Abstract
The pathogenic mycobacteria that cause tuberculosis (TB) and TB-like diseases in humans and animals elude sterilizing immunity by residing within an intracellular niche in host macrophages, where they are protected from microbicidal attack. Recent studies have emphasized microbial mechanisms for evasion of host defense; less is known about mycobactericidal mechanisms that remain intact during initial infection. To better understand macrophage mechanisms for restricting mycobacteria growth, we examined Mycobacterium marinum infection of Drosophila S2 cells. Among ≈1,000 host genes examined by RNAi depletion, the lysosomal enzyme β-hexosaminidase was identified as an important factor in the control of mycobacterial infection. The importance of β-hexosaminidase for restricting mycobacterial growth during mammalian infections was confirmed in macrophages from β-hexosaminidase knockout mice. β-Hexosaminidase was characterized as a peptidoglycan hydrolase that surprisingly exerts its mycobactericidal effect at the macrophage plasma membrane during mycobacteria-induced secretion of lysosomes. Thus, secretion of lysosomal enzymes is a mycobactericidal mechanism that may have a more general role in host defense.
Keywords: lysosome, Mycobacterium marinum, RNAi, S2, bacterial pathogenesis
More than one-third of the world is infected with Mycobacterium tuberculosis (Mtb), and tuberculosis (TB) kills more than 2 million people each year (1). Fewer than 0.1% of infected people die annually from infection, highlighting the intricate interplay of host and pathogen that leads to chronic infection. Once established, infection is lifelong, and adaptive immunity is required for control of disease. Innate immunity provides the first line of defense during initial infection, but Mtb has evolved mechanisms for countering this defense, even exploiting aspects of it to establish successful infection. Recent studies have emphasized the important aspects of virulence required for mycobacterial evasion of macrophage microbicidal activity, but less is understood about mechanisms of host defense required to control infection before onset of adaptive immunity. Reasons for the deficiency in understanding host response include slow growth of the bacterium, limitation of a natural host range to humans, and complexity of interactions between the bacterium and its human host.
Mycobacterium marinum (Mm), a bacterial pathogen of fish and amphibians, is one of the closest relatives to the Mtb complex, measured both by similarity in 16S rRNA and by more complex estimates of the phylogenetic tree (2). The genetic relatedness, together with similarity in macroscopic and microscopic pathology of diseases caused by Mm and Mtb, has resulted in wide acceptance of Mm infection as a model for pathogenesis of TB. Its fast growth rate (five times that of Mtb), and ease of genetic manipulation have facilitated studies of the organism. Additionally, Mm has a lower optimum growth temperature than Mtb, allowing it to cause disease in poikilotherms but remain innocuous to healthy humans (3).
A particular problem in understanding host–pathogen interactions in TB has been development of unbiased approaches for identification of factors required for host defense during mycobacterial infection. Certain cytokines, including IFN-γ and TNFα, essential for resistance to mycobacteria in studies of both human and murine susceptibility, were identified through testing of likely candidates (4) and studies of patients with increased susceptibility to infection by atypical mycobacteria (5). The putative Zn2+/Mg2+ transporter NRAMP1, required for resistance to several intracellular organisms including mycobacteria, was identified through analysis of increased susceptibility of certain inbred strains of mice to infection by intracellular pathogens (6). Despite these successes, systematic identification of novel genes involved in host resistance has been a challenge. RNAi screens in Drosophila S2 cells have become increasingly popular for identification of host factors involved in microbial pathogenesis, including genes required for infection by several intracellular bacterial pathogens, such as Listeria monocytogenes, Chlamydia trachomatis, Legionella pneumophila, and Mycobacterium fortuitum (a distant relative of Mm and the Mtb complex) (7–11). One drawback to using the S2 model is that these cells require temperatures of 25–28°C for optimal function; at higher temperatures S2 cells undergo a heat shock response that can result in cytotoxicity (12). Most human pathogens (including Mtb) grow optimally at higher temperatures similar to that of the human body. Because many pathogens will not undergo their normal intracellular life cycle at temperatures required by S2 cells, use of these cells often is limited to defining host genes required for early interactions between bacterium and host cell, such as receptor binding and microbial uptake.
Because Mm has (i) similarity to Mtb in phylogeny and disease manifestations and (ii) a consistent growth temperature with S2 cells, Drosophila RNAi libraries should be useful for unbiased identification of host genes involved in modulating the entire intracellular life cycle of this organism. We have found that Mm growth in S2 cells shares many properties with infection of mammalian macrophages. By using RNAi to determine roles for host genes during Mm growth in S2 cells, we identified a mycobactericidal role for the lysosomal enzyme β-hexosaminidase. After confirming its importance in restricting intracellular growth of Mm in mammalian macrophages, we show that β-hexosaminidase is a peptidoglycan hydrolase (PGH) that surprisingly is active at the macrophage surface during uptake of Mm. These findings establish a role for β-hexosaminidase in host defense against infection by Mm.
Results
Mm Infects and Proliferates in S2 Cells.
Hemocytes, from which S2 cells are derived, have been observed to ingest Mm during infection of Drosophila melanogaster (13), raising the possibility that S2 cells could be used as a model host for studying infection in vitro. S2 cells have been shown to ingest M. fortuitum (10), but consequences of uptake have not been well characterized. To determine whether S2 cells could be used to study Mm infection after uptake, we used fluorescence microscopy to track various stages of infection. Within 2 h of adding inoculum, GFP-expressing Mm (MmGFP) were internalized by S2 cells, and bacterial division could be observed over a 4-day period at 25°C (Fig. 1 A and B). Increasing the incubation temperature to 28°C resulted in increased bacterial growth with host cell lysis observed by 3–4 days after infection, kinetics consistent with Mm growth in murine macrophages. Incubation at temperatures >28°C resulted in early host cell death (data not shown), likely because of the Drosophila heat shock response (12).
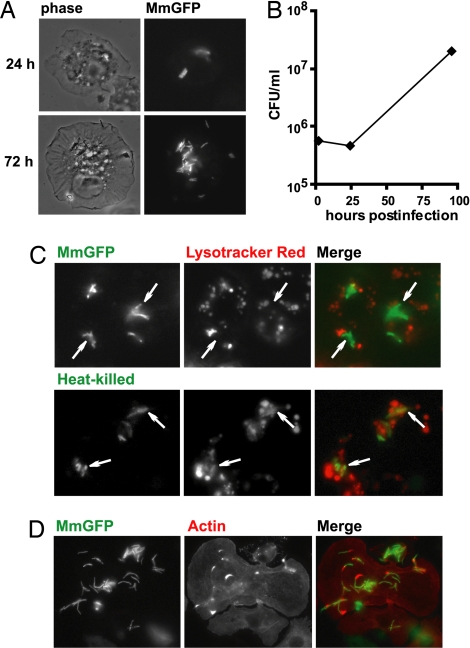
Fig. 1.
Mm infects and proliferates in S2 cells. (A) S2 cells were infected by MmGFP and visualized 24 and 72 h after infection. Phase-contrast images are shown on the left, and immunofluorescence is shown on the right. (B) Mm growth in S2 cells was enumerated by colony-forming units over a 4-day period. (C) MmGFP-infected S2 cells were stained with Lysotracker red. (Upper) Uptake of live bacilli. (Lower) Uptake of heat-killed bacilli. Fusion of phagosomes containing heat-killed Mm-containing with lysosomes is indicated by overlap of the entire bacterium with red fluorescence, demonstrating the low pH (5.5) of the phagosome. Examples of bacteria are indicated by arrows. (D) Infected S2 cells were fixed and stained with Alexa Fluor 594 phalloidin (red). Actin tails and clouds were found in proximity to intracellular bacteria.
A survival mechanism used by mycobacteria after macrophage uptake is the inhibition of phagosome–lysosome fusion. Staining of live S2 cells with Lysotracker showed that, whereas heat-killed bacteria were found primarily in acidic compartments, there was little colocalization of live MmGFP with acidic compartments (Fig. 1C), demonstrating that Mm were capable of inhibiting phagosome maturation in S2 cells.
Mm is unique among the studied pathogenic mycobacteria in that, after macrophage uptake, it escapes from the phagosome and uses host actin machinery to propel itself through the cytosol and into neighboring cells (14). To determine whether Mm could hijack S2 actin machinery, MmGFP-infected S2 cells were stained with phalloidin, and short actin tails and clouds were observed in close association with several bacteria (Fig. 1D). Some pathogens, such as L. monocytogenes, use cholesterol-dependent cytolysins for lysing phagosome membranes to facilitate escape into the cytosol. Efficient escape from S2 phagosomes by L. monocytogenes requires supplementation with exogenous cholesterol (15). However, cholesterol supplementation had no observable effect on the number of Mm with actin tails (data not shown). Forty-eight hours after infection, Mm were observed to propel themselves through S2 cytosol [supporting information (SI) Movie 1]. To confirm that Mm motility was actin-based, S2 expressing GFP-actin were infected; GFP-actin was found in the “comet tails” behind motile bacteria (SI Movie 2). Despite a lower frequency of actin tails observed in association with bacteria, the kinetics of actin-based motility was comparable in S2 and mammalian cells. These data demonstrate that the cell biology of Mm infection is similar in S2 and mammalian macrophages.
HEXO2 Is Important for Limiting Mm Growth in S2 Cells.
To establish whether flow cytometry was suitable for assessing Mm infection, S2 cells were infected with MmGFP, and intracellular growth was determined. Four hours after infection, GFP-positive cells were barely detectable by flow cytometry (data not shown). In contrast, 48 h after infection, there was a shift in population of GFP-positive cells to the right, with an 8-fold increase in mean fluorescence over that of uninfected cells (Fig. 2A). The overall increase in fluorescence demonstrates the robust growth of intracellular Mm in S2 cells.
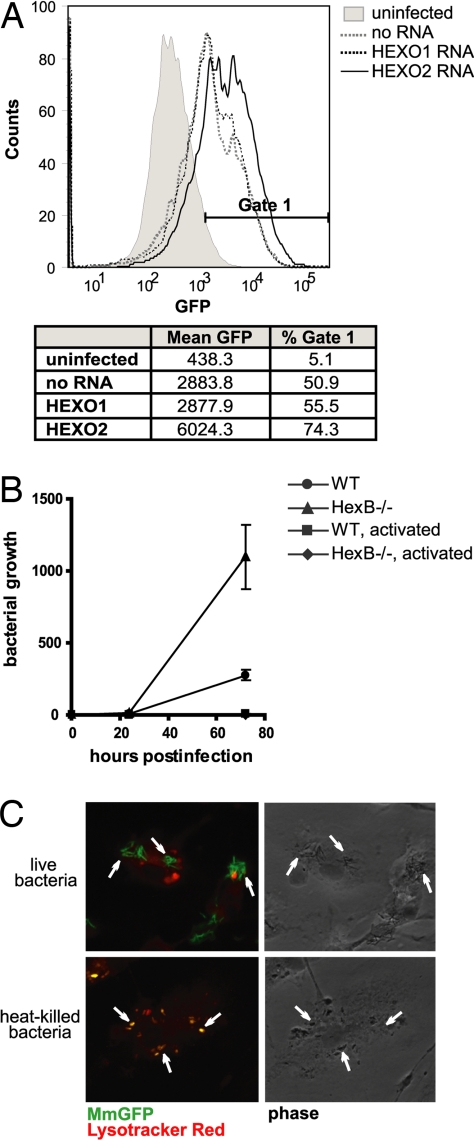
Fig. 2.
Effect of β-hexosaminidase on intracellular growth of Mm. (A) Flow cytometry histogram of S2 cells infected by MmGFP 48 h after initiation of infection. The fluorescence of Mm was greater in HEXO2 RNAi-treated S2 cells, despite equivalent initial uptake. Histogram is representative of three independent experiments. (B) Growth curve of Mm in WT or HexB−/− BMDM. Mm shows increased intracellular growth in HexB−/− BMDM. Activated BMDM (by treatment with IFN-γ and LPS) did not support growth of the bacilli. The graph shown is normalized for bacterial uptake (after washing of monolayer) and representative of three independent experiments. (C) MmGFP-infected HexB−/− BMDM were stained with Lysotracker red 24 h after initial bacterial uptake. (Upper) Uptake of live bacilli. (Lower) Uptake of heat-killed bacilli. Fusion of phagosomes containing heat-killed Mm with lysosomes is indicated by overlap of the entire bacterium with red fluorescence, demonstrating the low pH (5.5) of the phagosome. Examples of bacteria are indicated by arrows.
To identify host factors involved in limiting bacterial growth in S2 cells, cells were treated with RNAi directed at various Drosophila genes for 72 h before infection with Mm. Increased growth of Mm in these cells was taken as preliminary evidence for involvement of the knocked-down gene in restriction of bacterial growth. Among the first ≈1,000 genes screened, knockdown of two genes led to a consistent increase in bacterial growth: HEXO2 and ecdysone receptor. Because ecdysone receptor, which recognizes an insect-specific hormone, is not expressed in mammalian cells, we focused on HEXO2. HEXO2 encodes a homolog of the β-subunit of the mammalian lysosomal enzyme β-hexosaminidase. RNAi knockdown of HEXO2 resulted in a 2-fold increase in mean fluorescence of infected S2 cells, and a shift in the population of GFP-positive cells to the right, indicating increased bacterial load per cell (Fig. 2A). Depletion of HEXO2 by RNAi did not affect S2 viability, as determined by trypan blue exclusion and the Alamar blue cell viability assay (data not shown). RNAi directed toward HEXO1 (an alternate Drosophila homolog of β-hexosaminidase) did not affect growth of Mm (Fig. 2A), suggesting that the HEXO2 isoform was sufficient to control bacterial growth in S2 cells.
Mm Has Increased Survival in HexB−/− Murine Macrophages.
Mammalian β-hexosaminidase is found in two isoforms, composed of dimers of α- and β-subunits. The αβ heterodimer comprises β-hexosaminidase A, which degrades GM2 gangliosides in neurons. The ββ homodimer comprises β-hexosaminidase B, which degrades glycosphingolipids in the visceral organs. To determine whether β-hexosaminidase affected Mm growth in mammalian macrophages, bone marrow-derived macrophages (BMDM) from mice deficient in the HEXO2 homolog, HexB, were infected with Mm. Growth of ingested Mm was 2-fold greater in the HexB−/− BMDM by 24 h after infection and 4-fold by 72 h after infection (Fig. 2B). Mm did not grow in either WT or HexB−/− BMDM after treatment with IFN-γ (Fig. 2B), likely because of recruitment of additional host defenses, such as inducible nitric-oxide synthase (iNOS) or autophagy responses.
To determine whether absence of β-hexosaminidase led to a general defect in phagosome–lysosome fusion, we examined the fate of heat-killed Mm, which traffic to lysosomes in WT BMDM. Ingested heat-killed bacteria showed extensive colocalization with Lysotracker in HexB−/− cells (Fig. 2C). There was no difference in the numbers of heat-killed bacteria found in acidic compartments in WT and HexB−/− cells (87 ± 10% and 83 ± 9%, respectively), indicating that phagosome maturation was unaffected by the absence of β-hexosaminidase. HexB−/− cells that phagocytosed live Mm showed little colocalization of Lysotracker with ingested organisms, similar to live organisms in WT macrophages.
β-Hexosaminidase Is Secreted in Response to Mm.
An important aspect of the intracellular survival strategies of pathogenic mycobacteria is inhibition of phagosome–lysosome fusion. This raised the question of how, during normal infection, β-hexosaminidase might gain access to Mm to achieve its growth-regulatory effect. Several cytolysin- and hemolysin-containing bacterial pathogens have been reported to induce exocytosis of a subset of lysosomes from their cellular host (16, 17). Because Mm displays contact-dependent hemolysis (18), we hypothesized that it could induce the secretion of lysosomes from BMDM. Within 2 h of adding inoculum, dose-dependent secretion of β-hexosaminidase was observed. At the highest multiplicity of infection (MOI, 50), 27.7% of total β-hexosaminidase was secreted, compared with 2.3% from uninfected cells (Fig. 3A). Only 2.8% was secreted from cells to which an equal number of latex beads were added, demonstrating that secretion was specific to Mm, and not a general response to phagocytosis. Release of lysosomal contents was not a consequence of cell death, because cell viability was unaffected by infection (Fig. 3B). These data suggested that Mm may come in contact with β-hexosaminidase at the plasma membrane during ingestion, even though its ability to block phagosome–lysosome fusion prevents efficient delivery to the Mm-containing phagosome.
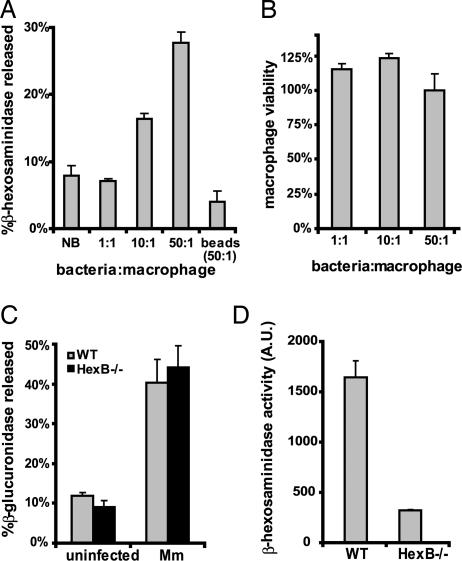
Fig. 3.
Mm induces secretion of lysosomes from macrophages. (A) Secretion of β-hexosaminidase by BMDM was measured 2 h after treatment with buffer (NB, no bacteria), Mm (MOI of 1, 10, or 50), or latex beads (beads, 50:1). (B) Viability of BMDM was measured 2 h after addition of inoculum. Cell viability is displayed as Absinfected/Absuninfected. (C) Secretion of lysosomal enzyme β-glucuronidase was measured 2 h after treatment of WT and HexB−/− BMDM with buffer (uninfected) or Mm (Mm). (D) Secretion of β-hexosaminidase was measured in Mm-infected WT or HexB−/− BMDM. β-Hexosaminidase activity is displayed in arbitrary units (A.U.). All assays were done in triplicate.
To determine whether the absence of β-hexosaminidase inhibited macrophage lysosome secretion in response to Mm, HexB−/− BMDM were incubated with Mm. Mm induced equivalent secretion of the lysosomal enzyme β-glucuronidase from HexB−/− and WT BMDM (Fig. 3C), demonstrating that Mm-induced exocytosis of lysosomes is intact in HexB−/− cells. As expected, HexB−/− BMDM secreted no β-hexosaminidase in response to Mm (Fig. 3D).
β-Hexosaminidase Is Bactericidal to Mm.
To determine whether β-hexosaminidase could affect bacterial viability directly, purified β-hexosaminidase was incubated with Mm for 2 h in acidic and neutral conditions, representing lysosomal and extracellular milieus, respectively. β-Hexosaminidase killed Mm in a dose-dependent manner (Fig. 4A). Although killing was more effective at low pH, mycobactericidal activity was significant even at neutral pH, suggesting that the enzyme could display bactericidal effects extracellularly.
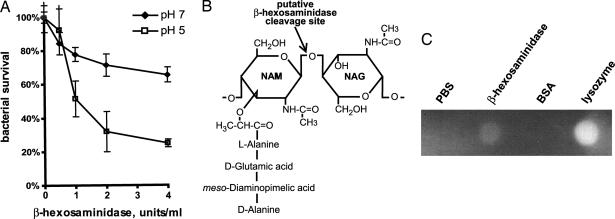
Fig. 4.
β-Hexosaminidase is a mycobactericidal peptidoglycan hydrolase. (A) Mm was incubated with 0, 0.5, 1, 2, and 4 units/ml human β-hexosaminidase. Bacterial survival was enumerated as colony-forming units on 7H10 agar. Assays were done in triplicate, at pH values of 5 and 7. (B) β-Hexosaminidase is predicted to hydrolyze the β-1,4-linked glycosidic bond between NAM and NAG, as shown. (C) PG hydrolase activity of β-hexosaminidase was assessed by zymography. PBS and BSA were negative controls, and lysozyme was used as a positive control.
As an N-acetyl-β-d-glucosaminidase, β-hexosaminidase is predicted to hydrolyze the β-1,4-linked glycosidic bond between the alternating N-acetylglucosamine (NAG) and N-acetylmuramic acid (NAM) subunits that make up the backbone of peptidoglycan (PG) (Fig. 4B) (19). To determine whether the bactericidal activity of β-hexosaminidase could result from degradation of PG, bacterial cell wall hydrolysis was assayed by zymography. Purified human β-hexosaminidase was spotted onto polyacrylamide embedded with PG, and PGH activity at pH 7 was determined by methylene blue staining. A zone of clearance, which represents PG hydrolysis, occurred in the presence of human β-hexosaminidase, but not BSA or buffer alone (Fig. 4C). Lysozyme, a muramidase, was used as a positive control. Thus, β-hexosaminidase has significant PGH activity, even at neutral pH. At pH 5, no methylene blue staining was detected anywhere in the gel (data not shown), precluding analysis of the effects of specific enzymes on PG degradation. These results demonstrate that mammalian β-hexosaminidase is a PGH that is active at neutral pH and may be responsible for damaging or killing mycobacteria.
Secreted β-Hexosaminidase Controls Mm Infection.
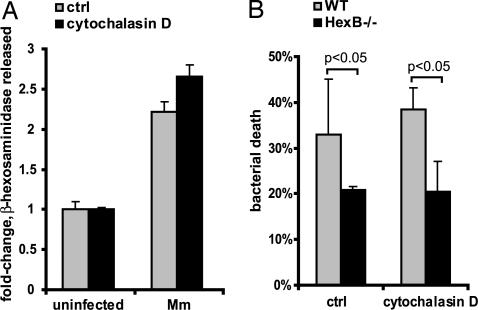
The ability of β-hexosaminidase to kill Mm at neutral pH, the secretion of lysosomes in response to macrophage interaction with Mm, and the failure of Mm-containing phagosomes to fuse with lysosomes suggested that the enzyme might have its bactericidal effect at the macrophage plasma membrane during the process of ingestion. To test this hypothesis, we compared Mm killing by WT and HexB−/− BMDM in the absence of phagocytosis. BMDM treated with cytochalasin D, an inhibitor of actin polymerization, showed markedly decreased uptake of Mm, but had no defect in secretion of lysosomes in response to Mm (Fig. 5A), suggesting that the signal for secretion occurs at the plasma membrane. To assess bacterial killing by extracellular β-hexosaminidase, BMDM were exposed to Mm, in the absence or presence of cytochalasin D, and colony-forming units were enumerated after 2 h of incubation. Even in the absence of phagocytosis, twice as many Mm were recovered from incubation with HexB−/− BMDM, demonstrating the importance of β-hexosaminidase in killing extracellular bacteria (Fig. 5B). No significant difference in mycobactericidal activity was observed between cytochalasin D-treated and untreated cells, suggesting that, even when phagocytosis occurred, the majority of HexB-dependent bacterial killing occurred at the cell surface.
Fig. 5.
β-Hexosaminidase exerts mycobactericidal effects at the cell surface in the absence of phagocytosis. (A) Secretion of β-hexosaminidase was measured in response to Mm (MOI of 50) in the presence or absence of 1 μM cytochalasin D. (B) Mm killing by WT and HexB−/− BMDM was assessed 2 h after initiation of interaction in the presence or absence of cytochalasin D. All assays were done in triplicate.
Discussion
We report the identification of a mycobactericidal role for the lysosomal enzyme β-hexosaminidase. We have found that macrophages secrete β-hexosaminidase in response to Mm, and this enzyme inhibits bacterial growth, presumably through its PGH activity. The finding that β-hexosaminidase restricted growth of Mm in Drosophila S2 cells was confirmed in vertebrate cells by using HexB−/− murine macrophages. Initially, these studies appeared to contain an anomaly, because Mm inhibits phagosome–lysosome fusion in both S2 cells and macrophages, yet β-hexosaminidase is a lysosomal enzyme. However, the resolution of the contradiction arose from our finding that Mm induces lysosome secretion and release of β-hexosaminidase into the extracellular milieu, where it manifests mycobactericidal activity. Our results suggest that the mycobacterial damage and killing that occurs at the cell surface during secretion of lysosomes is an important aspect of innate host defense.
In this context, reports that carriers of the Tay-Sachs gene have increased resistance to TB (20, 21) are very interesting. Tay-Sachs patients, who fail to make the α-chain and therefore lack the αβ-heterodimer form of β-hexosaminidase, have increased production of HexB (β-subunit) by as much as 200%, as assayed from both brain tissue (22) and fibroblasts (23). If the ββ isoform is most closely associated with host defense against Mtb, up-regulation of HexB could explain the possible increase in resistance to TB in individuals carrying the Tay-Sachs gene. Consistent with this hypothesis, we found no effect of RNAi-mediated depletion of HEXO1 (the Drosophila α-chain) on Mm infection of S2 cells. In addition to β-hexosaminidase, other lysosomal enzymes and other aspects of innate immunity also may contribute to bactericidal activity at the cell surface, but the marked defect of cell surface bacterial killing in the absence of HexB demonstrates that this enzyme has a central role in the process. Indeed, inhibition of phagocytosis demonstrated that the difference in bacterial killing between WT and HexB−/− cells was unaffected by inhibition of phagocytosis, revealing that the major role for β-hexosaminidase mycobactericidal activity does not require ingestion. These findings imply a previously unrecognized benefit of macrophage uptake for mycobacteria; entry into a compartment lacking β-hexosaminidase and subsequent inhibition of phagosome maturation result in an environment protected from β-hexosaminidase-mediated bacterial killing.
Because macrophages serve as the first line of defense against pathogenic mycobacteria, β-hexosaminidase likely exerts its antimycobacterial effects on initial encounter with the bacterium, either killing or sufficiently damaging it to overcome its normal survival strategies. Our in vitro assay for β-hexosaminidase killing of Mm demonstrates that the enzyme is cytotoxic to the bacterium, but does not assess the degree of damage inflicted to the cell wall. Previous reports have shown that mycobacteria with weakened cell walls are less resistant to the microbicidal effects of macrophages (24). Therefore, it is not unexpected that β-hexosaminidase-inflicted damage could render the bacterium more susceptible to macrophage killing. It is intriguing to consider that macrophage secretion of mycobactericidal enzymes is a host strategy that has evolved to counter the mycobacterial survival strategy of inhibiting phagosome–lysosome fusion. It has recently been shown that Mtb was recently shown to transiently colocalize with lysosomes early in infection of macrophages, before establishing its intracellular niche (25). Thus, the lysosome secretion we have noted may occur in the vicinity of the site of bacterial ingestion, and it is feasible that some bacterial killing may also occur in these early phagolysosomes.
Our data suggest that the role for β-hexosaminidase in restricting mycobacterial growth occurs early in infection, before the onset of adaptive immunity. Once macrophages are activated, the differences between WT and HexB−/− cells become negligible, as evidenced by the similar growth curves in IFN-γ-activated BMDM. Activation of BMDM results in additional mechanisms of bacterial killing, including iNOS and autophagy (26, 27), which result in significantly better control of infection. Therefore, the antimycobacterial effect of β-hexosaminidase is likely to have the greatest biological significance before macrophage activation by IFN-γ.
The in vitro bactericidal property of β-hexosaminidase appears to be specific to Mm, because neither L. monocytogenes nor Salmonella typhimurium were killed in the assay (data not shown). Resistance to β-hexosaminidase is not surprising for either organism, considering S. typhimurium has a protective outer membrane, and L. monocytogenes has a very thick layer of peptidoglycan. Both organisms also have very fast multiplication rates (20–40 min), which may allow them to multiply before sufficient damage can occur to kill the bacteria. Our attempts to extend our findings to Mtb have been unsuccessful (data not shown), perhaps because of the following possibilities: (i) Mtb has a three to five times slower growth rate than Mm. The incubation time for the in vitro assay may not be sufficient for bacterial killing, allowing Mtb to recover and form colonies on the agar plates. (ii) The localized concentration of β-hexosaminidase may be much higher in vivo than in the in vitro experiments. (iii) The cell walls of the two species are slightly different. For example, Mtb is more susceptible to acidic conditions than Mm for unknown reasons (28). Nonetheless, β-hexosaminidase-mediated damage to the Mtb cell wall may make the organism more susceptible to other macrophage killing mechanisms. β-Hexosaminidase is found predominantly in lysosomes, but it has also been detected in cytosol, plasma membranes, as well as human serum, blood plasma, and milk (29–32). The widespread localization of this enzyme in the human body makes it feasible that circulation of β-hexosaminidase, in conjunction with lysozyme and other secreted antimicrobial products, may have significant effects in limiting mycobacterial infection in general.
Materials and Methods
Bacterial Strains and Cell Culture.
Wild-type Mm (strain M) and MmGFP were cultured in Middlebrook 7H9 (Difco) supplemented with 0.2% glycerol/0.05% Tween 80/10% ADC enrichment (Fisher). Heat-killed bacteria were prepared by incubation for 30 min at 65°C, and loss of viability was confirmed by plating on 7H10 plates. S2 cells were cultured in Schneider's Drosophila media supplemented with 10% heat-inactivated FBS, as described in ref. 33. Macrophages were derived from the bone marrow of C57BL/6 or HexB−/− mice (34) and harvested after 7–21 days.
Microscopy.
Infected BMDM and S2 cells were fixed, permeabilized, and stained with Alexa Fluor 594 phalloidin (Molecular Probes) as described in ref. 14. Infected S2 cells were plated on Con A-coated coverslips for 1 h before fixation and staining (33). Coverslips were mounted with Prolong antifade reagent (Molecular Probes). For lysosome colocalization studies, Lysotracker Red (Molecular Probes) was used to label acidic compartments, as described in ref. 24. Images were acquired on a Nikon Eclipse TE300 microscope with an Evolution QEi cooled charge-coupled device (Media Cybernetics) with IPLAB acquisition software (Scanalytics).
RNAi Library Screen.
Drosophila S2 cells were plated at a concentration of 1 × 105 cells per well in 96-well cell culture plates. dsRNA was generated as previously described in ref. 35 and added to cells at a concentration of 10 μg/ml. Cells were cultured for 3 days at 28°C. dsRNA-treated cells were replated into 96-well plates and infected with Mm at an MOI of 20. After 2 h of incubation at 28°C, amikacin was added to the medium for 2 h at a final concentration of 200 μg/ml to kill uninternalized bacteria (24). After removal of residual amikacin by washing, fresh media and dsRNA were added to each well and incubated at 28°C for 48 h. A total of 10,000 cells from each well was analyzed on a Becton Dickinson LSR2 (BD Biosciences) fitted with a microplate sampler. Uninfected S2 cells were used to determined background autofluorescence. Mycobacterial growth in each sample was determined relative to infected cells without RNAi.
Macrophage Infections.
Bacteria were washed twice in HBSS without Ca2+ and Mg2+ and disrupted into single bacilli by passage through a 26-gauge needle, added to BMDM, centrifuged at 500 × g for 5 min, and incubated at 32°C in 5% CO2. After 2 h, the infected cells were washed with serum-free medium to remove extracellular bacteria. For growth curves, BMDM were incubated further in DMEM containing 2% FBS, 20 mM Hepes, 2% CMG14–12 SN (36) at 32°C in 5% CO2. Intracellular growth was measured by infecting BMDM with a MOI of 1 and hypotonically lysing the cells 4, 24, and 72 h after infection. Colony-forming units were counted on 7H10 agar.
Zymography.
A 7.5% polyacrylamide gel containing 0.07% cell wall from Micrococcus luteus (Sigma) was spotted with 40 μg of β-hexosaminidase, BSA, or lysozyme, in 5-μl volume. After the enzyme solutions were allowed to absorb into the gel matrix for 1 h, the gel was washed once in HBSS and incubated in HBSS for 24 h at 37°C. The gel was stained with methylene blue for 1 h, and destained in water for 1 h.
In Vitro β-Hexosaminidase Assay for Bactericidal Activity.
Mm was disaggregated by passage through a 26-gauge needle as described above. Mm, S. typhimurium, L. monocytogenes, or Mtb were mixed with 0, 0.5, 1, 2, and 4 units/ml human β-hexosaminidase (Sigma) in 200 μl of HBSS in a 96-well microtiter plate. After 1.5 h of incubation at 32°C, aliquots of bacteria were plated on to 7H10 or LB agar plates and enumerated by colony-forming units.
Lysosome Secretion Assays.
BMDM from C57BL/6 (WT) or HexB−/− mice (34) were plated at 5 × 104 cells per well in a 96-well plate. Bacteria washed in HBSS without Ca2+ and Mg2+ were spun onto BMDM monolayers in HBSS with Ca2+ and Mg2+ in the presence of absence of 1 μM cytochalasin D, as indicated. After 2 h of incubation at 30°C, supernatants were collected, and cells were lysed in 0.5% Triton X-100/0.1 M citrate buffer, pH 5. To measure β-hexosaminidase activity, 25 μl of 2 mM 4-methylumbelliferyl N-acetyl-β-d-glucosaminide (Sigma) in 0.2 M sodium acetate buffer, pH 4.8, was incubated with an equal volume of supernatant or lysate and incubated at 37°C for 1 h. The reaction was stopped with 100 μl of 0.1 M sodium carbonate buffer, pH 10.5, and released 4-methylumbelliferone was quantitated in a fluorometer with excitation at 355 nm and emission at 460 nm. The percentage of cellular β-hexosaminidase that had been secreted was calculated from enzyme activity of supernatants and lysates. Viability of infected cells was measured by using the CellTiter-Blue cell viability assay according to the manufacturer's instructions (Promega). All experiments were performed in triplicate.
Supplementary Material
ACKNOWLEDGMENTS.
We thank M. Pak for technical assistance, R. Proia (National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda) for sharing the parental breeding strains of HexB−/− mice, R. Vale (University of California, San Fransico) for GFP-actin S2 cells, J. De Risi and J. Weissman (University of California, San Francisco) for generous sharing of their high-throughput flow cytometer, and F. Carlsson and S. Joshi for critical reading of this manuscript. This work was supported by National Institutes of Health Grants AI55614 (to E.J.B.) and AI63302 and AI51667 (to J.S.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0708110105/DC1.
References
- 1.Frieden TR, Sterling TR, Munsiff SS, Watt CJ, Dye C. Lancet. 2003;362:887–899. doi: 10.1016/S0140-6736(03)14333-4. [DOI] [PubMed] [Google Scholar]
- 2.Stamm LM, Brown EJ. Microbes Infect. 2004;6:1418–1428. doi: 10.1016/j.micinf.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Ramakrishnan L, Falkow S. Infect Immun. 1994;62:3222–3229. doi: 10.1128/iai.62.8.3222-3229.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rook GA, Taverne J, Leveton C, Steele J. Immunology. 1987;62:229–234. [PMC free article] [PubMed] [Google Scholar]
- 5.Holland SM. Am J Med Sci. 2001;321:49–55. doi: 10.1097/00000441-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Forget A, Skamene E, Gros P, Miailhe AC, Turcotte R. Infect Immun. 1981;32:42–47. doi: 10.1128/iai.32.1.42-47.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agaisse H, Burrack LS, Philips JA, Rubin EJ, Perrimon N, Higgins DE. Science. 2005;309:1248–1251. doi: 10.1126/science.1116008. [DOI] [PubMed] [Google Scholar]
- 8.Cheng LW, Viala JP, Stuurman N, Wiedemann U, Vale RD, Portnoy DA. Proc Natl Acad Sci USA. 2005;102:13646–13651. doi: 10.1073/pnas.0506461102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elwell C, Engel JN. Cell Microbiol. 2005;7:725–739. doi: 10.1111/j.1462-5822.2005.00508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Philips JA, Rubin EJ, Perrimon N. Science. 2005;309:1251–1253. doi: 10.1126/science.1116006. [DOI] [PubMed] [Google Scholar]
- 11.Dorer MS, Kirton D, Bader JS, Isberg RR. PLoS Pathogens. 2006;2:e34. doi: 10.1371/journal.ppat.0020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Echalier G. Drosophila Cells in Culture. San Diego: Academic; 1997. [Google Scholar]
- 13.Dionne MS, Ghori N, Schneider DS. Infect Immun. 2003;71:3540–3550. doi: 10.1128/IAI.71.6.3540-3550.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stamm LM, Morisaki JH, Gao LY, Jeng RL, McDonald KL, Roth R, Takeshita S, Heuser J, Welch MD, Brown EJ. J Exp Med. 2003;198:1361–1368. doi: 10.1084/jem.20031072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng LW, Portnoy DA. Cell Microbiol. 2003;5:875–885. doi: 10.1046/j.1462-5822.2003.00327.x. [DOI] [PubMed] [Google Scholar]
- 16.Hakansson A, Bentley CC, Shakhnovic EA, Wessels MR. Proc Natl Acad Sci USA. 2005;102:5192–5197. doi: 10.1073/pnas.0408721102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roy D, Liston DR, Idone VJ, Di A, Nelson DJ, Pujol C, Bliska JB, Chakrabarti S, Andrews NW. Science. 2004;304:1515–1518. doi: 10.1126/science.1098371. [DOI] [PubMed] [Google Scholar]
- 18.Gao LY, Guo S, McLaughlin B, Morisaki H, Engel JN, Brown EJ. Mol Microbiol. 2004;53:1677–1693. doi: 10.1111/j.1365-2958.2004.04261.x. [DOI] [PubMed] [Google Scholar]
- 19.Karamanos Y. Res Microbiol. 1997;148:661–671. doi: 10.1016/S0923-2508(99)80065-5. [DOI] [PubMed] [Google Scholar]
- 20.Spyropoulos B. Nature. 1988;331:666. doi: 10.1038/331666a0. [DOI] [PubMed] [Google Scholar]
- 21.Rotter JI, Diamond JM. Nature. 1987;329:289–290. doi: 10.1038/329289a0. [DOI] [PubMed] [Google Scholar]
- 22.Okada S, O'Brien JS. Science. 1969;165:698–700. doi: 10.1126/science.165.3894.698. [DOI] [PubMed] [Google Scholar]
- 23.Utsumi K, Tsuji A, Kase R, Tanaka A, Tanaka T, Uyama E, Ozawa T, Sakuraba H, Komaba Y, Kawabe M, et al. Acta Neurol Scand. 2002;105:427–430. doi: 10.1034/j.1600-0404.2002.01097.x. [DOI] [PubMed] [Google Scholar]
- 24.Gao LY, Laval F, Lawson EH, Groger RK, Woodruff A, Morisaki JH, Cox JS, Daffe M, Brown EJ. Mol Microbiol. 2003;49:1547–1563. doi: 10.1046/j.1365-2958.2003.03667.x. [DOI] [PubMed] [Google Scholar]
- 25.van der Wel N, Hava D, Houben D, Fluitsma D, van Zon M, Pierson J, Brenner M, Peters PJ. Cell. 2007;129:1287–1298. doi: 10.1016/j.cell.2007.05.059. [DOI] [PubMed] [Google Scholar]
- 26.Flesch IE, Kaufmann SH. Infect Immun. 1991;59:3213–3218. doi: 10.1128/iai.59.9.3213-3218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. Cell. 2004;119:753–766. doi: 10.1016/j.cell.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 28.Piddington DL, Kashkouli A, Buchmeier NA. Infect Immun. 2000;68:4518–4522. doi: 10.1128/iai.68.8.4518-4522.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Massaccesi L, Lombardo A, Venerando B, Tettamanti G, Goi G. Clin Biochem. 2007;40:467–477. doi: 10.1016/j.clinbiochem.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Mahuran DJ. Biochim Biophys Acta. 1999;1455:105–138. doi: 10.1016/s0925-4439(99)00074-5. [DOI] [PubMed] [Google Scholar]
- 31.Lombardo A, Caimi L, Marchesini S, Goi GC, Tettamanti G. Clin Chim Acta. 1980;108:337–346. doi: 10.1016/0009-8981(80)90339-3. [DOI] [PubMed] [Google Scholar]
- 32.Wiederschain G, Newburg DS. Adv Exp Med Biol. 2001;501:573–577. doi: 10.1007/978-1-4615-1371-1_72. [DOI] [PubMed] [Google Scholar]
- 33.Rogers SL, Wiedemann U, Stuurman N, Vale RD. J Cell Biol. 2003;162:1079–1088. doi: 10.1083/jcb.200303023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sango K, Yamanaka S, Hoffmann A, Okuda Y, Grinberg A, Westphal H, McDonald MP, Crawley JN, Sandhoff K, Suzuki K, et al. Nat Genet. 1995;11:170–176. doi: 10.1038/ng1095-170. [DOI] [PubMed] [Google Scholar]
- 35.Foley E, O'Farrell PH. PLoS Biol. 2004;2:E203. doi: 10.1371/journal.pbio.0020203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takeshita S, Kaji K, Kudo A. J Bone Miner Res. 2000;15:1477–1488. doi: 10.1359/jbmr.2000.15.8.1477. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.