Abstract
The 5-hydroxytryptamine-3 (5-HT3) receptor mediates the fast excitatory neurotransmission of serotonin and is known to mediate the nausea/emesis induced by radio/chemotherapy and anesthetics. A polymorphism encoding the variation Y129S in the 5-HT3B subunit exists in high frequency in the general population and has been shown to be inversely correlated to the incidence of major depression in women. We show that 5-HT3AB(Y129S) receptors exhibit a substantially increased maximal response to serotonin compared with WT receptors in two fluorescence-based cellular assays. In electrophysiological recordings, the deactivation and desensitization kinetics of the 5-HT3AB(Y129S) receptor are 20- and 10-fold slower, respectively, than those of the WT receptor. Single-channel measurements reveal a 7-fold-increased mean open time of 5-HT3AB(Y129S) receptors compared with WT receptors. The augmented signaling displayed by 5-HT3AB(Y129S) receptors may confer protection against the development of depression. The variant also may influence the development and/or treatment of nausea and other disorders involving 5-HT3 receptors. Thus, the impact of the high-frequency variant 5-HT3B(Y129S) on 5-HT3AB receptor signaling calls for a search for additional phenotypes, and the variant may thus aid in establishing the role of the 5-HT3AB receptor in pathophysiology.
Keywords: 5-HT3, ligand-gated ion channel, polymorphism, serotonin, receptor kinetics
Serotonin [5-hydroxytryptamine (5-HT)] is a major neurotransmitter in both the CNS and the peripheral nervous system (PNS), where it plays a key role in basic functions such as mood, sleep, appetite regulation, and libido (1). Serotonergic signaling is mediated via a plethora of G protein-coupled receptors, whereas only one serotonin-gated ion channel has been identified: the 5-HT3 receptor (2).
5-HT3 receptors are expressed in both the CNS and the PNS. Presynaptic 5-HT3 receptors are known to modulate the synaptic release of various neurotransmitters (3–5), whereas postsynaptic 5-HT3 receptors are responsible for the fast excitatory response to serotonin (6). The role of the 5-HT3 receptor in general physiology and pathophysiology is not well established. However, the 5-HT3 receptor is known to mediate the nausea and emesis caused by radio/chemotherapy and anesthetics (7). Furthermore, 5-HT3 antagonists have proven effective in the treatment of irritable bowel disease (7). Finally, the 5-HT3 receptor has been suggested to be involved in anxiety, depression, pain, alcohol dependence, and eating disorders (7, 8).
The 5-HT3 receptor is a nonselective cation channel belonging to the superfamily of Cys-loop ligand-gated ion channels that includes receptors for the neurotransmitters ACh, γ-aminobutyric acid, and glycine. These receptors are pentameric assemblies, where the five subunits form a central ion channel path (9). To date, five 5-HT3 subunits have been cloned: 5-HT3A–5-HT3E (10–12). Transcripts of 5-HT3A, 5-HT3B, and 5-HT3C subunits have been demonstrated in both the CNS and the PNS (11–13), whereas 5-HT3D and 5-HT3E transcripts have been detected only in peripheral tissues (12). By using 5-HT3B-specific antibodies, the existence of the 5-HT3B subunit in the human hippocampus (14) as well as in the CNS in rodents (15) has been confirmed. Whereas homomeric 5-HT3A receptors are functional, the other subunits only form functional receptors when coexpressed with 5-HT3A (11, 16). The homomeric 5-HT3A and the heteromeric 5-HT3AB receptors are the best characterized 5-HT3 receptors, whereas the physiological relevance of subunits 5-HT3c-5-HT3E has just started to be unraveled. In heterologous expression systems, the 5-HT3A:5-HT3B stoichiometry and the relative positions of the subunits in the 5-HT3AB receptor have been determined to be 2:3 and B–B–A–B–A, respectively (17).
The impact of naturally occurring variations in the human genome has become increasingly evident after the sequencing of the human genome. Numerous variations in Cys-loop ligand-gated ion channels have been shown to be causative of channelopathies (18, 19). A single-nucleotide polymorphism in the human HTR3B gene giving rise to the nonsynonymous variation Y129S in the 5-HT3B subunit has been identified in very high frequencies in worldwide populations. The frequencies of the minor allele ranges from 0.17 in a Han Chinese sample to 0.43 in a Yoruba sample in Nigeria (rs1176744, NCBI dbSNP build 127) [supporting information (SI) Fig. 5]. Interestingly, the 5-HT3B subunits of mouse, rat, ferret, guinea pig, dog, and chimpanzee all have a Ser residue in the corresponding position.
Recently, the 5-HT3B(Y129S) polymorphism was reported to be associated with the incidence of major depression in women (20) and the incidence and severity of nausea after paroxetine treatment of psychiatric patients (21). In the present study, we have investigated the functional implications of the variant 5-HT3B(Y129S) on 5-HT3AB receptor signaling.
Results
Functional Characterization of 5-HT3AB Receptors in Fluorescence-Based Cellular Assays.
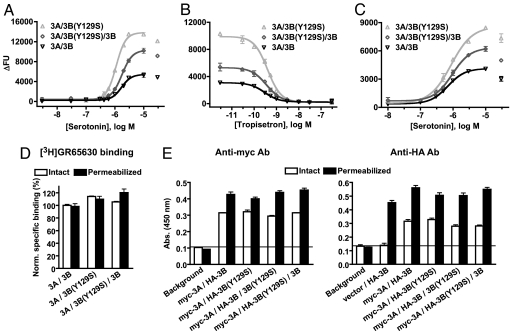
Initial characterization of heteromeric 5-HT3AB receptors containing the WT or the Y129S variant of 5-HT3B was performed in the FLIPR membrane potential (FMP) assay. tsA-201 (tsA) cells transiently coexpressing 5-HT3A and 5-HT3B(Y129S) displayed a substantially increased maximal response to serotonin (284 ± 17%; n = 3; P < 0.01) compared with cells expressing the WT 5-HT3AB receptor (100%) (Fig. 1A). To mimic the signaling of 5-HT3AB receptors in heterozygous individuals, the 5-HT3A subunit was coexpressed with a 1:1 mixture of WT 5-HT3B and 5-HT3B(Y129S). The maximal response to serotonin of these “heterozygous” receptors was intermediate to those of the WT and the “homozygous” 5-HT3AB(Y129S) receptors (172 ± 17%; n = 3; P < 0.05, compared with WT 5-HT3AB). The potency of serotonin at the 5-HT3AB receptors was not affected by the presence of the 5-HT3B(Y129S) subunit, with pEC50 values (mean ± SEM, n = 3) being 5.71 ± 0.03, 5.90 ± 0.03, and 5.74 ± 0.03 for WT 5-HT3AB, 5-HT3AB(Y129S), and “heterozygous” receptors, respectively. Furthermore, the competitive 5-HT3 receptor antagonist tropisetron displayed similar inhibitory potency at the three receptor combinations, with pKi values (mean ± SEM, n = 3) being 9.92 ± 0.03, 9.82 ± 0.02, and 9.80 ± 0.02, respectively (Fig. 1B).
Fig. 1.
The 5-HT3B(Y129S) variant augments the serotonin-induced maximal signaling of the heteromeric 5-HT3AB receptor. tsA cells were transiently transfected with human 5-HT3A and human 5-HT3B, 5-HT3B(Y129S), or a 1:1 mixture of 5-HT3B and 5-HT3B(Y129S) (A/B ratio 1:4). (A–C) The concentration–response curves for serotonin in the FMP assay (A), the concentration–inhibition curves for tropisetron in the FMP assay (B), and the concentration–response curves for serotonin in the [Ca2+]i assay (in the presence of 15 mM extracellular Ca2+) (C). ΔFU is the peak fluorescent response after application of serotonin minus baseline fluorescence. (D) Specific [3H]GR65630 binding to tsA cells transfected as described for the functional assays. Specific surface binding was determined by using intact cells, whereas specific binding to surface expressed and intracellular receptors was determined by using cells permeabilized with saponin. (E) Expression levels of myc-5-HT3A and HA-5-HT3B subunits in tsA cells (transfected with A/B cDNA ratios 1:4) determined in ELISA experiments (absorbance reading at 450 nm). Surface expressions were determined by using intact cells, whereas total expressions were determined in Triton X-100-permeabilized cells. All graphs display data from a representative experiment, and each data point or bar represents the mean ± SEM of duplicate or triplicate measurements.
5-HT3AB receptor signaling also was investigated in an intracellular Ca2+ ([Ca2+]i) assay by using Fluo-4 as the Ca2+ probe. The fluorescent response of 5-HT3AB receptors to 30 μM serotonin was very low by using an assay buffer containing 2 mM Ca2+, but increased with increasing extracellular Ca2+, with a maximum observed at 15 mM Ca2+ (SI Fig. 6A). At all Ca2+ concentrations tested, the 5-HT3B(Y129S)-containing receptors displayed higher maximal responses to serotonin than WT receptors, analogously to what was observed by using the FMP assay. Concentration–response relationships for serotonin were determined for the three 5-HT3AB receptor combinations in the presence of 15 mM Ca2+ (Fig. 1C). 5-HT3AB(Y129S) receptors displayed a substantially increased maximal response to serotonin (251 ± 76%; n = 4; P < 0.05) compared with cells expressing the 5-HT3AB receptor (100%), whereas the response of “heterozygous” receptors was nonsignificantly elevated compared with that of WT 5-HT3AB (202 ± 53%; n = 4). As observed in the FMP assay, the potency of serotonin at the 5-HT3AB receptors was not affected by the presence of the 5-HT3B(Y129S) subunit, with pEC50 values (mean ± SEM, n = 4) in the [Ca2+]i assay being 6.06 ± 0.08, 5.95 ± 0.11, and 5.92 ± 0.06 for WT 5-HT3AB, 5-HT3AB(Y129S), and “heterozygous” receptors, respectively. Similarly, the inhibitory potency of tropisetron at the 5-HT3AB receptors was unaffected by the 5-HT3B(Y129S) subunit in the [Ca2+]i assay (SI Fig. 6B).
Expression Analysis of 5-HT3AB Receptors.
The remarkable difference in the maximal responses to serotonin exhibited by the 5-HT3AB and 5-HT3AB(Y129S) receptors could potentially arise from differences in expression levels of the receptors. Hence, we determined the specific [2+H]GR65630 binding to intact and permeabilized tsA cells expressing the three 5-HT3AB receptor combinations (Fig. 1D). Specific binding was normalized to the specific binding of intact cells expressing WT 5-HT3AB receptors. Specific surface binding of 5-HT3AB(Y129S) and “heterozygous” receptors were 124 ± 12% (n = 3, P < 0.05, compared with WT 5-HT3AB) and 104 ± 8%, respectively, and the levels of specific binding to permeabilized cells were similar for all three combinations (n = 3, P = 0.17) (103 ± 6%, 133 ± 21%, and 114 ± 12% for cells expressing WT 5-HT3AB, 5-HT3AB(Y129S), and “heterozygous” receptors, respectively). We also quantified surface and total expression levels of myc-tagged 5-HT3A and HA-tagged 5-HT3B subunits transiently expressed in tsA cells by using an ELISA (Fig. 1E and Table 1). No differences in surface expression of the HA-tagged 5-HT3B subunits in cells transfected with myc-5-HT3A/HA-5-HT3B and myc-5-HT3A/HA-5-HT3B(Y129S) were observed. The surface expression of the “heterozygous” receptor combinations myc-5-HT3A/HA-5-HT3B/5-HT3B(Y129S) and myc-5-HT3A/5-HT3B/HA-5-HT3B(Y129S) also displayed similar surface levels of the HA-tagged subunits (Table 1). The total expression levels of HA-tagged 5-HT3B subunits (i.e., surface-expressed and intracellular receptors) in cells expressing the four different combinations of subunits were similar (n = 4, P = 0.17) (Table 1). The surface and total expression levels of myc-5-HT3A were similar in all four transfections (n = 4, P = 0.68; and n = 4, P = 0.46, respectively) (Table 1).
Table 1.
Surface and total expression of HA-5-HT3B and HA- 5-HT3B(Y129S) subunits coexpressed with myc-5-HT3A
| Antibody/receptor | Surface, % (mean ± SEM) | Total, % (mean ± SEM) |
|---|---|---|
| Anti-myc | ||
| myc-5-HT3A/HA-5-HT3B | 100 | 179 ± 26 |
| myc-5-HT3A/HA-5-HT3B(Y129S) | 100 ± 12 | 169 ± 37 |
| myc-5-HT3A/HA-5-HT3B/5-3B(Y129S) | 98 ± 16 | 193 ± 33 |
| myc-5-HT3A/HA-5-HT3B(Y129S)/3B | 92 ± 16 | 179 ± 30 |
| Anti-HA | ||
| vector/HA-5-HT3B | 11 ± 3 | 199 ± 21 |
| myc-5-HT3A/HA-5-HT3B | 100 | 233 ± 33 |
| myc-5-HT3A/HA-5-HT3B(Y129S) | 95 ± 7 | 231 ± 27 |
| myc-5-HT3A/HA-5-HT3B/5-3B(Y129S) | 83 ± 5 | 229 ± 24 |
| myc-5-HT3A/HA-5-HT3B(Y129S)/3B | 72 ± 5 | 208 ± 32 |
Expression levels of myc-5-HT3A are normalized to the surface level of myc-5-HT3A when coexpressed with HA-5-HT3B. Expression levels of HA-tagged subunits are normalized to the surface level of HA-5-HT3B when coexpressed with myc-5-HT3A. n = 4 independent experiments performed in triplicate.
Electrophysiological Characterization of 5-HT3AB and 5-HT3AB(Y129S) Receptors.
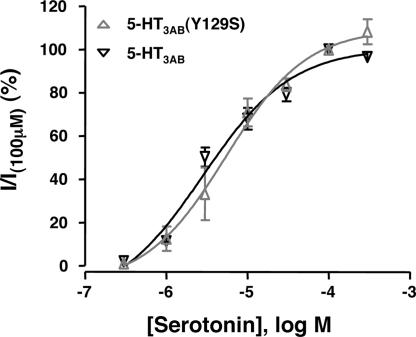
A 1-s application of 0.3–300 μM serotonin caused a concentration-dependent activation of currents recorded from whole cells expressing either WT 5-HT3AB or 5-HT3AB(Y129S) receptors. There was no significant difference in agonist potency between 5-HT3AB receptors (pEC50 = 5.55 ± 0.29, Hill slope = 0.8 ± 0.4) and 5-HT3AB(Y129S) receptors (pEC50 = 5.26 ± 0.1, Hill slope = 0.9 ± 0.2) (Fig. 2). There was no difference in the current density after a 1-s application of 300 μM (536 ± 62 pA pF−1; n = 6 and 474 ± 165 pA pF−1; n = 6; P = 0.73) for the WT and variant 5-HT3AB receptors, respectively, suggesting that the presence of the 5-HT3B(Y129S) subunit does not alter cell surface levels of functional 5-HT3AB receptors.
Fig. 2.
Concentration–response curves for serotonin as determined in whole-cell recordings from HEK293 cells transfected with 5-HT3A cDNA together with either 5-HT3B or 5-HT3B(Y129S) cDNA. Current amplitudes (I) are expressed as a percentage of those evoked at the respective receptors by application of 100 μM serotonin. Data points are mean ± SEM from three to six cells.
Kinetic Properties of 5-HT3AB and 5-HT3AB(Y129S) Receptors.
To determine whether the 5-HT3B(Y129S) subunit changed the kinetic properties of the 5-HT3AB receptor, we measured the time courses of activation and relaxation after rapid application of a high concentration of serotonin (300 μM) to whole cells expressing WT and variant 5-HT3AB receptors.
In the whole-cell recording configuration, no difference was observed in the time constants for activation of 5-HT3AB (4 ± 1 ms; n = 3) and 5-HT3AB(Y129S) receptors (5 ± 2 ms; n = 3) when activated by a saturating serotonin concentration (300 μM). Similar activation time constants for 5-HT3AB receptors were measured from outside-out patches (3 ± 1 ms; n = 3), significantly slower than an open-tip solution-exchange time (0.2 ± 0.1 ms; n = 3), suggesting that the whole-cell times are not limited by solution exchange times and are a true estimate of receptor activation.
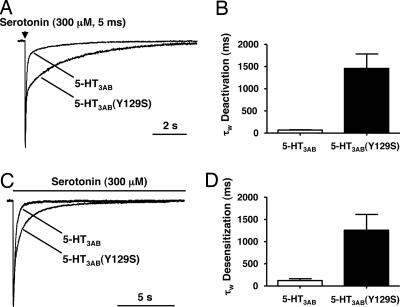
Postsynaptic receptors are exposed to brief but high concentrations of agonists. To mimic this event, we rapidly applied 300 μM serotonin for 5 ms to whole cells. Deactivation for 5-HT3AB receptors was biexponential, whereas the deactivation for 5-HT3AB(Y129S) receptors was best fitted to three exponentials (Fig. 3A). For comparison purposes, we examined the weighted average time constant (τw) of the two receptors. Receptors containing the 5-HT3B(Y129S) polymorphism deactivated ≈20-fold slower (1,455 ± 329 ms; n = 3) than WT 5-HT3AB receptors (67 ± 8 ms; n = 3; P = 0.01) (Fig. 3B). Desensitization during long exposure periods of high serotonin concentration (300 μM) was monoexponential in WT 5-HT3AB receptors, whereas it was multiexponential in 5-HT3B(Y129S) receptors (Fig. 3C). The weighted average time constant of desensitization for 5-HT3AB(Y129S) receptors (1,258 ± 354 ms; n = 3) was significantly slower than that for WT receptors (120 ± 44 ms; n = 3; P = 0.03) (Fig. 3D).
Fig. 3.
The 5-HT3B(Y129S) variant slows deactivation and desensitization of the 5-HT3AB receptor as determined in whole-cell recordings. (A) Examples of normalized average currents evoked by 300 μM serotonin applied for 5 ms to HEK293 cells expressing 5-HT3AB and 5-HT3AB(Y129S) receptors. (B) Bar graph showing the ≈20-fold-slower time constant for deactivation in receptors incorporating the 5-HT3B(Y129S) subunit compared with WT (n = 3). (C) Normalized currents from 5-HT3AB and 5-HT3AB(Y129S) channels showing the different decay times in the continued presence of 300 μM serotonin. (D) Desensitization is slowed ≈10-fold in 5-HT3AB(Y129S) receptors compared with 5-HT3AB receptors (n = 3).
Single-Channel Measurements of 5-HT3AB and 5-HT3AB(Y129S) Receptors.
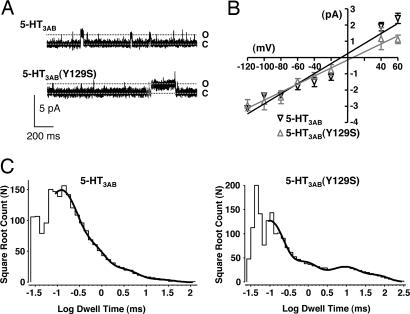
We examined the effects of the 5-HT3B(Y129S) variant on single-channel conductance and open-dwell times recorded from cell-attached patches (Fig. 4). For each patch, the unitary current amplitudes were obtained from all-points histograms and plotted against membrane potential (Fig. 4B). There was no change in the slope conductance, with WT 5-HT3AB and 5-HT3AB(Y129S) channels having conductances of 30 ± 2 pS (n = 6) and 25 ± 3 pS (n = 7), respectively (P = 0.13).
Fig. 4.
Single-channel properties of WT 5-HT3AB and 5-HT3AB(Y129S) channels. (A) Examples of single-channel recordings from HEK293 cells expressing either WT 5-HT3AB (Upper) or 5-HT3AB(Y129S) channels (Lower). Recordings made from cell-attached patches in the presence of 300 μM serotonin at a membrane potential of −60 mV. (B) Current–voltage relationship of WT 5-HT3AB and 5-HT3AB(Y129S) channels with slope conductance derived from the linear regression. (C) Histograms of open dwell times for open-channel events pooled from multiple patches at a membrane potential of −60 mV. The distribution was best described by the sum of four exponentials (thick dark line). Open times and their relative proportions are listed in Table 2.
Examination of the influence of the 5-HT3B(Y129S) variant on the distribution of single-channel open-dwell times at a predicted membrane potential of −60 mV showed that both the 5-HT3AB(Y129S) and WT open channels were best described by four exponential components (Fig. 4C). The mean open duration of 5-HT3AB(Y129S) channels (5.1 ms) was prolonged 7-fold compared with WT channels (0.74 ms). This change was due to the prolonged durations of τ3 and, more particularly, τ4 (Table 2).
Table 2.
Single-channel open dwell-time distributions of WT and 5-HT3B(Y129S) containing 5-HT3AB receptors in the presence of 300 μM serotonin
| Receptor | τ 1, ms (%) | τ 2, ms (%) | τ 3, ms (%) | τ 4, ms (%) |
|---|---|---|---|---|
| WT 5-HT3AB | 0.1 ± 0.05 (60 ± 2) | 0.42 ± 0.1 (28 ± 2) | 2.4 ± 0.2 (10 ± 1) | 15.8 ± 0.5 (2 ± 1) |
| 5-HT3AB(Y129S) | 0.1 ± 0.05 (60 ± 2) | 0.64 ± 0.12 (19 ± 2) | 7.7 ± 0.2 (13 ± 2) | 48.4 ± 0.2 (8 ± 2) |
The time constants of each component and its relative proportion (as percentage in parentheses) are given as mean ± SEM. The data contained 207,009 events (5-HT3AB channels) or 176,605 events [5-HT3AB(Y129S) channels].
Probing the Functional Importance of Residue 129 in the 5-HT3B Subunit of 5-HT3AB Receptors.
To elucidate the role of residue 129 in 5-HT3B for 5-HT3AB receptor signaling further, nine additional 5-HT3B(Y129X) mutants were constructed, and the functional properties of the mutant 5-HT3AB receptors were characterized in the FMP assay (Table 3). The maximal responses to serotonin exhibited by these mutant receptors varied considerably. Interestingly, of the 11 different 5-HT3AB receptor combinations studied, the lowest maximal response was observed for the WT 5-HT3AB receptor. The mutant 5-HT3AB(Y129W) displayed a slightly increased (2.5-fold) serotonin potency compared with that of the WT 5-HT3AB receptor, whereas the other nine mutants displayed similar EC50 values for serotonin as the WT receptor (Table 3).
Table 3.
The serotonin-mediated 5-HT3AB(Y129X) receptor signaling determined in the FMP assay
| Receptor | Maximal response, % (mean ± SEM) | pEC50 (mean ± SEM) |
|---|---|---|
| 5-HT3AB | 100 | 5.95 ± 0.06 |
| 5-HT3AB(Y129S) | 197 ± 8 | 6.04 ± 0.04 |
| 5-HT3AB(Y129C) | 171 ± 8 | 5.91 ± 0.04 |
| 5-HT3AB(Y129D) | 222 ± 11 | 6.12 ± 0.03 |
| 5-HT3AB(Y129A) | 165 ± 7 | 6.09 ± 0.06 |
| 5-HT3AB(Y129H) | 190 ± 17 | 6.04 ± 0.03 |
| 5-HT3AB(Y129L) | 173 ± 7 | 6.08 ± 0.04 |
| 5-HT3AB(Y129F) | 174 ± 13 | 6.03 ± 0.05 |
| 5-HT3AB(Y129N) | 145 ± 6 | 5.94 ± 0.10 |
| 5-HT3AB(Y129E) | 194 ± 34 | 5.85 ± 0.08 |
| 5-HT3AB(Y129W) | 218 ± 25 | 6.34 ± 0.03 |
Maximal responses are normalized to that of WT 5-HT3AB.
Discussion
In the present study, we investigated the expression and signaling properties of the human 5-HT3AB receptor containing a variant 5-HT3B subunit, 5-HT3B(Y129S). The variant is highly interesting because it is encoded by a worldwide high-frequency polymorphism, and heterozygous individuals (Y/S) as well as individuals homozygous for the variation (S/S) have been detected in high numbers in multiple ethnic groups. In fact, in three of five populations investigated, the number of carriers of the 5-HT3B(Y129S) variation equals or exceeds the number of Y/Y individuals (SI Fig. 5B).
The substantially increased maximal responses to serotonin exhibited by 5-HT3B(Y129S)-containing 5-HT3AB receptors (“homozygous” and “heterozygous”) compared with WT 5-HT3AB receptors in the FMP and [Ca2+]i assays demonstrate that the polymorphism has a pronounced effect on 5-HT3AB signaling (Fig. 1). The similar potencies displayed by serotonin at WT and 5-HT3B(Y129S)-containing receptors in the fluorescence-based assays were confirmed in electrophysiological recordings (Fig. 2). The fact that neither serotonin nor the competitive 5-HT3 antagonist tropisetron exhibit different potencies at WT and 5-HT3B(Y129S)-containing 5-HT3AB receptors suggests that the observed difference between the maximal responses to serotonin is not caused by a change in the orthosteric site of the variant receptor (Fig. 1 and SI Fig. 6). Furthermore, the functional differences between WT and variant 5-HT3AB receptors do not appear to arise from different surface expression levels because the receptors were found to be expressed at similar levels in the [2+H]GR65630-binding assay and the ELISA experiments and displayed similar whole-cell current densities. Thus, the increased maximal responses to serotonin displayed by the 5-HT3B(Y129S)-containing receptors seem to arise from changes in the conductance or the gating kinetics of the receptor.
The electrophysiological measurements at WT and variant 5-HT3AB receptors revealed that the augmented signaling of 5-HT3AB(Y129S) receptors in the fluorescence-based assays most likely can be ascribed to a dramatic slowing of its deactivation and desensitization kinetics (Fig. 3). The prolonged time of relaxation may be due to the observed increase in the mean open time of 5-HT3AB(Y129S) channels compared with WT channels (Fig. 4). The four exponential components needed to describe the open distributions indicate that there should be at least four separate open states in any future kinetic scheme.
Mutating residue 129 in 5-HT3B to 10 different amino acid residues showed that this position is indeed important for the 5-HT3AB receptor signaling (Table 3). The EC50 values of serotonin at all 5-HT3AB(Y129X) receptors were similar to those at the WT receptor, further substantiating that the binding of serotonin to the 5-HT3AB receptor is unaffected by the identity of residue 129 in 5-HT3B. An alignment of the amino acid sequences of human 5-HT3B and the ACh-binding protein of Lymnaea stagnalis (22) suggests that residue 129 is located within the β5 strand, and thus it is situated at or in close proximity to the A/B and B/B subunit interfaces of the 5-HT3AB receptor. Although it is tempting to speculate that the residue may affect gating kinetics through an involvement in intersubunit interactions, further investigations into the molecular architecture of this particular region of the 5-HT3AB receptor are needed to address this issue.
In view of the kinetic properties displayed by 5-HT3B(Y129S)-containing 5-HT3AB receptors in this study, we propose that individuals carrying the variant allele may experience alterations in neurotransmitter release and/or neuronal excitability. Thus, it is interesting that a recent article reported an association between female major depression and a haplotype block of seven polymorphisms in HTR3B, including the Y129S polymorphism (20). Among these polymorphisms, 5-HT3B(Y129S) was the only one encoding an amino acid change. Y/Y individuals were found in a significantly higher frequency in a group of women diagnosed with major depression than in the control group. Thus, the variant 5-HT3B(Y129S) allele may be protective against the development of depression (20). In the present study, we offer a possible explanation for this finding because the maximal responses of both homozygous and heterozygous 5-HT3AB(Y129S) receptors were substantially increased compared with that of the WT receptor. The other six polymorphisms of the HTR3B haplotype block were either located to introns or were silent variations in the coding region, and the possibility that these could have in vivo effects on 5-HT3 receptor signaling cannot be completely ruled out. However, the polymorphism encoding a change in the amino acid sequence 5-HT3B(Y129S) seems the most likely to cause an effect on 5-HT3AB receptor signaling. Considering the importance of the serotonergic system in depression and for the medical treatment of it, this link between a variant serotonin receptor with pronounced augmented signaling characteristics and depression is highly interesting. In a previous study (23), a polymorphism in the regulatory region of HTR3A shown to increase gene expression (24) was found to affect the improvement observed in depressed patients upon paroxetine treatment. In addition, other studies have suggested a link between 5-HT3 receptor activation and the treatment/etiology of depression (5, 25–27). Our data in combination with the association study by Yamada et al. (20) suggest that the role of the 5-HT3AB receptor in the etiology and treatment of depression may be interesting to investigate in greater detail.
In contrast to the limited insight into the roles of 5-HT3 receptors in the CNS, their physiological functions in the PNS are somewhat better understood. For example, the receptor is a well established target in the treatment of nausea and emesis. The association between the 5-HT3B(Y129S) polymorphism and various forms of nausea and/or emesis (induced by selective serotonin-reuptake inhibitors or chemotherapy) has been investigated with divergent results (21, 28, 29). Future studies are required to determine the possible contributions of the 5-HT3B(Y129S) polymorphism to the development and/or the treatment efficacy of the nausea and emesis known to be common side effects of radio/chemotherapy, anesthetics, and antidepressants.
In conclusion, we have characterized a naturally occurring variation of HTR3B, 5-HT3B(Y129S), which occurs with a high frequency in the general population. When incorporated into heteromeric 5-HT3AB receptors, the 5-HT3B(Y129S) subunit conferred a profound increase in the time constants of deactivation and desensitization. This delay in relaxation was possibly due to the observed increase in mean open time of the 5-HT3B(Y129S)-containing channel. WT and variant 5-HT3AB receptors did not display any essential differences in ligand potencies, conductances, or expression levels. Our findings may provide a functional explanation to the reported higher prevalence of female major depression in Y/Y individuals compared with carriers of the 5-HT3B(Y129S) allele. The 5-HT3B(Y129S) polymorphism may further affect the severity and the treatment of other indications involving the 5-HT3AB receptor. This aspect, coupled with the high prevalence of the polymorphism in the general population, calls for a number of association studies in a search for a phenotype of the 5-HT3B(Y129S) polymorphism. Such studies could shed light on the roles of the 5-HT3AB receptor in general physiology and pathology.
Materials and Methods
Chemicals and Drugs.
Serotonin, tropisetron, and quipazine were purchased from Sigma–Aldrich, whereas [2+H]GR65630 was purchased from PerkinElmer.
Molecular Biology.
The cDNAs of human 5-HT3A (GenBank accession no. NM_000869) and 5-HT3B (GenBank accession no. NM_006028) were kind gifts from J. Egebjerg (H. Lundbeck A/S, Copenhagen, Denmark) and E. F. Kirkness (J. Craig Venter Institute, Rockville, MD), respectively. The generation of the myc-5-HT3A construct has been described previously (30). The HA epitope was inserted in 5-HT3B between residues P27 and Q28. The Y129X mutations were introduced into 5-HT3B by using the QuikChange Site-Directed Mutagenesis Kit (Stratagene). All cDNAs were in the pCI-neo vector. The fidelity of all cDNAs was verified by DNA sequencing.
Cell Culture and Transfection. FMP and [Ca2+]i assays, [3H]GR65630-binding assay, and ELISA.
tsA cells were maintained in GlutaMAX-I DMEM supplemented with 10% dialyzed FBS, 100 units·ml−1 penicillin, and 100 μg·ml−1 streptomycin in a humidified atmosphere of 5% CO2 at 37°C. Exponentially growing tsA cells were transfected with 5-HT3A and 5-HT3B cDNAs (1:4 ratio) by using SuperFect (Qiagen) according to the manufacturer's protocol, and the cells were used for experiments 40–48 h after transfection.
Electrophysiological recordings.
HEK293 cells, cultured as described for the tsA cells, were transfected by using the calcium phosphate precipitation method with 5-HT3A and 5-HT3B cDNAs (1:1 ratio), along with GFP cDNA. Cells were used for experiments 24–72 h after transfection.
FMP Assay.
Twenty-four hours after transfection, tsA cells were plated into poly-d-lysine-coated black 96-well plates with clear bottoms (BD Biosciences) (7 × 104 cells per well). The following day, cells were loaded with 0.3 mg·ml−1 FMP Blue dye (Molecular Devices) in assay buffer [HBSS with 2 mM CaCl2, 0.5 mM MgCl2, and 20 mM Hepes (pH 7.4)] at 37°C for 30 min. In the tropisetron experiments, the antagonist was added to the cells together with the dye. The cellular responses to serotonin addition were assayed at 30°C in a NOVOstar plate reader (BMG Labtechnologies) by using excitation/emission at 530/560 nm. Experiments were performed in duplicate.
[Ca2+]i Assay.
The assay was performed as the FMP assay until dye loading. Cells were loaded with 4 μM Fluo-4 AM (Molecular Probes) in the presence of 0.04% Pluronic F-127 (Molecular Probes) in assay buffer containing 2.5 mM probenecid (Sigma–Aldrich). After 1 h incubation at 37°C, cells were washed once with probenecid-containing assay buffer, and 100 μl of this buffer was added to each well (in the tropisetron experiments, the antagonist was added at this point). The cellular responses to serotonin addition were determined in a NOVOstar plate reader by using excitation/emission at 485/520 nm. Experiments were performed in duplicate.
[3H]GR65630-Binding Assay.
The assay was performed essentially as described previously (30) by using a saturating final concentration of 5 nM [2+H]GR65630 in a volume of 0.6 ml containing 3 × 105 cells. Ten micromolar quipazine was used for the determination of nonspecific binding. Specific binding was determined to intact as well as to permeabilized (saponin-treated) cells. All experiments were performed in duplicate.
Quantification of 5-HT3AB Expression by ELISA.
The ELISA was performed essentially as described previously (30) by using tsA cells transfected with myc-5-HT3A and HA-5-HT3B constructs (A/B cDNA ratio, 1:4) and by using mouse anti-myc antibody (Invitrogen) or mouse anti-HA antibody (Nordic Biosite) as primary antibodies (both diluted 1:1,000). All experiments were performed in triplicate.
Electrophysiological Measurements.
Macroscopic currents.
Culture media were replaced by an extracellular solution [140 mM NaCl, 4.7 mM KCl, 1.2 mM MgCl2, 2.5 mM CaCl2, 11.0 mM glucose, and 10 mM Hepes (pH 7.4)]. Patch electrodes were filled with intracellular recording solution [140 mM KCl, 2.0 mM MgCl2, 11 mM EGTA, and 10 mM Hepes (pH 7.4)]. Currents were recorded by using an Axopatch 200B amplifier (Molecular Devices), low-pass-filtered at 1–2 kHz and digitized at 10 kHz with a digidata 1320 A/D converter. 5-HT was rapidly applied to cells via a piezo-driven pipette that achieves nominal whole-cell solution exchange times of 2 ± 0.2 ms (n = 3) as measured by using a change in potassium-driving force through endogenous voltage-activated potassium channels as previously described (31). Experiments were performed at room temperature with the cells voltage-clamped at −60 mV. For the concentration-response determinations, serotonin was pressure-applied for 1 s to cells via a modified micropipette.
Single-channel recordings.
Cell-attached patches were formed on HEK293 cells expressing 5-HT3AB receptors. Cells were bathed in an extracellular recording solution [140 mM KCl, 4.7 mM NaCl, 1.2 mM MgCl2, 2.5 mM CaCl2, 11.0 mM glucose, and 10 mM Hepes (pH 7.4)]. The high potassium-recording solution effectively neutralizes the transmembrane potential. Sylgard-coated electrodes were filled with extracellular solution containing 300 μM serotonin. Single-channel currents were low-pass-filtered at 5 kHz and digitized at 40 kHz.
Data Analysis.
Concentration–response relationships.
Responses from the functional assays were calculated as the difference in fluorescent units (ΔFU) between peak fluorescence after serotonin addition and the baseline fluorescence. In the electrophysiological recordings, peak amplitudes of 5-HT-evoked responses were normalized to 100 μM serotonin. Concentration–response data were fitted by using nonlinear regression to a sigmoidal curve with a variable slope by using Graphpad Prism version 4 (GraphPad). Ki values were calculated from the inhibition curves by using the functional equivalent of the Cheng–Prusoff equation (32): Ki = IC50/(1+[agonist]/EC50).
Macroscopic currents.
Time constants for activation, deactivation, and desensitization were determined by fitting exponential functions to the corresponding component of the current wave form by using the Levenberg–Marquardt algorithm with least squares minimization (Clampfit Ver. 9.2; Molecular Devices). The activation phase of the macroscopic current was fitted by a single exponential function. Some decay components were best fit to two exponentials, but others were fitted by one or more than two exponentials. To allow for comparisons, weighted time constants (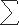 τnAn/
τnAn/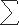 An) were calculated, where τn and An are the time constants and amplitudes of each component (n).
An) were calculated, where τn and An are the time constants and amplitudes of each component (n).
Single-channel recordings.
Single-channel currents were analyzed by using Clampfit version 9.2. All-points histograms were fitted with multiple Gaussians (least squares minimization) to determine the unitary current amplitudes. The slope conductance was determined from the unitary current amplitudes from patches where the pipette potential ranged from 120 to −60 mV.
Single-channel events were detected by using the 50% threshold-detection method (33). From the single-channel events list, histograms of channel open-dwell time distributions were plotted and fitted by using a maximum-likelihood procedure employing correction for missed events (34). The minimum number of exponentials required to fit the distribution was determined by χ2 statistics. A dead time of 0.09 ms was imposed on the fitting routine. Mean open durations were calculated from the fitted components of the open-dwell time histograms as a weighted mean from the durations and proportions of each component.
Statistical analysis.
Data from electrophysiological recordings were analyzed post hoc by using Graphpad Prism version 4. Statistical analysis was performed by using Student's t test, with statistical significance set at P < 0.05. Statistical analysis of data from the FMP and [Ca2+]i assays, the [2+H]GR65630-binding assay, and the ELISA experiments was performed by using GraphPad InStat version 3 (GraphPad). A one-way ANOVA was performed as a randomized complete block design when blocking was effective. The null hypothesis was rejected at P < 0.05, and the differences between means were analyzed by Bonferroni's multiple comparisons test for selected pairs of means.
Supplementary Material
ACKNOWLEDGMENTS.
We thank Dr. Jan Egebjerg for inspiring discussions. This work was supported by the Center for Pharmacogenomics, Denmark (K.K. and H.B.-O.), the Lundbeck Foundation and the Danish Medical Research Council (A.A.J.), and the Department of Anesthesia and Critical Care (P.A.D. and P.L.F.-Z.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0708454105/DC1.
References
- 1.Lucki I. Biol Psychiatry. 1998;44:151–162. doi: 10.1016/s0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- 2.Hoyer D, Hannon JP, Martin GR. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- 3.Turner TJ, Mokler DJ, Luebke JI. Neuroscience. 2004;129:703–718. doi: 10.1016/j.neuroscience.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 4.Funahashi M, Mitoh Y, Matsuo R. Methods Find Exp Clin Pharmacol. 2004;26:615–622. doi: 10.1358/mf.2004.26.8.863726. [DOI] [PubMed] [Google Scholar]
- 5.Dremencov E, Weizmann Y, Kinor N, Gispan-Herman I, Yadid G. Curr Drug Targets. 2006;7:165–175. doi: 10.2174/138945006775515491. [DOI] [PubMed] [Google Scholar]
- 6.Sugita S, Shen KZ, North RA. Neuron. 1992;8:199–203. doi: 10.1016/0896-6273(92)90121-s. [DOI] [PubMed] [Google Scholar]
- 7.Färber L, Haus U, Späth M, Drechsler S. Scand J Rheumatol Suppl. 2004:2–8. [PubMed] [Google Scholar]
- 8.Thompson AJ, Lummis SC. Expert Opin Ther Targets. 2007;11:527–540. doi: 10.1517/14728222.11.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reeves DC, Lummis SC. Mol Membr Biol. 2002;19:11–26. doi: 10.1080/09687680110110048. [DOI] [PubMed] [Google Scholar]
- 10.Miyake A, Mochizuki S, Takemoto Y, Akuzawa S. Mol Pharmacol. 1995;48:407–416. [PubMed] [Google Scholar]
- 11.Davies PA, Pistis M, Hanna MC, Peters JA, Lambert JJ, Hales TG, Kirkness EF. Nature. 1999;397:359–363. doi: 10.1038/16941. [DOI] [PubMed] [Google Scholar]
- 12.Niesler B, Frank B, Kapeller J, Rappold GA. Gene. 2003;310:101–111. doi: 10.1016/s0378-1119(03)00503-1. [DOI] [PubMed] [Google Scholar]
- 13.Tecott LH, Maricq AV, Julius D. Proc Natl Acad Sci USA. 1993;90:1430–1434. doi: 10.1073/pnas.90.4.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brady CA, Dover TJ, Massoura AN, Princivalle AP, Hope AG, Barnes NM. Neuropharmacology. 2007;52:1284–1290. doi: 10.1016/j.neuropharm.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Doucet E, Latremoliere A, Darmon M, Hamon M, Emerit MB. Eur J Neurosci. 2007;26:355–366. doi: 10.1111/j.1460-9568.2007.05659.x. [DOI] [PubMed] [Google Scholar]
- 16.Niesler B, Walstab J, Combrink S, Möller D, Kapeller J, Rietdorf J, Bönisch H, Göthert M, Rappold G, Brüss M. Mol Pharmacol. 2007;72:8–17. doi: 10.1124/mol.106.032144. [DOI] [PubMed] [Google Scholar]
- 17.Barrera NP, Herbert P, Henderson RM, Martin IL, Edwardson JM. Proc Natl Acad Sci USA. 2005;102:12595–12600. doi: 10.1073/pnas.0503253102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Combi R, Dalprà L, Tenchini ML, Ferini-Strambi L. J Neurol. 2004;251:923–934. doi: 10.1007/s00415-004-0541-x. [DOI] [PubMed] [Google Scholar]
- 19.Lynch JW. Physiol Rev. 2004;84:1051–1095. doi: 10.1152/physrev.00042.2003. [DOI] [PubMed] [Google Scholar]
- 20.Yamada K, Hattori E, Iwayama Y, Ohnishi T, Ohba H, Toyota T, Takao H, Minabe Y, Nakatani N, Higuchi T, et al. Biol Psychiatry. 2006;60:192–201. doi: 10.1016/j.biopsych.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 21.Sugai T, Suzuki Y, Sawamura K, Fukui N, Inoue Y, Someya T. Pharmacogenomics J. 2006;6:351–356. doi: 10.1038/sj.tpj.6500382. [DOI] [PubMed] [Google Scholar]
- 22.Brejc K, van Dijk WJ, Klaassen RV, Schuurmans M, van Der Oost J, Smit AB, Sixma TK. Nature. 2001;411:269–276. doi: 10.1038/35077011. [DOI] [PubMed] [Google Scholar]
- 23.Kato M, Fukuda T, Wakeno M, Fukuda K, Okugawa G, Ikenaga Y, Yamashita M, Takekita Y, Nobuhara K, Azuma J, et al. Neuropsychobiology. 2006;53:186–195. doi: 10.1159/000094727. [DOI] [PubMed] [Google Scholar]
- 24.Niesler B, Flohr T, Nöthen MM, Fischer C, Rietschel M, Franzek E, Albus M, Propping P, Rappold GA. Pharmacogenetics. 2001;11:471–475. doi: 10.1097/00008571-200108000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Varea E, Blasco-Ibánez JM, Gómez-Climent MA, Castillo-Gómez E, Crespo C, Martínez-Guijarro FJ, Nácher J. Neuropsychopharmacology. 2007;32:803–812. doi: 10.1038/sj.npp.1301183. [DOI] [PubMed] [Google Scholar]
- 26.Ishihara K, Sasa M. Neurosci Lett. 2001;307:37–40. doi: 10.1016/s0304-3940(01)01902-4. [DOI] [PubMed] [Google Scholar]
- 27.Faris PL, Eckert ED, Kim SW, Meller WH, Pardo JV, Goodale RL, Hartman BK. J Affect Disord. 2006;92:79–90. doi: 10.1016/j.jad.2005.12.047. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki Y, Sawamura K, Someya T. Neuropsychopharmacology. 2006;31:825–831. doi: 10.1038/sj.npp.1300919. [DOI] [PubMed] [Google Scholar]
- 29.Tremblay PB, Kaiser R, Sezer O, Rosler N, Schelenz C, Possinger K, Roots I, Brockmoller J. J Clin Oncol. 2003;21:2147–2155. doi: 10.1200/JCO.2003.05.164. [DOI] [PubMed] [Google Scholar]
- 30.Krzywkowski K, Jensen AA, Connolly CN, Bräuner-Osborne H. Pharmacogenet Genomics. 2007;17:255–266. doi: 10.1097/FPC.0b013e3280117269. [DOI] [PubMed] [Google Scholar]
- 31.Li X, Czajkowski C, Pearce RA. Anesthesiology. 2000;92:1366–1375. doi: 10.1097/00000542-200005000-00027. [DOI] [PubMed] [Google Scholar]
- 32.Craig DA. Trends Pharmacol Sci. 1993;14:89–91. doi: 10.1016/0165-6147(93)90070-z. [DOI] [PubMed] [Google Scholar]
- 33.Colquhoun D, Sakmann B. J Physiol. 1985;369:501–557. doi: 10.1113/jphysiol.1985.sp015912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colquhoun D, Sigworth FJ. In: Single-Channel Recording. Sakmann B, Neher E, editors. New York: Plenum; 1995. pp. 483–587. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.