.no). Overall, therefore, there is probably no north–south gradient in vitamin D status in Norway. This finding seems consistent with the lack of north–south gradient in both cancer incidence and prognosis, which is discussed later (36, 39, 40).
Different clinical searches for a latitudinal gradient in vitamin D status do not agree. Zittermann et al. (44) found a negative 25(OH)D gradient with increasing latitude, as expected, whereas others found the opposite (45). Our review of international data (Fig. 1B) shows no significant gradient. It is surprising that mean population levels of vitamin D are similar in sunny regions like Florida (46), Australia (47), and Northern Europe (48). We found earlier that the incidence rates of the three major forms of skin cancer increase from Norway to Australia, which is in agreement with a large increase in annual UV fluence (16). Thus, because the action spectrum of pre-vitamin D photosynthesis and that of squamous cell carcinoma are similar (43, 49), one should expect to find a vitamin D gradient. The answer to this puzzle may be found either in the pattern of sun exposure or in differences in vitamin D intake. The most likely explanation of the discrepancy, however, is probably that 25(OH)D determinations are not standardized well enough for international or interlaboratorial comparisons (50, 51).
Pre-vitamin D and vitamin D are photolabile (52). These compounds and some of their metabolites can be photodegraded or photochemically changed while they are in the skin, where solar radiation can reach them. Photolability may be the reason that sun-induced vitamin D intoxication has rarely or never been reported. Such intoxication was wrongly proposed to be the evolutionary reason for dark skin colors of humans living close to the equator (53). However, the photolability of pre-vitamin D and vitamin D is not likely to explain the lack of latitude gradients in 25(OH)D levels because the vitamin D generation is almost linear up to UV exposures as high as three or four minimum erythema doses (54). However, it should be noted that this reference concerns a narrow wavelength band of around 295 nm. In human skin radiation, around 295 nm can convert ≈65% of the 7-dehydrocholestrol to pre-vitamin D, whereas solar radiation can convert only ≈20% (43). In future investigations, one should take into account the increase in skin darkness of populations from north to south. Moreover, in assessing latitude variations of vitamin D levels, one should focus mainly on summer values or winter–summer differences. Doing so would minimize the role of different vitamin D intakes.
North–South Gradients of Cancer Incidence, Mortality, and Prognosis.
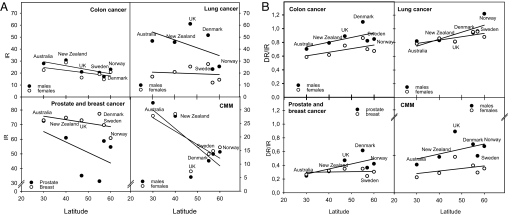
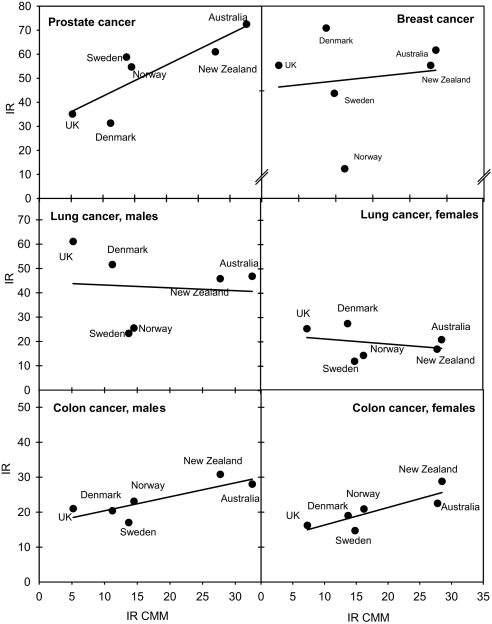
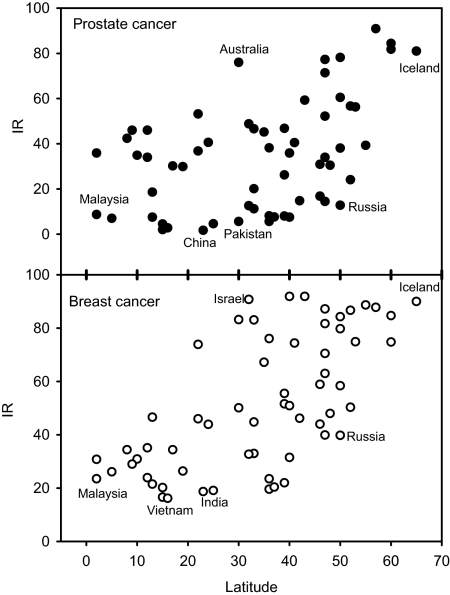
A number of investigations (6, 55, 56) indicate that, in some populations, the incidence and/or mortality of a number of cancers (prostate, breast, colon, etc.) increase with increasing latitude. However, in contrast to this theory, the similarity of cancer incidence and mortality rates in Australia/New Zealand and U.K./Scandinavia should be noticed (Fig. 2). For some cancers, there may even be a significant inverse latitude gradient (Fig. 2). Differences in sun exposure habits and skin types can probably not explain this observation because the inverse relationship remains when we use CMM incidence rates as a crude measure for real UV exposures (Fig. 3). However, taking more countries into consideration, we see that no reliable north–south gradient can be extracted (Fig. 4). There is a large variation of the incidence rates by factors of ≈50 and 5 for prostate cancer and breast cancer, respectively. Even for countries at the same latitude, large differences are found. From this epidemiological variation, we can conclude that genetical, dietary, and environmental factors, other than sun exposure, play major roles and may completely mask the effects of vitamin D.
Fig. 2.
Cancer incidence and death rates as a function of latitude. (A) Incidence rates of four cancer forms in different countries as functions of the mean latitude of the country. Only countries populated predominantly by individuals with skin types I and II are included: Australia, New Zealand, Sweden, Norway, Denmark, and U.K. cancer data represent averages for the period 1987–1997. The data are from Cancer Incidence in Five Continents (ref. 34; see www-dep.iarc.fr). (B) The ratios of death rates to incidence rates for the same countries as considered in A. Death rates are collected 2 years after incidence rates and represent averages for the period 1989–1999. Cancer mortality data are obtained from a WHO database (see www-dep.iarc.fr).
Fig. 3.
The incidence rates of prostate, breast, lung, and colon cancers as functions of the incidence rates of CMM. Data are from the same sources as those in Fig. 2.
Fig. 4.
Incidence rates of prostate and breast cancers in different countries as functions of the mean latitude of the country. Cancer data are obtained from Globocan 2002 (see www-dep.iarc.fr).
There might be a method to approach the problem from a different point of view, namely by looking at prognosis. According to our experience with the Norwegian epidemiological data, this method may be sensitive enough to study the connection between vitamin D and cancer. The ratio of death rate to incidence rate is a crude estimate of prognosis. In populations with white skin tone included in Fig. 2B, there is an increasing ratio of death rates to incidence rates with increasing latitude. This finding indicates improved prognosis with decreasing latitude (i.e., with increasing UV exposure). We find it unlikely that cancer treatment is better in Australia than in the U.K. Further, we conclude that the observations in Fig. 2B indicate, although weakly, the beneficial role of sun-induced vitamin D for cancer prognosis, in agreement with epidemiological findings (35–40).
The Rise of Incidence Rates of Skin Cancer and Internal Cancers.
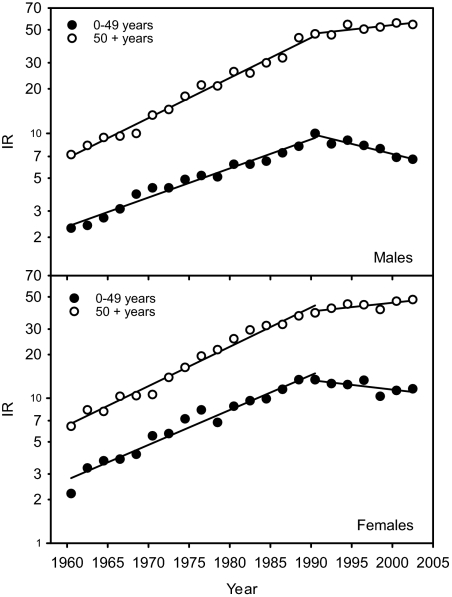
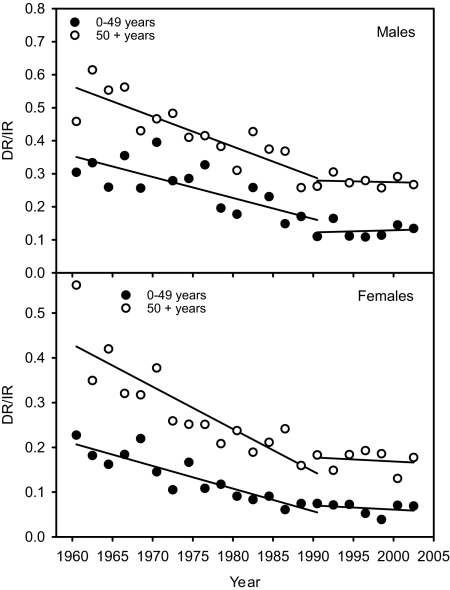
It is well known that the incidence rates of most cancers have been increasing with time over many decades. Until 1990, this fact also was universally true for all three major forms of skin cancer (see ref. 16 and www.kreftregisteret.no). This finding clearly indicates that, before 1990, the sun exposure of people was constantly increasing with time. One would then expect increasing vitamin D levels and decreasing rates of internal cancers, the opposite of what is found. However, in addition to vitamin D, a number of dietary and environmental factors need to be considered. In many countries, people certainly consume more and more fat and sugar (the vitamin D status worsens with increasing BMI) (57, 58), exercise less, and also may be more exposed to environmental carcinogens. It should be noted that, in the mentioned time period, melanoma rates increased much faster than those of internal cancers (16). However, changing diagnostic criteria and increased detection pressure may play roles. From 1990–2004, the increase in melanoma incidence stopped in several countries, notably in the young population (Australia, New Zealand, Canada, United States, and Norway) (59–62). In Norway, there is even a decreasing trend (Fig. 5). Also, the incidence rates of nonmelanoma skin cancer do not increase any longer in Australia, although this finding is less certain because of the lack of collection of these types of data (63). Hence, the “be aware of skin cancer” campaigns have had an impact. The improvement of the prognosis of melanoma that was evident over several decades (possibly because of earlier diagnosis) seemed to stop in 1990 in Norway (Fig. 6), while the incidence rates of melanoma flattened out (Fig. 5). Thus, improved melanoma prognosis may be related not only to earlier diagnosis, but also to increased sun exposure, which is in agreement with the findings of Berwick et al. (64). Whether the seeming lack of improvement of prognosis after 1990 (Fig. 5) is because of decreasing sun exposure remains to be evaluated in the future.
Fig. 5.
Incidence rates of CMM in Norway as a function of time. The rates are averaged over 2 years and shown for the period 1960–2003.
Fig. 6.
The ratios of death rates to incidence rates of CMM in Norway. The rates are averaged over 2 years for the period 1960–2003.
Conclusions
So far, epidemiological data for cancer argue for an overall positive role of sun-induced vitamin D. There may be more beneficial than adverse effects of moderately increased sun exposure, even for total cancer mortality (65). This message should be addressed to populations at risk for vitamin D deficiency. Trends need to be closely followed in the future. In view of the supposedly long latency times for cancer manifestation, decades are needed for final evaluation of the impacts of the antisun campaigns with respect to melanoma incidence, cancer prognosis, and other possible positive or adverse health effects. Authorities should pay attention not only to skin cancer research, but also to research on vitamin D–sun–health relationships occurring worldwide.
Materials and Methods
Data Sources.
Age-adjusted incidence and death rates from six countries populated by whites were obtained from the International Association for Research on Cancer database (see www-dep.iarc.fr). Incidence data are collected by cancer registries worldwide, whereas mortality data are extracted from the World Health Organization (WHO) databank (see www-dep.iarc.fr). The data are presented as averages for the period 1987–1997.
The age-adjusted (world standard population) incidence rates of CMM for Norway are obtained from The Norwegian Cancer Registry (see www.kreftregisteret.no) and are presented as 2-year averages for the period 1960–2003. Mortality data are retrieved from the WHO mortality database (see www-dep.iarc.fr) and are presented as 2-year averages for the period 1960–2003.
Data on seasonal variation of 25(OH)D were collected from a number of investigations done in healthy individuals ages 30–50 years.
Cancer data were plotted against latitude or the age-adjusted incidence rates of CMM as a measure of the UV exposure achieved. Simple linear regression Sigma Plot 10 (Systat) was used to investigate the relationship.
Vitamin D Photosynthesis.
We calculated the annual fluence of vitamin D-generating solar radiation as a function of latitude by using the action spectrum for generation of pre-vitamin D in human skin (43) by applying a radiation transfer model (66, 67). Global solar exposure (direct plus diffuse exposure) was determined, approximating the human body by a horizontal cylinder, excluding top and bottom. Total ozone columns measured by the TOMS satellite instruments were used in the calculations. The daily average cloud cover for each site was derived from measured reflectivities at an ozone-insensitive channel of the same instrument. Further details of the calculations can be found elsewhere (68, 69).
ACKNOWLEDGMENTS.
This work was supported by Sigval Bergesen D.Y. og hustru Nankis Foundation, The Research Foundation of the Norwegian Radiumhospital, and Helse-Sør Norway. Brookhaven National Laboratory is operated by Brookhaven Associates, under contract with the U.S. Department of Energy.
Footnotes
The authors declare no conflict of interest.
References
- 1.Diffey B. Do we need a revised public health policy on sun exposure? Br J Dermatol. 2006;154:1046–1051. doi: 10.1111/j.1365-2133.2006.07268.x. [DOI] [PubMed] [Google Scholar]
- 2.Gillie O. A new government policy is needed for sunlight and vitamin D. Br J Dermatol. 2006;154:1052–1061. doi: 10.1111/j.1365-2133.2006.07261.x. [DOI] [PubMed] [Google Scholar]
- 3.Garbe C, Eigentler TK. Diagnosis and treatment of cutaneous melanoma: State of the art 2006. Melanoma Res. 2007;17:117–127. doi: 10.1097/CMR.0b013e328042bb36. [DOI] [PubMed] [Google Scholar]
- 4.Cummins DL, et al. Cutaneous malignant melanoma. Mayo Clin Proc. 2006;81:500–507. doi: 10.4065/81.4.500. [DOI] [PubMed] [Google Scholar]
- 5.Bouillon R, et al. Vitamin D, cancer. J Steroid Biochem Mol Biol. 2006;102:156–162. doi: 10.1016/j.jsbmb.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 6.Giovannucci E. The epidemiology of vitamin D, cancer incidence and mortality: A review (United States). Cancer Causes Control. 2005;16:83–95. doi: 10.1007/s10552-004-1661-4. [DOI] [PubMed] [Google Scholar]
- 7.Holick MF. Vitamin D: Importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79:362–371. doi: 10.1093/ajcn/79.3.362. [DOI] [PubMed] [Google Scholar]
- 8.Jablonski NG, Chaplin G. The evolution of human skin coloration. J Hum Evol. 2000;39:57–106. doi: 10.1006/jhev.2000.0403. [DOI] [PubMed] [Google Scholar]
- 9.Jablonski NG. A possible link between neural tube defects and ultraviolet light exposure. Med Hypotheses. 1999;52:581–582. doi: 10.1054/mehy.1997.0697. [DOI] [PubMed] [Google Scholar]
- 10.Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet. 1982;1:74–76. doi: 10.1016/s0140-6736(82)90214-8. [DOI] [PubMed] [Google Scholar]
- 11.Chen TC, et al. Factors that influence the cutaneous synthesis and dietary sources of vitamin D. Arch Biochem Biophys. 2007;460:213–217. doi: 10.1016/j.abb.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moan J, Porojnicu AC, Dahlback A. In: Skin Cancer Prevention. Ringborg U, Brandberg Y, Breitbart EW, Greinert R, editors. New York: Informa Healthcare; 2006. pp. 179–201. [Google Scholar]
- 13.Moan J, Porojnicu AC, Dahlback A. In: Sunlight, Vitamin D, Skin Cancer. Reichrath J, editor. Austin, TX: Landes Bioscience; 2007. [Google Scholar]
- 14.Beral V, Robinson N. The relationship of malignant melanoma, basal and squamous skin cancers to indoor and outdoor work. Br J Cancer. 1981;44:886–891. doi: 10.1038/bjc.1981.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elwood JM, Jopson J. Melanoma and sun exposure: An overview of published studies. Int J Cancer. 1997;73:198–203. doi: 10.1002/(sici)1097-0215(19971009)73:2<198::aid-ijc6>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 16.Moan J, Dahlback A. In: Environmental UV Photobiology. Bjørn LO, Moan J, Nultsch W, Young AR, editors. New York: Plenum; 1993. pp. 255–192. [Google Scholar]
- 17.Green A, et al. A case-control study of melanomas of the soles and palms (Australia and Scotland). Cancer Causes Control. 1999;10:21–25. doi: 10.1023/a:1008872014889. [DOI] [PubMed] [Google Scholar]
- 18.Yu GP, Hu DN, McCormick SA. Latitude and incidence of ocular melanoma. Photochem Photobiol. 2006;82:1621–1626. doi: 10.1562/2006-07-17-RA-970. [DOI] [PubMed] [Google Scholar]
- 19.Shah CP, et al. Intermittent and chronic ultraviolet light exposure and uveal melanoma: A meta-analysis. Ophthalmology. 2005;112:1599–1607. doi: 10.1016/j.ophtha.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 20.Lea CS, et al. Ambient UVB, melanoma risk in the United States: A case-control analysis. Ann Epidemiol. 2007;17:447–453. doi: 10.1016/j.annepidem.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 21.Ono Y, et al. Seasonal changes of serum 25-hydroxyvitamin D, intact parathyroid hormone levels in a normal Japanese population. J Bone Miner Metab. 2005;23:147–151. doi: 10.1007/s00774-004-0553-8. [DOI] [PubMed] [Google Scholar]
- 22.Carnevale V, et al. Longitudinal evaluation of vitamin D status in healthy subjects from southern Italy: Seasonal and gender differences. Osteoporos Int. 2001;12:1026–1030. doi: 10.1007/s001980170012. [DOI] [PubMed] [Google Scholar]
- 23.Bischof MG, Heinze G, Vierhapper H. Vitamin D status and its relation to age and body mass index. Horm Res. 2006;66:211–215. doi: 10.1159/000094932. [DOI] [PubMed] [Google Scholar]
- 24.Bouillon RA, Auwerx JH, Lissens WD, Pelemans WK. Vitamin D status in the elderly: Seasonal substrate deficiency causes 1,25-dihydroxycholecalciferol deficiency. Am J Clin Nutr. 1987;45:755–763. doi: 10.1093/ajcn/45.4.755. [DOI] [PubMed] [Google Scholar]
- 25.Finch PJ, et al. Blunted seasonal variation in serum 25-hydroxy vitamin D, increased risk of osteomalacia in vegetarian London Asians. Eur J Clin Nutr. 1992;46:509–515. [PubMed] [Google Scholar]
- 26.Rejnmark L, et al. Vitamin D insufficiency in Greenlanders on a westernized fare: Ethnic differences in calcitropic hormones between Greenlanders and Danes. Calcif Tissue Int. 2004;74:255–263. doi: 10.1007/s00223-003-0110-9. [DOI] [PubMed] [Google Scholar]
- 27.Hine TJ, Roberts NB. Seasonal variation in serum 25-hydroxy vitamin D3 does not affect 1,25-dihydroxy vitamin D. Ann Clin Biochem. 1994;31:31–34. doi: 10.1177/000456329403100105. [DOI] [PubMed] [Google Scholar]
- 28.Savolainen K, Maenpaa PH, Alhava EM, Kettunen K. A seasonal difference in serum 25-hydroxyvitamin D3 in a Finnish population. Med Biol. 1980;58:49–52. [PubMed] [Google Scholar]
- 29.Lamberg-Allardt C. Vitamin D intake, sunlight exposure and 25-hydroxyvitamin D levels in the elderly during one year. Ann Nutr Metab. 1984;28:144–150. doi: 10.1159/000176796. [DOI] [PubMed] [Google Scholar]
- 30.Vik T, Try K, Stromme JH. The vitamin D status of man at 70 degrees north. Scand J Clin Lab Invest. 1980;40:227–232. doi: 10.3109/00365518009095571. [DOI] [PubMed] [Google Scholar]
- 31.Brustad M, et al. Vitamin D status of middle-aged women at 65–71 degrees N in relation to dietary intake and exposure to ultraviolet radiation. Public Health Nutr. 2004;7:327–335. doi: 10.1079/PHN2003536. [DOI] [PubMed] [Google Scholar]
- 32.Johansson L, Solvoll K. National survey for nutrition and physical activity. 1999:45. [Google Scholar]
- 33.Moan J, Lagunovaz, Porojnicu AC. Proceedings of the meeting “Sunlight Vitamin D, Health”; London: House of Commons; 2006. pp. 33–40. [Google Scholar]
- 34.Curado MP, et al., editors. Cancer Incidence in Five Continents. Vol 9. Lyon, France: Int Agency Res Cancer; 2007. [Google Scholar]
- 35.Robsahm TE, Tretli S, Dahlback A, Moan J. Vitamin D3 from sunlight may improve the prognosis of breast-, colon- and prostate cancer (Norway). Cancer Causes Control. 2004;15:149–158. doi: 10.1023/B:CACO.0000019494.34403.09. [DOI] [PubMed] [Google Scholar]
- 36.Lagunova Z, et al. Prostate cancer survival is dependent on season of diagnosis. Prostate. 2007;67:1362–1370. doi: 10.1002/pros.20577. [DOI] [PubMed] [Google Scholar]
- 37.Moan J, et al. Solar radiation, vitamin D, survival rate of colon cancer in Norway. J Photochem Photobiol B. 2005;78:189–193. doi: 10.1016/j.jphotobiol.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 38.Porojnicu AC, Robsahm TE, Hansen Ree A, Moan J. Season of diagnosis is a prognostic factor in Hodgkin lymphoma. A possible role of sun-induced vitamin D. Br J Cancer. 2005;93:571–574. doi: 10.1038/sj.bjc.6602722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Porojnicu AC, et al. Seasonal and geographical variations in lung cancer prognosis in Norway. Does vitamin D from the sun play a role? Lung Cancer. 2007;55:263–270. doi: 10.1016/j.lungcan.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 40.Porojnicu AC, et al. Changes in risk of death from breast cancer with season and latitude: Sun exposure and breast cancer survival in Norway. Breast Cancer Res Treat. 2007;102:323–328. doi: 10.1007/s10549-006-9331-8. [DOI] [PubMed] [Google Scholar]
- 41.Lim HS, et al. Cancer survival is dependent on season of diagnosis and sunlight exposure. Int J Cancer. 2006;119:1530–1536. doi: 10.1002/ijc.22052. [DOI] [PubMed] [Google Scholar]
- 42.Zhou W, et al. Vitamin D is associated with improved survival in early-stage non-small cell lung cancer patients. Cancer Epidemiol Biomarkers Prev. 2005;14:2303–2309. doi: 10.1158/1055-9965.EPI-05-0335. [DOI] [PubMed] [Google Scholar]
- 43.MacLaughlin JA, Anderson RR, Holick MF. Spectral character of sunlight modulates photosynthesis of previtamin D3 and its photoisomers in human skin. Science. 1982;216:1001–1003. doi: 10.1126/science.6281884. [DOI] [PubMed] [Google Scholar]
- 44.Zittermann A, Schleithoff SS, Koerfer R. Putting cardiovascular disease and vitamin D insufficiency into perspective. Br J Nutr. 2005;94:483–492. doi: 10.1079/bjn20051544. [DOI] [PubMed] [Google Scholar]
- 45.Lips P, et al. A global study of vitamin D status and parathyroid function in postmenopausal women with osteoporosis: Baseline data from the multiple outcomes of raloxifene evaluation clinical trial. J Clin Endocrinol Metab. 2001;86:1212–1221. doi: 10.1210/jcem.86.3.7327. [DOI] [PubMed] [Google Scholar]
- 46.Levis S, et al. Vitamin d deficiency and seasonal variation in an adult South Florida population. J Clin Endocrinol Metab. 2005;90:1557–1562. doi: 10.1210/jc.2004-0746. [DOI] [PubMed] [Google Scholar]
- 47.Pasco JA, et al. Seasonal periodicity of serum vitamin D, parathyroid hormone, bone resorption, and fractures: The Geelong Osteoporosis Study. J Bone Miner Res. 2004;19:752–758. doi: 10.1359/JBMR.040125. [DOI] [PubMed] [Google Scholar]
- 48.Beadle PC, Burton JL, Leach JF. Correlation of seasonal variation of 25-hydroxycalciferol with UV radiation dose. Br J Dermatol. 1980;103:289–293. doi: 10.1111/j.1365-2133.1980.tb07246.x. [DOI] [PubMed] [Google Scholar]
- 49.de Gruijl FR, et al. Wavelength dependence of skin cancer induction by ultraviolet irradiation of albino hairless mice. Cancer Res. 1993;53:53–60. [PubMed] [Google Scholar]
- 50.Binkley N, et al. Assay variation confounds the diagnosis of hypovitaminosis D: A call for standardization. J Clin Endocrinol Metab. 2004;89:3152–3157. doi: 10.1210/jc.2003-031979. [DOI] [PubMed] [Google Scholar]
- 51.Hollis BW. Editorial: The determination of circulating 25-hydroxyvitamin D: No easy task. J Clin Endocrinol Metab. 2004;89:3149–3151. doi: 10.1210/jc.2004-0682. [DOI] [PubMed] [Google Scholar]
- 52.Holick MF. Vitamin D: Photobiology, metabolism, and clinical application. In: Arias IM, Boyer JL, Fausto N, Jakoby WB, Schachter D, Shafritz DA, editors. The Liver: Biology and Pathobiology. 3rd Ed. New York: Raven; 1994. pp. 543–562. [Google Scholar]
- 53.Loomis WF. Skin-pigment regulation of vitamin-D biosynthesis in man. Science. 1967;157:501–506. doi: 10.1126/science.157.3788.501. [DOI] [PubMed] [Google Scholar]
- 54.Adams JS, Clemens TL, Parrish JA, Holick MF. Vitamin-D synthesis and metabolism after ultraviolet irradiation of normal and vitamin-D-deficient subjects. N Engl J Med. 1982;306:722–725. doi: 10.1056/NEJM198203253061206. [DOI] [PubMed] [Google Scholar]
- 55.Garland CF, et al. The role of vitamin D in cancer prevention. Am J Public Health. 2006;96:252–261. doi: 10.2105/AJPH.2004.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schwartz GG, Skinner HG. Vitamin D status and cancer: New insights. Curr Opin Clin Nutr Metab Care. 2007;10:6–11. doi: 10.1097/MCO.0b013e328011aa60. [DOI] [PubMed] [Google Scholar]
- 57.Wortsman J, et al. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 58.Ybarra J, Sanchez-Hernandez J, Perez A. Hypovitaminosis D, morbid obesity. Nurs Clin North Am. 2007;42:19–27. doi: 10.1016/j.cnur.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 59.Coory M, et al. Trends for in situ and invasive melanoma in Queensland, Australia, 1982–2002. Cancer Causes Control. 2006;17:21–27. doi: 10.1007/s10552-005-3637-4. [DOI] [PubMed] [Google Scholar]
- 60.Marks R. The changing incidence and mortality of melanoma in Australia. Recent Results Cancer Res. 2002;160:113–121. doi: 10.1007/978-3-642-59410-6_15. [DOI] [PubMed] [Google Scholar]
- 61.Marrett LD, Nguyen HL, Armstrong BK. Trends in the incidence of cutaneous malignant melanoma in New South Wales, 1983–1996. Int J Cancer. 2001;92:457–462. doi: 10.1002/ijc.1203. [DOI] [PubMed] [Google Scholar]
- 62.Pearce J, Barnett R, Kingham S. Slip! Slap! Slop! Cutaneous malignant melanoma incidence and social status in New Zealand, 1995–2000. Health Place. 2006;12:239–252. doi: 10.1016/j.healthplace.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 63.Staples MP, et al. Non-melanoma skin cancer in Australia: The 2002 national survey and trends since 1985. Med J Aust. 2006;184:6–10. doi: 10.5694/j.1326-5377.2006.tb00086.x. [DOI] [PubMed] [Google Scholar]
- 64.Berwick M, et al. Sun exposure and mortality from melanoma. J Natl Cancer Inst. 2005;97:195–199. doi: 10.1093/jnci/dji019. [DOI] [PubMed] [Google Scholar]
- 65.Giovannucci E, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98:451–459. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- 66.Dahlback A, Stamnes K. A new spherical model for computing the radiation field available for photolysis and heating rate at twilight. Planet Space Sci. 1991;39:671–683. [Google Scholar]
- 67.Stamnes K, Tsay SC, Wiscombe W, Jayaweera K. Numerically stable algorithm for discrete-ordinate-method for radiative transfer in multiple scattering and emitting layered media. Appl Opt. 1988:2502–2509. doi: 10.1364/AO.27.002502. [DOI] [PubMed] [Google Scholar]
- 68.Moan J, Dahlback A, Henriksen T, Magnus K. Biological amplification factor for sunlight-induced nonmelanoma skin cancer at high latitudes. Cancer Res. 1989;49:5207–5212. [PubMed] [Google Scholar]
- 69.Moan J, Dahlback A. The relationship between skin cancers, solar radiation and ozone depletion. Br J Cancer. 1992;65:916–921. doi: 10.1038/bjc.1992.192. [DOI] [PMC free article] [PubMed] [Google Scholar]