Abstract
A major question regarding the sensitivity of solid tumors to targeted kinase inhibitors is why some tumors respond and others do not. The observation that many tumors express EGF receptor (EGFR), yet only a small subset with EGFR-activating mutations respond clinically to EGFR inhibitors (EGFRIs), suggests that responsive tumors uniquely depend on EGFR signaling for their survival. The nature of this dependence is not understood. Here, we investigate dependence on EGFR signaling by comparing non-small-cell lung cancer cell lines driven by EGFR-activating mutations and genomic amplifications using a global proteomic analysis of phospho-tyrosine signaling. We identify an extensive receptor tyrosine kinase signaling network established in cells expressing mutated and activated EGFR or expressing amplified c-Met. We show that in drug sensitive cells the targeted tyrosine kinase drives other RTKs and an extensive network of downstream signaling that collapse with drug treatment. Comparison of the signaling networks in EGFR and c-Met-dependent cells identify a “core network” of ≈50 proteins that participate in pathways mediating drug response.
Keywords: EGF receptor, c-Met, oncogene dependence, proteomics, stable isotope labeling with amino acids in cell culture
Selective tyrosine kinase inhibitors have shown promise in treating cancers driven by activated tyrosine kinases such as Bcr-Abl in chronic myelogenous leukemia (CML), c-Kit in gastrointestinal stromal tumors (GIST), and EGF receptor (EGFR) in non-small-cell lung cancer (NSCLC). Gefitinib (Iressa) and erlotinib (Tarceva) are selective inhibitors of the EGFR receptor tyrosine kinase currently used to treat NSCLC patients. Although most NSCLC patients express EGFR in their tumors, only a subfraction of patients with tumors dependent on EGFR for growth and survival respond clinically to EGFR inhibitors (EGFRIs). These patients appear to contain EGFR-activating mutations or have undergone amplification of EGFR gene copy number (1–3). Similar to EGFR and NSCLC, a subfraction of gastric cancers are known to exhibit amplification at the c-Met locus. Recently, it has been shown that gastric cancer cell lines exhibiting c-Met amplification are sensitive to the c-Met inhibitor PHA-665752, suggesting that patients with c-Met-amplified tumors may respond to selective c-Met inhibitors (4).
Why activating mutations in EGFR or amplification of c-Met predict response to targeted kinase inhibitors is not well understood (5). For EGFR, increased affinity of mutant receptor for drug (6, 7) and altered signaling from mutant EGFR have been observed (8), and the production of positive and negative signals that differ in time course of activity have been proposed (9). The dependence of cancer cells on oncogenic tyrosine kinases such as activated Bcr-Abl, EGFR, or c-Met has been proposed to result from adaptations in cell circuitry that create dependence on the activated tyrosine kinase (10). Because oncogenic tyrosine kinases sit at the top of many different signaling pathways, the key drug-induced changes that confer sensitivity and dependence have been difficult to identify and remain largely unknown.
In this study, we characterize phosphotyrosine signaling downstream of the EGFR receptor in EGFRI-sensitive and -resistant NSCLC cell lines and downstream of c-Met in the gastric cancer cell line MKN45. We use a global phosphoproteomic method to identify hundreds of downstream targets of the activated tyrosine kinases. Although other studies have investigated signaling downstream of EGFR (11–13), our study focuses on signaling in EGFRI-sensitive cell lines. We identify large signaling networks present in sensitive cell lines, and show that the oncogenic kinase drives multiple receptor tyrosine kinases and a large downstream network that collapse upon drug treatment.
Results
Profiling Phosphotyrosine Signaling in EGFRI-Sensitive and -Resistant NSCLC Cell Lines.
To understand signaling differences underlying drug sensitivity, we measured the sensitivity of seven NSCLC cell lines with different EGFR and KRAS mutational status (see Table 1) to the EGFR inhibitor gefitinib. Growth inhibition assays [supporting information (SI) Fig. 5A] show that two EGFR mutant cell lines, H3255 and HCC827, are sensitive to gefitinib, with IC50 <0.1 μM, and two other EGFR mutant cell lines, H1650 and H1975, are resistant to gefitinib. The KRAS mutant cell lines H358 and H1734 are also resistant to gefitinib, with IC50 >5 μM. The EGFR WT and BRAF mutant (G466V) cell line H1666 was resistant to gefitinib, with an IC50 value close to 4 μM.
Table 1.
Summary of phospho-tyrosine profiling of 7 NSCLC cell lines
| Cell line | Mutation status of EGFR, KRAS | Gefitinib sensitivity (IC50, μM) | Phospho-peptides | Nonredundant phospho-proteins | Nonredundant phospho-peptides | Nonredundant phospho-sites |
|---|---|---|---|---|---|---|
| H358 | KRAS: G12V | ≈10 | 222 | 72 | 89 | 84 |
| H1650 | EGFR: E746-A750del | >10 | 321 | 180 | 251 | 220 |
| H1666 | EGFR: wt; KRAS: wt | ≈4 | 374 | 219 | 303 | 278 |
| H1734 | KRAS: G13C | >10 | 317 | 201 | 270 | 249 |
| H1975 | EGFR: L858R, T790M | >10 | 371 | 202 | 302 | 275 |
| HCC827 | EGFR: E746_A750del | <0.1 | 762 | 369 | 575 | 508 |
| H3255 | EGFR: L858R | <0.1 | 754 | 373 | 547 | 509 |
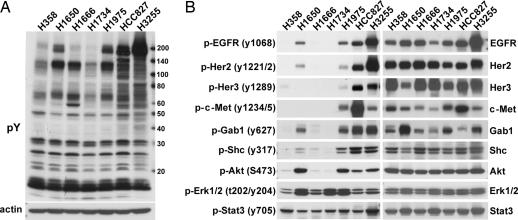
We next profiled tyrosine phosphorylation in the seven NSCLC cell lines using PhosphoScan, a recently established phospho-proteomics method (14). The total number of tyrosine phosphorylated peptides observed as well as the number of different phosphosites and different phosphoproteins identified are shown in Table 1. More phosphoproteins were found in the gefitinib-sensitive and EGFR mutant cell lines. Western blot analysis with phospho-tyrosine antibody (Fig. 1A) confirms that the EGFR mutant cell lines HCC827 and H3255 have stronger p-Tyr intensity, supporting the PhosphoScan results.
Fig. 1.
Comparison of phosphotyrosine signaling among EGFRI-sensitive and -resistant cell lines. (A) Western blot analysis of phospho-tyrosine (pY) signaling in serum-starved NSCLC cell lines. (B) Western blot confirmation of specific signaling molecules and selected phosphorylation sites identified by PhosphoScan experiments.
Signaling Associated with EGFR Dependence.
We used a semiquantitative spectral counting approach (15) summing all phosphotyrosine-containing peptides observed for a given protein and comparing total protein phosphorylation between different NSCLC cell lines. SI Table 3 shows a selected set of differentially tyrosine phosphorylated proteins, and the complete phospho-peptide information is shown in SI Table 4. H3255 and HCC827 cells show higher levels of tyrosine phosphorylation on many proteins than two other EGFR mutant cell lines, H1975 and H1650 (Table 1 and confirmed in Fig. 1A), possibly because of EGFR gene amplification. EGFR tyrosine phosphorylation is higher in cell lines with EGFR kinase domain-activating mutations (SI Table 3 and confirmed in Fig. 1B) and several sites on EGFR appear to be phosphorylated only in mutant cell lines (SI Fig. 5B). Two KRAS mutant cell lines H358 and H1734 show lower-level EGFR phosphorylation (Fig. 1B and SI Table 3), suggesting mutually exclusive activation of EGFR and KRAS (16, 17). EGFR family members Her2 and Her3 as well as c-Met showed higher levels of tyrosine phosphorylation in gefitinib-sensitive EGFR mutant cell lines HCC827 and H3255 (SI Table 3) that was confirmed by Western blot analysis (Fig. 1B).
Adaptor proteins differentially phosphorylated include Her3, Gab1, and Shc1, important transmitters of growth and survival signals downstream of EGFR (18). Western blot analysis using antibodies to specific tyrosine phosphorylated sites confirm that EGFR, Her3, Gab1, Shc1, and Akt are all highly phosphorylated only in the four EGFR mutant cell lines (Fig. 1B). In agreement with MS results (SI Table 3) phosphorylated Erk1/2 is present in all seven cell lines (Fig. 1B).
The effects of gefitinib at three different concentrations on EGFR, Akt, and Erk phosphorylation in each of the NSCLC cell lines (SI Fig. 6A) confirmed previously reported sensitivities for HCC827 and H3255 and showed that, in H1650 cells, gefitinib blocked Erk phosphorylation but not Akt phosphorylation, suggesting additional inputs to the Akt pathway, such as possible inactivation of PTEN. PTEN status was determined in the seven NSCLC cell lines by Western blot analysis, and H1650 was found to be PTEN null (SI Fig. 6B). This is consistent with observations from glioblastoma patients, where sensitivity to EGFRIs require functional PTEN (19). Interestingly, RAS mutant cell lines H358 and H1734 show decreased sensitivity of p-ERK to gefitinib, especially in H1734 cells, suggesting that ERK is driven by deregulated RAS in these cells (SI Fig. 6A).
In addition to the well described signaling of EGFR to Akt and Erk, many other proteins were phosphorylated on tyrosine in mutant EGFR cell lines. Highly phosphorylated proteins include adhesion and scaffolding molecules and cytoskeletal proteins such as CTNND1, plakophilin 2, 3, 4, DCBLD2, Erbin, and occludin (SI Table 3). Another class of proteins highly phosphorylated in mutant EGFR cell lines are negative regulators of EGFR including Spry1, 2, 4, and Mig6. We observed Spry1 (Y53), Spry2 (Y55), and Spry4 (Y75) in H3255 and HCC827 cells but not other cell lines (SI Table 3). Mig6 has been reported to be a negative regulator of EGFR and may function as a tumor suppressor (20). Mig6 is tyrosine phosphorylated at Y394 in mutant EGFR cell lines (SI Table 3 and SI Fig. 5C), but not in H1666 or H358. Many proteins not previously known to be involved in EGFR signaling are also differentially phosphorylated between EGFR mutant and WT cell lines. Examples are: serine/threonine kinases MINK and NRK, cell cycle regulators septin 2/7/9, G protein regulators ARHGAP5, ARHGEF5, Rab7, RAB34, and lipid-binding proteins PLEKHA5, PLEKHA6, and the cell-surface proteins LDLR and cx43.
Effects of EGFR Inhibitors on Downstream Signaling.
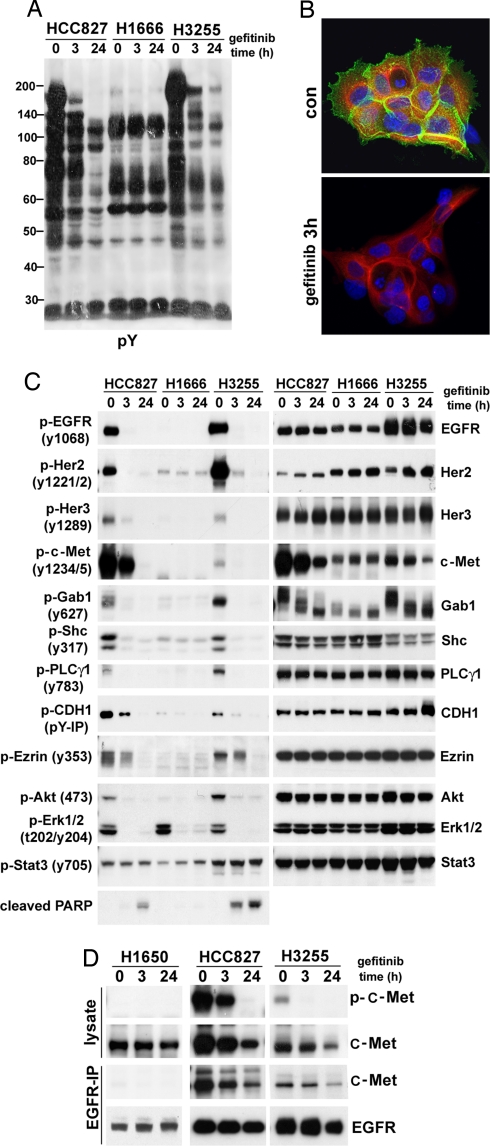
To quantitatively measure changes induced by the EGFR inhibitor gefitinib, we merged our immunoaffinity method (PhosphoScan) with quantitative MS method SILAC (stable isotope labeling with amino acids in cell culture) (21). We measured the effects of gefitinib on hundreds of different tyrosine phosphorylation sites after 3 and 24 h of drug treatment in two gefitinib-sensitive EGFR mutant cell lines, HCC827 and H3255. Both cell lines gave similar results, and the results for H3255 (Table 2 and SI Tables 5 and 6) show that tyrosine phosphorylation of many proteins decrease dramatically at 3 and 24 h after Iresssa treatment. After 3 h of gefitinib treatment, we identified 110 sites that were reduced by at least 2.5-fold, and 84 sites that were reduced by at least 5-fold (Table 2). Western blot analysis and immunofluorescence using a phospho-tyrosine antibody supports these findings (Fig. 2 A and B). The majority of tyrosine phosphorylation is found at cell membrane and cell–cell junctions and is blocked by drug treatment in HCC827 and H3255 cell lines.
Table 2.
Summary of the PhosphoScan-SILAC results for H3255 treated with gefitinib and for MKN45 treated with Su11274
| H3255 (gefitinib) |
MKN45 (Su11274) |
|||||
|---|---|---|---|---|---|---|
| 3-h and 24-h combined | 3-h | 24-h | 3-h and 24-h combined | 3-h | 24-h | |
| p-Peptides | 393 | 308 | 337 | 1181 | 976 | 903 |
| p-Proteins | 185 | 160 | 167 | 535 | 469 | 447 |
| p-Sites | 301 | 245 | 267 | 945 | 793 | 756 |
| Sites affected >2.5-fold | 110 | 161 | 586 | 603 | ||
| Sites affected >5-fold | 84 | 133 | 423 | 462 | ||
Fig. 2.
Western blot analysis confirms PhosphoScan-SILAC results. Cells were serum-starved overnight and then treated with 1 μM gefitinib for 3 or 24 h. (A) Phospho-tyrosine Western blot analysis of HCC827, H1666, and H3255 cells treated with gefitinib. (B) Immunofluorescence staining using a phosphotyrosine antibody shows membrane and cytoskeleton staining in H3255 cells that is reduced by 3-h gefitinib (1 μM) treatment. Green shows p-Tyr antibody staining, red shows β-tubulin antibody staining, and blue shows staining of nucleus. (C) The effect of gefitinib (1 μM) on selected phosphorylation sites and protein levels in HCC827, H1666, and H3255 cells. (D) Coimmunoprecipitation reveals association of Met and EGFR in gefitinib-sensitive cell lines HCC827 and H3255.
We immediately noticed that the set of proteins showing higher phosphorylation in mutant EGFR cell lines was inhibited by gefitinib treatment. Examples are shown in SI Table 5. As expected, EGFR phosphorylation decreased dramatically (5- to 200-fold) after gefitinib treatment. The adaptor proteins Her3, Gab1, and Shc1 all showed decreased phosphorylation by gefitinib, as did adhesion, scaffolding, and cytoskeletal proteins such as CTNND1, plakophillins, claudin, and desmoplakin 3. Fig. 2C shows Western blot confirmation of phosphorylation change for some sites in HCC827, H1666, and H3255 cell lines. Most sites differentially phosphorylated between mutant and WT cell lines responded to gefitinib treatment, whereas shared sites such as Stat3 (Y705) in general did not respond.
SILAC experiments also identified decreased phosphorylation on many proteins not previously associated with the EGFR signaling pathway. For example, the MAGUK family proteins DLG3, SAP97, and MPP7 all showed dramatic dephosphorylation upon drug treatment. These proteins cluster active NMDA receptors together at the neuronal synapse and play organizational roles in the assembly of membrane specializations such as tight junctions (22, 23) and could conceivably serve to cluster activated receptor tyrosine kinase and other activated signaling molecules. Other proteins effected by drug treatment include the protease CPD, the Ser/Thr kinase MINK, surface protein CD46, LDLR, transcription regulator calgizzarin, EBNA-2 coactivator, and other proteins of unknown function such as LISCH, LISCH7, CRIP2, and LMBRD2.
Inhibiting EGFR with gefitinib also inhibited phosphorylation of Her2, Her3, and c-Met. These results were confirmed by Western blot analysis (Fig. 2C). Interestingly, c-Met phosphorylation was inhibited only in gefitinib-sensitive mutant EGFR cell lines. In H1666 cells, Met is not phosphorylated and is not down-regulated by gefitinib treatment (Fig. 2C). Furthermore, c-Met associates with EGFR in HCC827 and H3255, and the association is decreased by gefitinib treatment (Fig. 2D), possibly because of the reduced level of c-Met after gefitinib treatment. Control IgG did not pull down either protein in coimmunoprecipitation experiment (SI Fig. 6C). In the gefitinib-resistant and EGFR mutant cell line H1650, c-Met is not associated with EGFR and is not phosphorylated (Fig. 2D). Several other RTKs including EphA2, EphB4, and Tyro3, also showed decreased phosphorylation at tyrosine sites after 24 h of gefitinib treatment (SI Table 6). Surprisingly, although we observed phosphorylation of many nonreceptor tyrosine kinases including Src, FAK, Lyn, and Pyk2, their phosphorylation was not decreased by drug treatment (SI Fig. 7 and Table 6), suggesting they are not drug sensitive. Although Stat3 acts downstream of EGFR (13), as measured by PhosphoScan-SILAC, Stat3 phophorylation at Y705 did not change upon gefitinib treatment in both HCC827 and H3255 cells (SI Table 6) and was confirmed by Western blot analysis (Fig. 2C).
Signaling Networks Downstream of c-Met.
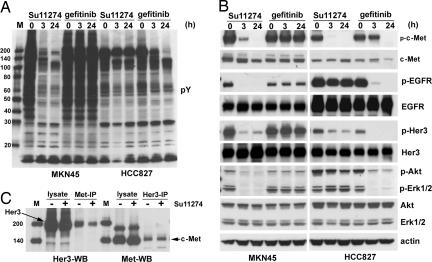
To explore whether similar changes occur in other RTK-dependent cell lines, we investigated a c-Met-driven gastric cancer cell line, MKN45, where genomic amplification of c-Met confers sensitivity to the c-Met inhibitor PHA-665752 (4). We profiled MKN45 cells using phosphotyrosine PhosphoScan and identified 516 nonredundant phosphosites from 355 phosphoproteins (SI Table 7). Many proteins phosphorylated in HCC827 and H3255 cells were also phosphorylated in MKN45 cells, including EGFR, Her2, Her3, etc. PhosphoScan (SI Tables 4 and 7) and Western blot analysis (Fig. 3 A and B) show that MKN45 cells, like HCC827 cells, express both c-Met and EGFR but respond differently to EGFR and c-Met inhibitors as assessed by cell growth inhibition assay (SI Fig. 8). MKN45 cells respond dramatically to the c-Met inhibitor Su11274 (24) but not to gefitinib, whereas the EGFR mutant cell line HCC827 responds to gefitinib but not to Su11274.
Fig. 3.
The sensitivity of MKN45 cells to tyrosine kinase inhibitors correlates with extensive changes in tyrosine phosphorylation. MKN45 and HCC827 cells were serum-starved overnight, treated with 1 μM Su11274 or 1 μM gefitinib for 3 or 24 h, respectively. (A) Phosphotyrosine Western blot analysis of MKN45 and HCC827 cells treated with Su11274 and gefitinib. (B) The effects of Su11274 and gefitinib on selected phosphorylation sites and proteins in MK45 cells (Left) and HCC827 cells (Right). (C) Coimmunoprecipitation and Western blot analysis of c-Met and Her3 association in MKN45 cells. M indicates the protein marker lane.
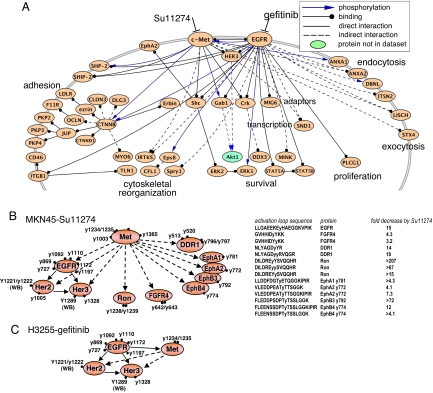
We next performed PhosphoScan-SILAC experiments to characterize tyrosine phosphorylation in Su11274-treated MKN45 cells (SI Table 8). In agreement with Western blot analysis (Fig. 3A), the majority of identified phosphotyrosine sites decreased dramatically with Su11274 treatment (Table 2 and SI Tables 5 and 8). Of 793 tyrosine phosphorylation sites identified at 3 h of Su11274 treatment, 423 sites decreased >5-fold. The majority of sites that changed with gefitinib treatment in H3255 cells also changed with Su11274 treatment of MKN45 cells, revealing networks that overlap extensively in the areas of adhesion and cytoskeletal reorganization, vesicle trafficking, and survival and proliferation (Fig. 4A and SI Figs. 9 and 10).
Fig. 4.
Regulatory networks sensitive to tyrosine kinase inhibitors in H3255 and MKN45 cells revealed by PhosphpScan-SILAC study. (A) Core signaling molecules inhibited by both gefitinib in H3255 cells and Su11274 in MKN45 cells. The proteins indicated show at least one phosphotyrosine site reduced >2.5-fold at 3 h of drug treatment. Signaling pathway connections are from PhosphoSite (31), with additional protein–protein interactions extracted from PROTEOME. (B) RTK networks revealed by PhosphoScan-SILAC in MKN45 cells (Left). Receptor tyrosine kinase activation loop tyrosine sites inhibited by Su11274 in MKN45 cells (Right). (C) RTK networks revealed by PhosphoScan-SILAC in H3255 cells. In B and C, WB indicates the sites identified by Western blot analysis. All other sites are from PhosphoScan-SILAC study. Solid arrow, known interaction; dashed arrow, predicted interaction.
Western blot analysis confirmed changes in many sites identified in our PhosphoScan-SILAC analysis (Fig. 3B). Src family tyrosine kinases, although highly phosphorylated, were not significantly inhibited as observed in EGFR-dependent cell lines (SI Fig. 7A). Su11274 inhibits phosphorylation of EGFR, Her2, and Her3 in MKN45 cells but not in HCC827 or H3255 cells (Fig. 3B and data not shown). Coimunoprecipitation experiments (Fig. 3C and data not shown) show that Met, EGFR, and Her3 appear to interact or exist in a protein complex in MKN45 cells. Tyrosine phosphorylation of Ron, DDR1, EphA2, and Tyro3 also decreases after 3 h of treatment with Su11274, suggesting that c-Met regulates the phosphorylation of multiple receptor tyrosine kinases in MKN45 cells (Fig. 4B and SI Table 8). Sites on receptor tyrosine kinase inhibited by Su11274 all occur on the activation loop (Fig. 4B).
Discussion
Quantitative and semiquantitative MS/MS comparison of EGFR mutant and WT EGFR cells identified a large network of activated signaling molecules sensitive to EGFRIs present in EGFR mutant cells but not in WT EGFR cells. The signaling networks activated and assembled by mutant EGFR are blocked by EGFRIs in sensitive cell lines and provide insights into the unique dependence and drug sensitivity these cell lines demonstrate. We also show similar effects in a gastric cancer cell line, MKN45, dependent on amplified c-Met. As observed for EGFR cell lines, c-Met also assembles a large signaling network that is blocked by treatment with the c-Met inhibitor Su11274, and this network shares many signaling components with the gefitinib-sensitive network that may underlie common aspects of drug sensitivity.
Our studies also show that amplified c-Met drives the activity of EGFR family members and, conversely, that mutated and amplified EGFR can drive c-Met activity. In both cases, we find that the oncogenic kinase sits at the top of a hierarchical network driving the phosphorylation and activity of additional receptor tyrosine kinases, potentially explaining why in these cell lines the inhibitors so effectively block Akt phosphorylation and downstream survival signaling. This relationship may also explain the perplexing observation that EGFR- and c-Met-dependent cell lines express many other phosphorylated and presumably active receptor tyrosine kinases that could substitute survival signaling to Akt and ERK pathways and render EGFR and c-Met inhibitors ineffective (25).
Although most tumors responsive to EGFRIs carry EGFR-activating mutations, these mutations alone may be insufficient to drive high-level EGFR signaling and dependence on EGFR. Of the four mutant EGFR cell lines we examined, all contain additional genetic alterations that boost EGFR signaling, and some of these changes also confer resistance to EGFRIs. For example, both HCC827 and H3255 exhibit amplification at the EGFR locus, dramatically enhancing EGFR signaling, whereas H1975 carries the T790M mutation that, when combined in cis with L858R or del746–750 mutations, boosts EGFR signaling (26). The T790M mutation also confers resistance to gefitinib by blocking drug binding (SI Fig. 11 and ref. 27) but not to the irreversible EGFR inhibitors EKB569 and CI-1033 (SI Fig. 11 A and B and ref. 28). For these reasons, H1975 can be considered dependent on EGFR signaling and sensitive to EGFRIs, consistent with signaling similarities to other sensitive cell lines (Fig. 1B, SI Fig. 11C, and SI Tables 3 and 4). H1650 cells have inactivated the tumor suppressor PTEN, amplifying EGFR signals through enhanced PI3K signaling. The resistance of H1650 to EGFRIs may result from the absence of a EGFR-organized network (SI Fig. 11C) and the presence of parallel RTK activity as observed in glioblastoma (29). Taken together, our data suggest that drugs targeting a single kinase will be most effective when the targeted kinase assembles and uniquely controls the downstream signaling networks as observed in HCC827, H3255, and MKN45 cells.
Because drug-sensitive cell lines studied here were derived from late-stage disease, the altered signaling networks identified may result from changes underlying both early and late aspects of cancer development. Activation of c-Met has been associated with metastasis, and many elements shared between H3255 and MKN45 signaling networks may represent pathways important for cell adhesion, motility, and invasion. The observation that EGFR drives c-Met and that gefitinib blocks both EGFR and c-Met signaling may explain important clinical benefits in patients with tumors driven by similar signaling networks.
Materials and Methods
Cell Culture and Reagents.
All cell culture reagents were purchased from Invitrogen. H358, H1650, H1666, H1734, and H1975 were obtained from American Type Culture Collection. HCC827 and gastric cancer cell line MNK45 were purchased from DSMZ (German Collection of Microorganisms and Cell Cultures). For the immunoaffinity precipitation and immunoblot experiments, cells were grown to 80% confluence and then starved in medium without FBS overnight before harvesting.
Western Blot and Protein Immunoprecipitation Analysis.
Total Her3 antibody was purchased from Santa Cruz, all other antibodies were from Cell Signaling Technology, Inc. (CST). Western blot and protein immunoprecipitation analysis were performed after the CST protocol. Details are in SI Methods.
Immunofluorescence Analysis.
Immunofluorescence staining with pTyr-100, β-tubulin, and Draq5 DNA dye in untreated and gefitinib-treated H3255 cells was done after CST protocols, for details see SI Methods.
Phosphopeptide Immunoprecipitation and Analysis by LC-MS/MS.
Phosphopeptide immunoprecipitation from different cell lines was performed as described (14) by using PhosphoScan Kit (P-Tyr-100) from CST. Cells were serum-starved overnight before harvesting. The enriched phospho-peptides were concentrated by using a ZipTip column (Millipore) and analyzed by LC-MS/MS. Mass spectra were collected with an LTQ ion trap mass spectrometer (ThermoFinnigan).
PhosphoScan-SILAC Analysis of H3255 Cells and MKN45 Cells Treated with Gefitinib and Su11274.
Equal numbers of cells were grown in either light (supplemented with regular l-lysine and l-arginine (Sigma) or heavy [supplemented with l-arginine (U-13C6,98%) and l-lysine (U-13C6,98%; U-15N2,98%) (Cambridge Isotope Laboratories) SILAC medium]. The cells were grown for at least five generations to reach 100 million cells in each medium type. Cells grown in the light medium were serum-starved overnight and treated with 1 μM gefitinib for 3 or 24 h. Cells grown in the heavy medium were starved but not treated with drug. Lysate was prepared after the PhosphoScan kit protocol. Equal amounts of untreated and treated lysate were combined for phosphopeptide immunoprecipitation experiments as described above.
MS analysis and data acquisition are described in ref. 30. Peptides were analyzed by using a hybrid linear ion trap-7 Tesla ion cyclotron resonance Fourier transform instrument (LTQ-FT; ThermoFinnigan). MS2 spectra were searched against a human database by using Sequest with methionine oxidation and heavy lysine and arginine as dynamic modifications. A composite target/decoy database approach was used to establish filtering criteria such that estimated peptide false-positive rate was <1%. Peptide hits passing those criteria were submitted to an in-house quantification program, Vista (C. E. Bakalarski and S.P.G., unpublished data) to calculate peak areas and an abundance ratio between light and heavy forms of each peptide. Identified peptides with signal-to-noise in the MS scan <10 were not considered for quantification. Peptide ratios (H/L) were manually inspected and averaged for each peptide; these values are referred to as SILAC ratio (H/L).
Supplementary Material
ACKNOWLEDGMENTS.
We thank Drs. Ting-lei Gu, Yu Li, and Jeffrey Silva for reading the manuscript and for discussion and Dr. Lewis Cantley for providing H3255 cells. This work was partially supported by Innovative Technologies for the Molecular Analysis of Cancer Program of the National Cancer Institute Grant Number R44CA101106.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Data reported in this paper were deposited in the PhosphoSite database and can be dowloaded from http://ms.phosphosite.org:5080/.
This article contains supporting information online at www.pnas.org/cgi/content/full/0707270105/DC1.
References
- 1.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, et al. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 2.Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et al. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 3.Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, Singh B, Heelan R, Rusch V, Fulton L, et al. Proc Natl Acad Sci USA. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smolen GA, Sordella R, Muir B, Mohapatra G, Barmettler A, Archibald H, Kim WJ, Okimoto RA, Bell DW, Sgroi DC, et al. Proc Natl Acad Sci USA. 2006;103:2316–2321. doi: 10.1073/pnas.0508776103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riely GJ, Politi KA, Miller VA, Pao W. Clin Cancer Res. 2006;12:7232–7241. doi: 10.1158/1078-0432.CCR-06-0658. [DOI] [PubMed] [Google Scholar]
- 6.Yun CH, Boggon TJ, Li Y, Woo MS, Greulich H, Meyerson M, Eck MJ. Cancer Cell. 2007;11:217–227. doi: 10.1016/j.ccr.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mulloy R, Ferrand A, Kim Y, Sordella R, Bell DW, Haber DA, Anderson KS, Settleman J. Cancer Res. 2007;67:2325–2330. doi: 10.1158/0008-5472.CAN-06-4293. [DOI] [PubMed] [Google Scholar]
- 8.Sordella R, Bell DW, Haber DA, Settleman J. Science. 2004;305:1163–1167. doi: 10.1126/science.1101637. [DOI] [PubMed] [Google Scholar]
- 9.Sharma SV, Gajowniczek P, Way IP, Lee DY, Jiang J, Yuza Y, Classon M, Haber DA, Settleman J. Cancer Cell. 2006;10:425–435. doi: 10.1016/j.ccr.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weinstein B. Science. 2002;297:63–64. doi: 10.1126/science.1073096. [DOI] [PubMed] [Google Scholar]
- 11.Blagoev B, Ong SE, Kratchmarova I, Mann M. Nat Biotechnol. 2004;22:1139–1145. doi: 10.1038/nbt1005. [DOI] [PubMed] [Google Scholar]
- 12.Thelemann A, Petti F, Griffin G, Iwata K, Hunt T, Settinari T, Fenyo D, Gibson N, Haley JD. Mol Cell Proteomics. 2005;4:356–376. doi: 10.1074/mcp.M400118-MCP200. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Wolf-Yadlin A, Ross PL, Pappin DJ, Rush J, Lauffenburger DA, White FM. Mol Cell Proteomics. 2005;4:1240–1250. doi: 10.1074/mcp.M500089-MCP200. [DOI] [PubMed] [Google Scholar]
- 14.Rush J, Moritz A, Lee KA, Guo A, Goss VL, Spek EJ, Zhang H, Zha XM, Polakiewicz RD, Comb MJ. Nat Biotechnol. 2005;23:94–101. doi: 10.1038/nbt1046. [DOI] [PubMed] [Google Scholar]
- 15.Old WM, Meyer-Arendt K, Aveline-Wolf L, Pierce KG, Mendoza A, Sevinsky JR, Resing KA, Ahn NG. Mol Cell Proteomics. 2005;4:1487–1502. doi: 10.1074/mcp.M500084-MCP200. [DOI] [PubMed] [Google Scholar]
- 16.Pao W, Wang TY, Riely GJ, Miller VA, Pan Q, Ladanyi M, Zakowski MF, Heelan RT, Kris MG, Varmus HE. PLoS Med. 2005;2:e17. doi: 10.1371/journal.pmed.0020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soung YH, Lee JW, Kim SY, Seo SH, Park WS, Nam SW, Song SY, Han JH, Park CK, Lee JY, et al. Virchows Arch. 2005;446:483–488. doi: 10.1007/s00428-005-1254-y. [DOI] [PubMed] [Google Scholar]
- 18.Engelman JA, Janne PA, Mermel C, Pearlberg J, Mukohara T, Fleet C, Cichowski K, Johnson BE, Cantley LC. Proc Natl Acad Sci USA. 2005;102:3788–3793. doi: 10.1073/pnas.0409773102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mellinghoff IK, Wang MY, Vivanco I, Haas-Kogan DA, Zhu S, Dia EQ, Lu KV, Yoshimoto K, Huang JH, Chute DJ, et al. N Engl J Med. 2005;353:2012–2024. doi: 10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- 20.Ferby I, Reschke M, Kudlacek O, Knyazev P, Pante G, Amann K, Sommergruber W, Kraut N, Ullrich A, Fassler R, Klein R. Nat Med. 2006;12:568–573. doi: 10.1038/nm1401. [DOI] [PubMed] [Google Scholar]
- 21.Ong SE, Blagoev B, Kratchmarova I, Kristensen DB, Steen H, Pandey A, Mann M. Mol Cell Proteomics. 2002;1:376–386. doi: 10.1074/mcp.m200025-mcp200. [DOI] [PubMed] [Google Scholar]
- 22.Fanning AS, Anderson JM. Curr Top Microbiol Immunol. 1998;228:209–233. doi: 10.1007/978-3-642-80481-6_9. [DOI] [PubMed] [Google Scholar]
- 23.Garner CC, Nash J, Huganir RL. Trends Cell Biol. 2000;10:274–280. doi: 10.1016/s0962-8924(00)01783-9. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Le P, Liang C, Chan J, Kiewlich D, Miller T, Harris D, Sun L, Rice A, Vasile S, et al. Mol Cancer Ther. 2003;2:1085–1092. [PubMed] [Google Scholar]
- 25.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen J, et al. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 26.Godin-Heymann N, Bryant I, Rivera MN, Ulkus L, Bell DW, Riese DJ, II, Settleman J, Haber DA. Cancer Res. 2007;67:7319–7326. doi: 10.1158/0008-5472.CAN-06-4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, Zakowski MF, Kris MG, Varmus H. PLoS Med. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwak EL, Sordella R, Bell DW, Godin-Heymann N, Okimoto RA, Brannigan BW, Harris PL, Driscoll DR, Fidias P, Lynch TJ, et al. Proc Natl Acad Sci USA. 2005;102:7665–7670. doi: 10.1073/pnas.0502860102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stommel JM, Kimmelman AC, Ying H, Nabioullin R, Ponugoti AH, Wiedemeyer R, Stegh AH, Bradner JE, Ligon KL, Brennan C, et al. Science. 2007;318:287–290. doi: 10.1126/science.1142946. [DOI] [PubMed] [Google Scholar]
- 30.Neher SB, Villen J, Oakes EC, Bakalarski CE, Sauer RT, Gygi SP, Baker TA. Mol Cell. 2006;22:193–204. doi: 10.1016/j.molcel.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 31.Hornbeck PV, Chabra I, Kotrhauser JM, Skrzypek E, Zhang B. Proteomics. 2004;4:1551–1561. doi: 10.1002/pmic.200300772. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.