Abstract
Studies have shown that α-synuclein (α-syn) deposited in Lewy bodies in brain tissue from patients with Parkinson disease (PD) is extensively phosphorylated at Ser-129. We used recombinant Adeno-associated virus (rAAV) to overexpress human wild-type (wt) α-syn and two human α-syn mutants with site-directed replacement of Ser-129 to alanine (S129A) or to aspartate (S129D) in the nigrostriatal tract of the rat to investigate the effect of Ser-129 phosphorylation state on dopaminergic neuron pathology. Rats were injected with rAAV2/5 vectors in the substantia nigra pars compacta (SNc) on one side of the brain; the other side remained as a nontransduced control. The level of human wt or mutant α-syn expressed on the injected side was about four times the endogenous rat α-syn. There was a significant reduction of dopaminergic neurons in the SNc and dopamine (DA) and tyrosine hydroxylase (TH) levels in the striatum of all S129A-treated rats as early as 4 wk postinjection. Nigral DA pathology occurred more slowly in the wt-injected animals, but by 26 wk the wt α-syn group lost nigral TH neurons equivalent to the mutated S129A group at 8 wk. In stark contrast, we did not observe any pathological changes in S129D-treated animals. Therefore, the nonphosphorylated form of S129 exacerbates α-syn-induced nigral pathology, whereas Ser-129 phosphorylation eliminates α-syn-induced nigrostriatal degeneration. This suggests possible new therapeutic targets for Parkinson Disease.
Keywords: AAV, dopamine, Lewy body, tyrosine hydroxylase
Parkinson disease is a progressive neurodegenerative disorder characterized by a loss of dopamine (DA)-expressing neurons in the substantia nigra pars compacta (SNc) and depletion of DA in the striatum. Aside from DA depletion, the major pathological hallmark in postmortem tissue from patients with Parkinson disease (PD) is the presence of the α-synuclein (α-syn)-positive Lewy bodies (LBs) and Lewy neurites in surviving neurons. α-syn is a 140-aa presynaptic and nuclear protein that has been implicated in early onset of PD in humans if its gene is triplicated or contains one of several missense mutations: A53T, A30P, or E46K (reviewed in ref. 1). The functions of α-syn and the events leading from α-syn expression to the selective degeneration of nigral neurons are not well understood. The genetic data from humans have been verified by experimental studies on various animal models, which demonstrated that targeted overexpression of human wild-type (wt) α-syn or the mutant forms in the SNc leads to Parkinson-like neurodegeneration (1–3). Studies have suggested that α-syn can form soluble oligomers and fibrillar species that may be precursors to Lewy bodies and are associated with the neurotoxicity seen in animals and humans. A number of studies have suggested that soluble oligomers or protofibrils are likely to be the toxic species and that fibrils and Lewy bodies may be protective by sequestering excess α-syn (4, 5).
Studies on α-syn knockout mice (6) and primary neurons (7) suggested that α-syn is involved in DA neurotransmission. α-syn appears to be associated with synaptic vesicles, and there is evidence that it regulates the size of the synaptic vesicular pool (7). Furthermore, α-syn can interact with presynaptic membranes, indicating that one of its functions may be in the regulation of DA release and reuptake (8–10). There is also evidence that α-syn can modulate expression of genes involved in DA synthesis (11) and can affect enzymes involved in chromatin remodeling (12) and signal transduction (13).
α-syn can undergo phosphorylation at several residues including Ser-129 (14, 15). Phosphorylation at Ser-129 is a specific marker of all α-synucleinopathy lesions including PD (16). Alteration of Ser-129 to the negatively charged aspartate to mimic phosphorylation and Ser-129 phosphorylation by G protein-coupled receptor kinase 2 significantly enhanced α-syn toxicity in the Drosophila model of Parkinson disease (17). In vitro studies of Ser1129 phosphorylation have shown that it leads to α-syn fibril formation (18), comparable to the effect of PD-associated mutations and oxidative stress.
In this study we examined the role of α-syn phosphorylation at Ser-129 in a mammalian model of PD. We demonstrated that recombinant adeno-associated virus (rAAV)-mediated α-syn expression in the SNc of rats and primates produced Parkinson-like neurodegeneration (2, 3). Here, we show that the S129A mutant is extremely toxic in the rat model, whereas constitutive phosphorylation of Ser-129 produces no toxicity even when α-syn is overexpressed.
Results
The pseudotype rAAV2/5 was used as the gene delivery vehicle because a comparison with the previously used rAAV 2 serotype demonstrated that serotype 5 capsids increased both the number and volume of neurons that were transduced (19). Rats were injected with equal numbers of rAAV vectors that expressed GFP, the human wild-type (wt) α-syn protein or human α-syn mutants containing substitutions of Ser-129 with alanine (S129A) or aspartate (S129D). Injections were done in the SNc only on one side of the brain to determine their effect on neurodegeration; the other side was kept as a nontransduced internal control. Animals were killed at 4, 8, and 26 wk after injections. These time points were selected based on our results (3), which demonstrated that expression of α-syn reached a maximum at 3–4 wk after nigral injection and neural degeneration became significant at 8–10 wk.
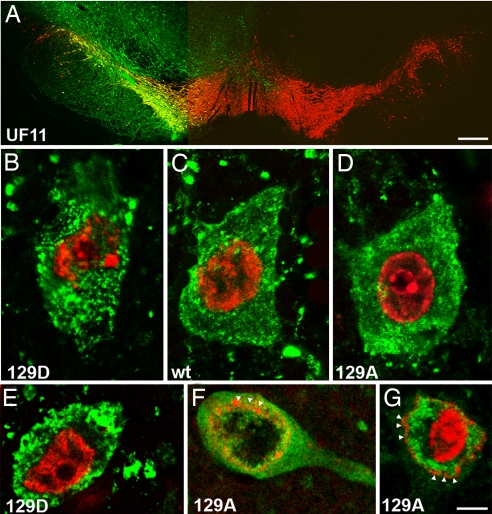
Control injections with vectors expressing GFP showed GFP-positive (GFP+) cell bodies in virtually all tyrosine hydroxylase positive neurons of the SNc (Fig. 1A). However, the transduction area of GFP+ neurons was not limited to the SNc, because there were GFP+ neurons in the mesencephalic reticular formation and in the ventral tegmental area (Fig. 1A). These data are in agreement with our findings that showed that rAAV5 exhibits higher transduction frequencies than rAAV2 (19).
Fig. 1.
Fluorescence microscopy of tyrosine hydroxylase (TH)-positive neurons expressing α-syn mutants. (A) Photomicrographs showing the expression of the GFP transgene on the injected side of rat brain. GFP (green) was expressed in the majority of the TH-positive neurons (red) in SNc and also can be seen in cells of the mesenphalic tegmentum and in the SN pars reticulata. (B–G) Confocal images illustrate α-syn expression in SNc neurons 4 (B–D) and 8 (E–G) wk after injection. In B–G, α-syn is stained green and nuclei are stained with propidium iodide (red). S129D is more prone to make punctate aggregates at 4 wk (B), becoming more dense and larger at 8 wk (E) compared with wt (C) and 129A (D, F, and G). (F and G) Examples of neurodegenerative changes in SNc neurons of 129A-injected animals at 8 wk that have diffuse α-syn immunoreactivity and vacuolated nuclei. containing both diffuse and aggregated α-syn. α-syn was found both inside and outside of the nuclear membrane (arrowheads indicate propidium iodide staining at the nuclear membrane). (Scale bar: A, 0.5 mm; B–G, 5 μm.)
The Level of Expression of Human α-Syn Was 3- to 5-Fold Higher than Endogenous Levels.
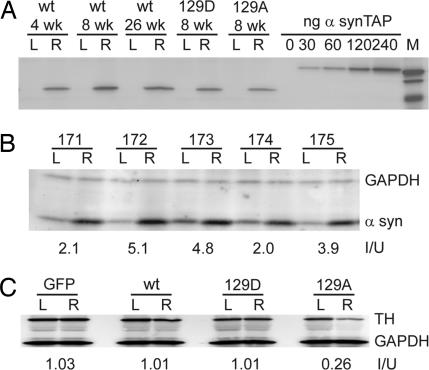
Quantitative Western blot analysis of α-syn expression was performed with an antibody that is specific for human α-syn in pooled striatal tissues obtained at 4, 8, and 26 wk after injection. The level of human α-syn expression in the striatum 4 wk after injection with wt α-syn vector was ≈140 ng/100 μg of cellular protein (Fig. 2A). This was ≈3.9-fold higher than values reported for endogenous α-syn in normal mice [36 ng/100 μg (20)]. As expected, no human α-syn expression was detected on the uninjected left side (Fig. 2A, L lanes). In addition, there was no significant difference between the levels of expression of human wt α-syn and the S129A and S129D mutants at 8 wk postinjection (140 and 130 ng/100 μg, respectively), and no significant difference in the expression of human wt α-syn at different times after injection when normalized to total protein (Fig. 2A).
Fig. 2.
Measurement of α-syn and TH expression in α-syn-injected animals. (A) Pooled samples of striatal extracts taken from the uninjected left side (L) and injected right side (R) were electrophoresed on acrylamide gels. Fifty micrograms of protein from animals injected with wt, 129A, and 129D at the indicated time points were Western blotted with an antibody specific for human α-syn and a fluorescent secondary antibody, and compared with purified samples of a human α-syn-TAP fusion protein of known concentration. The concentration of α-syn was calculated from the fluorescence measurements by using ImagQuant software. (B) SNc tissue was excised from uninjected (L) and injected (R) sides of individual animals (171–175) that had been injected with wt α-syn at 4 wk postinjection. Fifty micrograms of tissue extract was Western blotted with antibodies that recognize rat α-syn or GAPDH. The ratio of α-syn on the injected and uninjected sides (I/U) was calculated for each animal. (C) Pooled striatal tissue extracts from 4-wk animals was Western blotted with TH and GAPDH antibodies. The amount of TH enzyme was normalized to GAPDH and the ratio of the injected vs. uninjected sides was calculated (I/U). At 4 wk only the 129A samples showed a reduction in TH compared with total protein.
To get a more direct estimate of the increase in α-syn on the injected side, we used an anti-rat α-syn antibody that recognizes an epitope present in both endogenous rat and exogenous human α-syn. The ratio of α-syn on the injected to uninjected side was measured at 4 wk postinjection in SNc tissue of five individual animals injected with wt α-syn, when neurodegeneration was not expected to be significant. The ratio varied between 2 and 5 with an average increase in total α-syn expression of 3.6-fold (Fig. 2B).
Immunohistochemistry with an α-syn antibody that recognizes both rat and human α-syn also revealed an increase in α-syn expression on the injected side and a distribution of α-syn immunoreactivity (IR) in the SNc and surrounding brain regions similar to that seen with GFP (Fig. 3 A–C). We also note that the use of AAV5 produced uniform staining of the SNc with antibodies to α-syn or GFP (Figs. 1 and 3), suggesting that there was no major variation of expression across the injected region. We concluded that we had expressed significant amounts of human α-syn or its mutants in virtually all of the TH+ neurons of the SNc and surrounding regions. Taken together, the data suggested that α-syn levels increased ≈4-fold on the injected side because of expression of the human gene.
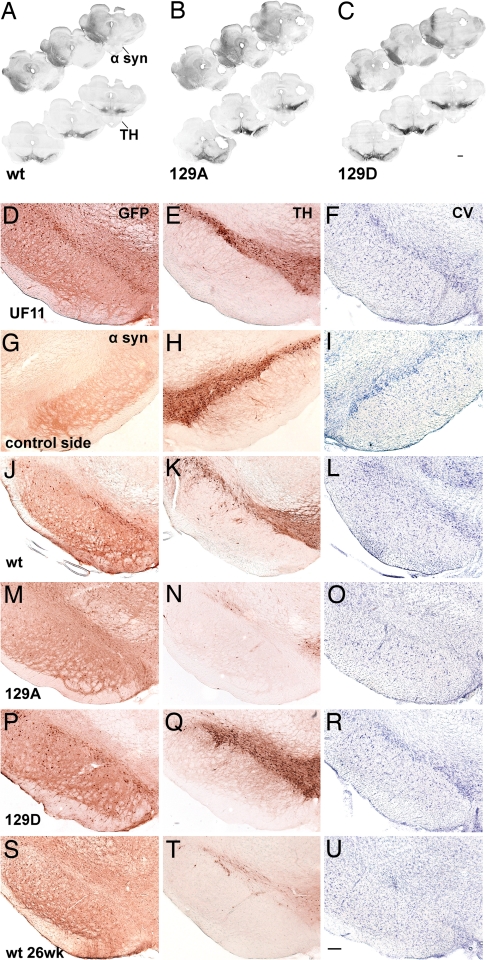
Fig. 3.
Photomicrographs showing nigral degeneration in the rAAV-α-syn-injected animals. (A–C) Montages of rostral-to-caudal coronal sections illustrate transduction volume in different experimental groups at 8 wk postinjection (α-syn-stained sections, Upper) and a reduction of TH immunoreactivity on the injected side of parallel sections (Lower). These sections were labeled with standard DAB immunohistochemistry (see Materials and Methods). (D–J) Each row contains neighboring sections, taken from the same animal, that illustrate cells immunostained for GFP (D), α-syn (G, J, M, P, S) and TH (E, H, K, N, Q, T), and CV-stained cells (F, I, L, O, R, U) in the SN at 8 (D–R) and 26 (S–U) wk postinjection. The sections were uninjected (G–I) or injected with UF11 (which expresses GFP, D–F), wt-α-syn (J–L, S–U), 129A (M–O), 129D (P–R). Whereas expression of GFP protein (D–F) or 129D (P–R) did not alter the number of TH-positive neurons or CV-stained neurons, the expression of 129A (M–O) or wt (J–L and S–U) α-syn led to loss of TH-positive neurons and CV staining in the SNc, compared with the contralateral intact side (G–I). (Scale bars: A–D, 1 mm; E–V, 250 μm.)
S129A Mutant Protein Is More Diffuse and Makes Fewer Inclusions than S129D.
Examination of sections stained with human α-syn antibody by confocal microscopy at 4 wk after injection identified a large number of α-syn-positive (α-syn+) aggregates in the cytoplasm (Fig. 1 B and C). α-syn+ aggregates were seen in both wt and mutant α-syn-injected animals. However, S129D, unlike wt and S129A, appeared to form punctate α-syn inclusions within the cytoplasm (compare Fig. 1 B and E with Fig. 1 D, F, and G). Additionally, at 8 wk postinjection S129A appeared to be present in the nucleus at a higher level than S129D. Furthermore, cells expressing S129A that survived at 8 wk often had vacuolated nuclei with reduced or absent chromatin density (compare Fig. 1E with Fig. 1 F and G). Examination of α-syn staining by light microscopy showed a similar diffuse pattern of staining in nigral tissue for wt and the S129A mutant. S129D, however, showed a more focused pattern of staining, suggesting that S129D might aggregate more readily to form intracellular inclusions (Fig. 3 A–C, compare nigral staining patterns of S129A and S129D). This was not in agreement with the data reported from the Drosophila PD model (17). As in previous studies, we observed α-syn+ cytoplasmic inclusions, swollen α-syn+ dystrophic neurites, and picnotic α-syn-immunoreactive cytoplasm (data not shown). These were present in wt and S129A-injected animals. None of these changes were found in GFP or the uninjected side of any of the animals and, with the exception of cytoplasmic inclusions, in S129D-expressing neurons.
S129A Mutant Is Toxic, S129D Is Not, and wt Is Intermediate.
Unbiased estimation of nigral TH-positive (TH+) cells in the vector-injected SNc was compared with the uninjected SNc for each rat. No significant differences were seen between animals injected with the control GFP vector at the 4-, 8-, and 26-wk time points (F[2,14] = 0.125, P = 0.883; data not shown). All GFP time points showed an ≈10% reduction of TH neurons compared with the uninjected side, and a pooled GFP control group that included all time points was used for further comparisons.
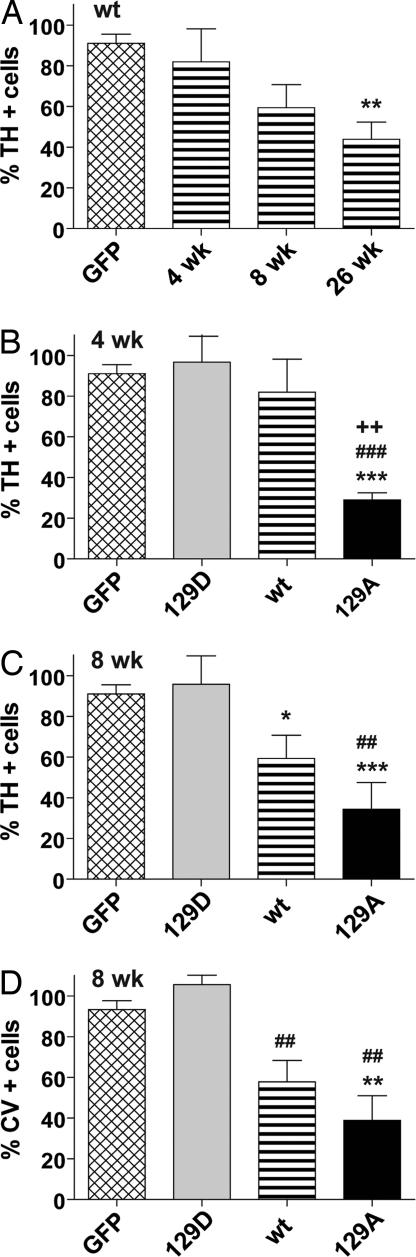
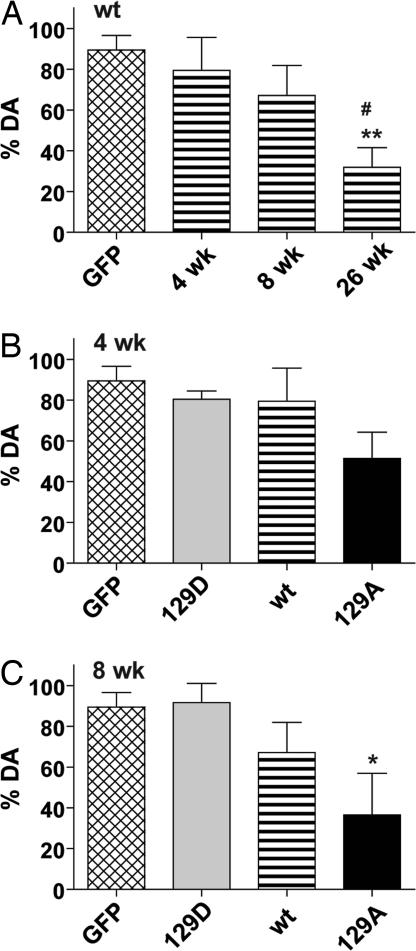
Animals injected with wt α-syn showed a progressive loss of TH+ neurons that reached significance only at the 26-wk time point (60% reduction) compared with the pooled GFP control group (Fig. 4A; P < 0.01). When wt α-syn was compared with GFP and the mutant α-syn genes at 4 wk postinjection, significant loss of TH neurons (70%) was seen only with the α-syn S129A mutant, which was significantly different from all other groups at 4 wk (Fig. 4B; P < 0.01–0.001). Typically, AAV-mediated gene expression reaches maximum expression between 2 and 4 wk, and, therefore, this suggested that the S129A mutant exerted a rapid effect on the level of TH expression and was unusually toxic.
Fig. 4.
Unbiased estimation of nigral TH+ cells in SNc of animals injected with wt or mutant human α-syn. Tissue slices were labeled with antibody to TH (A–C) or stained with cresyl violet (D) as described in Materials and Methods and the percentage of surviving cells was calculated by comparison with the uninjected side in the same animal. The graphs display a comparison of wt injected animals compared with GFP as a function of time postinjection (A), and a comparison of the wt, 129A, and 129D animals compared with GFP at 4 wk (B) and 8 wk (C) postinjection. The GFP control in A–C is the same and includes 4-, 8-, and 26-wk samples as these did not vary significantly. The wt 4-wk and 8-wk groups in B and C are the same as in A and are repeated to facilitate comparison. (D) Eight-week samples stained with cresyl violet. Group ANOVA statistics were for (A) F[3,34] = 5.973, P = 0.002; (B) F[3,32] = 13.53, P = 0.001; (C) F[3, 29] = 9.22, P = 0.0002; (D) F[3,15] = 9.431, P = 0.001. Tukey's post hoc results are indicated as *, **, *** = P < 0.05, 0.01, and 0.001, respectively vs. GFP; #, ##, ### = P < 0.05, 0.01, and 0.001 vs. 129D; ++ = P < 0.01 vs. wt 4 wk.
At the 8-wk time point, both wt and the S129A mutant were significantly different from GFP-injected animals (Fig. 4C) with significant differences between GFP and S129A (P < 0.001), GFP and wt α-syn (P < 0.05), and S129A and S129D (P < 0.01). In contrast, there was no significant difference between S129D and GFP at either the 8- or 4-wk time points (Fig. 4 B and C). Thus, S129D appeared to completely lack toxicity at any time point, whereas S129A showed 70–80% loss of TH+ neurons in the SNc as early as 4 wk postinjection.
The loss of TH+ neurons in wt α-syn injections at 8 wk was only 40% of GFP controls compared with an almost 70% loss seen with S129A (Fig. 4B). However, when wt-injected animals were analyzed at 26 wk, the loss of TH+ neurons had increased to 60% (Fig. 4B), comparable to what had been seen with S129A at 8 wk.
Unbiased stereology counts of cresyl violet (CV)-stained sections confirmed that significant loss of neurons had occurred in the SNc of wt α-syn- and S129A-injected animals compared with GFP and S129D animals at the 8-wk time point (Fig. 4D; P < 0.01). The percentage of CV-positive cells in both wt and mutant injected animals was nearly the same as that seen with TH immunohistochemistry (compare Fig. 4 C and D). Similar results were also seen when sections were stained for the vesicular monoamine transporter protein (data not shown).
The results of cell counting were confirmed by visual inspection of SNc sections stained with TH antibody or CV (Fig. 3). Animals injected with S129A showed little TH+ staining at 8 wk compared with uninjected or GFP-injected controls (compare Fig. 3N with Fig. 3 H and E). In contrast, S129D animals showed robust staining of the SNc for TH at 8 wk (compare Fig. 3Q with Fig. 3 H and E). Similar results were seen when CV staining was performed (Fig. 3 Right). Degeneration of TH+ cells in wt-injected animals were consistently intermediate between S129D and S129A at 8 wk and approached the level of degeneration seen with S129A only at 26 wk postinjection (compare Fig. 3 K, N, Q, and T)
Striatal DA Levels Mirror the Number of Surviving TH+ Cells.
Given the apparent loss of TH+ neurons in animals injected with wt or S129A α-syn, we anticipated that there would be a comparable decrease in DA levels on the injected side. DA was extracted from striatal tissues taken from injected and uninjected sides, fractionated by HPLC, and measured by using electrochemical detection.
In general, the levels of DA reflected the level of TH+ cells. Animals that had been injected with S129A or wt α-syn displayed the most severe reduction in DA pools compared with GFP. At 4 wk postinjection, only the S129A-reated animals demonstrated a trend toward greater DA loss (51% compared with a 70% reduction in TH+ cells). At 8 wk postinjection, the level of DA on the injected side compared with the uninjected side was in agreement with the level of TH+ cells that remained in that group. Thus, DA was depleted in S129A-injected animals by 63% (P < 0.05 compared with GFP), which was accompanied by ≈66% loss of TH+ cells (compare Figs. 4C and 5C). Similarly, wt-injected animals showed a DA depletion of ≈68% at 26 wk postinjection (P < 0.01 compared with GFP), similar to the 56% loss of TH+ cells. As expected, there also was no significant difference at 8 wk in DA levels in animals injected with S129D compared with the GFP control group. Finally, there also appeared to be a progressive depletion of DA in wt-injected animals between 8 and 26 wk that mirrored the progressive loss of TH+ cells at the same time points. Thus, the reduced level of DA agreed well with the number of surviving TH+ neurons.
Fig. 5.
Measurement of striatal dopamine. The amount of dopamine in striatal tissue was measured on the injected and uninjected sides of individual animals as described in Materials and Methods and is displayed as the mean percentage of dopamine remaining on the injected side compared with the uninjected side plus standard error. Tukey's post hoc results are indicated as in Fig. 4. Group ANOVA statistics were F[3,28] = 5.949, P = 0.0029 (A); F[3,33] = 2.867, P = 0.0513 (B); and F[3,24] = 4.079, P = 0.0179 (C).
Striatal TH Protein Levels Are Depressed in S129A Animals but Not in S129D Animals.
To see whether there were differences in the levels of TH protein in animals expressing the different forms of α-syn, immunoblotting was used to compare the amount of TH protein in striatal tissue on the injected vs. uninjected sides 4 wk postinjection. Striatal tissues were pooled from several animals in each experimental group, immunoblotted with TH antibody, and quantified by fluorescent phosphoimaging. GAPDH was used to normalize the amount of total protein used for analysis and the ratio of the two sides (injected/uninjected) was calculated (Fig. 2C). No difference in striatal TH levels was seen between uninjected and injected sides in animals that received the GFP vector or the S129D mutant. Also, in agreement with nigral TH+ neuronal numbers (Fig. 4B) and striatal DA levels (Fig. 5B), there was no neurodegenerative effect of nigral wt α-syn overexpression at 4 wk postinjection as measured by quantitative TH immunoblot (Fig. 2C). In contrast, animals injected with S129A showed significantly lower TH levels at 4 wk (Fig. 2C). The lower levels of TH protein in S129A at 4 wk is in agreement with the lower number of TH+ cells (Fig. 4B) in this group and the lower DA levels (Fig. 5B). Measurement of TH levels in individual animals from the 8- and 26-wk time points were also consistent with the level of TH+ cells and DA levels seen in these groups at the later time points (data not shown). For example, animals injected with wt α-syn had a ratio (injected/uninjected) of 0.64 at the 8-wk time point and 0.36 at the 26-wk time point.
Discussion
We have examined the toxicity of human α-syn that is phosphorylated at Ser-129 in a rodent animal model of PD. Overexpression of α-syn with a substitution of the serine residue with alanine, which cannot be phosphorylated, produced a rapid onset of toxicity characterized by a loss of TH+ neurons and depletion of DA on the affected side. Nigral overexpression with wt α-syn also produced toxicity, but its onset was slower, taking up to three times longer to produce a comparable loss of TH+ neurons in the SNc. In contrast, nigral overexpression of α-syn with a substitution of the Ser-129 residue with aspartate, which mimics constitutive phosphorylation, produced no obvious toxicity, including no loss of TH+ neurons and no change in striatal DA on the injected side. Because wt α-syn and the two mutant forms were overexpressed in the injected tissue to approximately the same levels (≈3–4 times endogenous levels), the toxicity of α-syn over the time scale studied appears to be caused exclusively by the state of phosphorylation of Ser-129. Curiously, the formation of α-syn inclusions was decreased in the S129A-expressing animals and S129A was more likely to be localized to the nucleus. We note, however, that these were primarily immunohistochemical observations and we did not quantitatively determine the amount of α-syn that was present in different kinds of aggregated species.
These results are opposite to those seen with the same genes in a transgenic Drosophila model of PD (17) and suggest that in rodents phosphorylation of Ser-129 may protect nigral DA neurons from neurodegeneration. Phosphorylated S129 was also found to accumulate in a transgenic mouse model expressing the A53T mutant of human α-syn (20). In this study, phosphorylation of endogenous α-syn was not detected but the human mutant α-syn was phosphorylated on Ser-129 and, in contrast to our study, seemed to preferentially localize to the nucleus. However, like our study, the accumulation of phosphorylated α-syn was not associated with toxicity (20).
Several other observations related to α-syn phosphorylation have been reported. One of the features of PD is the formation of detergent-insoluble aggregates called Lewy bodies that contain a high proportion of α-syn. Several groups have reported that Lewy bodies predominately consist of α-syn that is phosphorylated at Ser-129 (16, 18), whereas soluble, monomeric α-syn contains relatively little of the S129-phosphorylated form (15). In addition, coexpression of human α-syn with G protein-receptor kinase 5 (GRK5), one of several kinases that is capable of phosphorylating Ser-129 (14, 20), increased the formation of α-syn aggregates in cultured cells (21). In another study, coexpression of synphilin 1 with the S129A mutation of α-syn in cultured cells reduced aggregation and interaction with synphilin 1 (22), another component found in Lewy bodies. Finally, expression of S129D α-syn in cultured cells produced an increase of ubiquitinated α-syn conjugates and aggregate formation compared with wt α-syn (16, 23). Taken together, these observations and those presented here suggest that the phosphorylated form of α-syn is more prone to be aggregated, but that these aggregates are actually not toxic to dopaminergic neurons and may be neuroprotective. The concept that aggregation might be neuroprotective has been suggested before (1); however, the precise mechanism by which S129-phosphorylated α-syn might reduce neuronal toxicity in the substantia nigra is not clear.
The search for a mechanism is complicated by the fact that the normal function of α-syn is not known. α-syn has been implicated in a variety of cellular processes that include regulation of DA homeostasis through regulation of DA transporter activity (8), tyrosine hydroxylase activity (11), or vesicle formation (24). α-syn has also been implicated in ER-Golgi trafficking (25), tubulin trafficking (26), interaction with mitochondrial proteins (27), and control of nuclear transcription (12, 28). Disruption of any of these processes through expression of a nonphosphorylated mimic could, in principle, lead to cell death.
Finally, it is also not clear which cellular kinases and phosphatases control the steady-state level of S129 phosphorylation. Casein kinase 1 and 2 and GRK2 and 5 are all capable of phosphorylating S129 (14, 15, 17, 20, 21), and no specific phosphatase has yet been identified. Given the multiple cellular locations of α-syn, it is possible that several kinases are functional in vivo. However, identification of the specific kinases and phosphatases that are involved will potentially provide new drug targets for treating PD.
Materials and Methods
rAAV Vectors.
The rAAV vector expressing the wt human α-syn was described in ref. 3. α-syn 129A and 129D mutants were constructed by overlap extension PCR by using rAAV-wt α-synuclein plasmid as a template. Primer sequences are available on request.
Virus was grown and purified as described in ref. 19 by using a helper plasmid expressing the AAV5 capsid gene. The final titers were 1.0 × 1013, 1.7 × 1013, and 0.9 × 1013 vector genome (vg) per ml for the S129A, S129D, and wt α-syn virus stocks, respectively.
Intracerebral Injection of AAV Vectors.
All surgical procedures were performed by using aseptic techniques and isofluorane gas anesthesia as described in ref. 3. Animals were injected with a total of 1.5 μl containing 1.4 × 1010 vector genomes of the appropriate gene.
Isolation and Processing of Tissues.
Animals were deeply anesthetized by pentobarbital injection. Brains were removed and divided into two parts by a coronal blade cut at approximately −3.5 mm behind bregma. The caudal part containing the SNc was fixed in the ice-cold 4% paraformaldehyde in 0.1 M phosphate buffer (PB), pH 7.4. The rostral piece of brain tissue was used immediately to dissect the right and left striatum. The striatum from each hemisphere was homogenized and separated into two separate tubes. The tissue pieces were weighed, frozen separately on dry ice, and kept at −80°C until assayed for protein expression or DA content. The fixed part of brains were stored overnight at 4°C and then transferred into 30% sucrose in 0.1 M PB for cryoprotection. Coronal sections (40 μm thick) were cut on a freezing stage sliding microtome and processed for immunohistochemistry.
Immunohistochemistry.
For the bright-field microscopy analysis, sections were preincubated first with 1% H2O2/10% methanol for 15 min and then with 5% normal goat serum for 1 h. Sections were incubated overnight at room temperature with mouse anti-TH (Chemicon, 1:2,000 dilution) or mouse anti-α-syn (BD Laboratories) antibodies. Incubation with biotinylated secondary anti-mouse antibody was followed by incubation with avidin–biotin–peroxidase complex (ABC; Vector Laboratories). Reactions were visualized by using 3,3-diaminobenzidine (DAB) as a chromagen. All manipulations of contrast and illumination on color images and color replacement were made by using Adobe PhotoShop CS software.
For confocal microscopy, sections were incubated with the indicated primary antibodies for human α-syn, TH, vesicular monoamine transporter (VMAT), or DAPI and a secondary antibody labeled with Cy3 or Cy2 (Jackson Immunoresearch Laboratories). The sections were examined with a laser-scanning confocal microscope and images were processed with Adobe Photoshop 9 software.
Unbiased Stereology.
The unbiased stereological estimation of the total number of the TH+ neurons in SNc was performed by using the optical fractionator method, as described in ref. 3, with the MicroBrightfield Stereo Investigator System. The estimate of the total number of neurons and coefficient of error due to the estimation was calculated according to the optical fractionator formula as described in ref. 19. Because α-syn expression might down-regulate TH expression and lead to a mistaken sense of cell loss, we also evaluated the number of neurons in the SNc by using sections stained with cresyl violet.
Immunoblotting.
Tissues were suspended in 300 μl of lysis buffer (50 mM Tris, pH 7.5, 0.15 M NaCl) containing protease mixture (0.1 mM PMSF, 0.5 μg/ml leupeptin, 0.7 μg/ml pepstatin A) (Roche) and homogenized for 10 s. Each aliquot was adjusted to a final concentration of 1% Nonidet P-40, 0.1% SDS, incubated on ice for 30 min, and centrifuged for 15 min at 4°C. Lysates from each group were pooled (n = 3–5 per group), and protein concentrations were determined by the Bradford protein assay. Fifty micrograms of each protein pool was separated on Bio-Rad precast 4–20% SDS/PAGE gradient gel, transferred to PVDF-LFP (Amersham) membranes, and immunoblotted. In some cases lysates from single animals were also analyzed. Mouse anti-α-syn (BD Transduction Laboratories and Zymed Laboratories), mouse anti-GAPDH, and goat anti-TH (Chemicon) were used as recommended by the supplier. Cy5- and cy3-conjugated goat anti-mouse and rabbit anti-goat secondary antibodies were purchased from GE Bioscience. Immunblotted α-syn was detected and quantified with a Typhoon scanner (Amersham) by using purified α-syn-TAP protein as a standard. Western blots used for TH quantitation included GAPDH for normalization.
Striatal DA Measurements.
DA samples were thawed and the equivalent of 3 mg of starting tissue was diluted into 1 ml of 0.1 N HClO4 containing dihydrobenzylamine as an internal control, and thoroughly homogenized. DA and 3, 4-dihydroxyphenylacetic acid (DOPAC) were measured as described in ref. 3.
Statistical Analysis.
Dopamine measurements and estimates of surviving neurons were analyzed by using one-way ANOVA with Tukey's post hoc analysis; n = 4–10 per group.
ACKNOWLEDGMENTS.
We thank Craig Meyer for able technical assistance. This work was supported by National Institutes of Health Program Project Grant PO1 NS36302 (to N.M.).
Footnotes
Conflict of interest statement: N.M. is an inventor of patents related to recombinant AAV technology and owns equity in a gene therapy company that is commercializing AAV for gene therapy applications.
References
- 1.Cookson MR. The biochemistry of Parkinson's disease. Annu Rev Biochem. 2005;74:29–52. doi: 10.1146/annurev.biochem.74.082803.133400. [DOI] [PubMed] [Google Scholar]
- 2.Eslamboli A, et al. Long-term consequences of human alpha-synuclein overexpression in the primate ventral midbrain. Brain. 2007;130:799–815. doi: 10.1093/brain/awl382. [DOI] [PubMed] [Google Scholar]
- 3.Kirik D, et al. Parkinson-like neurodegeneration induced by targeted overexpression of alpha-synuclein in the nigrostriatal system. J Neurosci. 2002;22:2780–2791. doi: 10.1523/JNEUROSCI.22-07-02780.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Periquet M, Fulga T, Myllykangas L, Schlossmacher MG, Feany MB. Aggregated alpha-synuclein mediates dopaminergic neurotoxicity in vivo. J Neurosci. 2007;27:3338–3346. doi: 10.1523/JNEUROSCI.0285-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fredenburg RA, et al. The impact of the E46K mutation on the properties of alpha-synuclein in its monomeric and oligomeric states. Biochemistry. 2007;46:7107–7118. doi: 10.1021/bi7000246. [DOI] [PubMed] [Google Scholar]
- 6.Abeliovich A, et al. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- 7.Murphy DD, Rueter SM, Trojanowski JQ, Lee VM. Synucleins are developmentally expressed, and alpha-synuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. J Neurosci. 2000;20:3214–3220. doi: 10.1523/JNEUROSCI.20-09-03214.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wersingerand C, Sidhu A. Attenuation of dopamine transporter activity by alpha-synuclein. Neurosci Lett. 2003;340:189–192. doi: 10.1016/s0304-3940(03)00097-1. [DOI] [PubMed] [Google Scholar]
- 9.Chandra S, Gallardo G, Fernandez-Chacon R, Schluterand OM, Sudhof TC. Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell. 2005;123:383–396. doi: 10.1016/j.cell.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 10.Yavich L, Tanila H, Vepsalainen S, Jakala P. Role of alpha-synuclein in presynaptic dopamine recruitment. J Neurosci. 2004;24:11165–11170. doi: 10.1523/JNEUROSCI.2559-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng X, Tehranian R, Dietrich P, Stefanis L, Perez RG. Alpha-synuclein activation of protein phosphatase 2A reduces tyrosine hydroxylase phosphorylation in dopaminergic cells. J Cell Sci. 2005;118:3523–3530. doi: 10.1242/jcs.02481. [DOI] [PubMed] [Google Scholar]
- 12.Kontopoulos E, Parvin JD, Feany MB. Alpha-synuclein acts in the nucleus to inhibit histone acetylation and promote neurotoxicity. Hum Mol Genet. 2006;15:3012–3023. doi: 10.1093/hmg/ddl243. [DOI] [PubMed] [Google Scholar]
- 13.Iwata A, MaruyamaI M, Kanazawa, Nukina N. Alpha-synuclein affects the MAPK pathway and accelerates cell death. J Biol Chem. 2001;276:45320–45329. doi: 10.1074/jbc.M103736200. [DOI] [PubMed] [Google Scholar]
- 14.Pronin AN, Morris AJ, Surguchov A, Benovic JL. Synucleins are a novel class of substrates for G protein-coupled receptor kinases. J Biol Chem. 2000;275:26515–26522. doi: 10.1074/jbc.M003542200. [DOI] [PubMed] [Google Scholar]
- 15.Okochi M, et al. Constitutive phosphorylation of the Parkinson's disease associated alpha-synuclein. J Biol Chem. 2000;275:390–397. doi: 10.1074/jbc.275.1.390. [DOI] [PubMed] [Google Scholar]
- 16.Anderson JP, et al. Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. J Biol Chem. 2006;281:29739–29752. doi: 10.1074/jbc.M600933200. [DOI] [PubMed] [Google Scholar]
- 17.Chenand L, Feany MB. Alpha-synuclein phosphorylation controls neurotoxicity and inclusion formation in a Drosophila model of Parkinson disease. Nat Neurosci. 2005;8:657–663. doi: 10.1038/nn1443. [DOI] [PubMed] [Google Scholar]
- 18.Fujiwara H. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;4:160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- 19.Burger C, et al. Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol Ther. 2004;10:302–317. doi: 10.1016/j.ymthe.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 20.Wakamatsu M, et al. Accumulation of phosphorylated alpha-synuclein in dopaminergic neurons of transgenic mice that express human alpha-synuclein. J Neurosci Res. 2007;85:1819–1825. doi: 10.1002/jnr.21310. [DOI] [PubMed] [Google Scholar]
- 21.Arawaka S, et al. The role of G-protein-coupled receptor kinase 5 in pathogenesis of sporadic Parkinson's disease. J Neurosci. 2006;26:9227–9238. doi: 10.1523/JNEUROSCI.0341-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cappai R. Dopamine promotes alpha-synuclein aggregation into SDS-resistant soluble oligomers via a distinct folding pathway. FASEB J. 2005;19:1377–1379. doi: 10.1096/fj.04-3437fje. [DOI] [PubMed] [Google Scholar]
- 23.Liu C. Assembly of lysine 63-linked ubiquitin conjugates by phosphorylated alpha-synuclein implies Lewy body biogenesis. J Biol Chem. 2007;282:14558–14566. doi: 10.1074/jbc.M700422200. [DOI] [PubMed] [Google Scholar]
- 24.Lotharius J, et al. Effect of mutant alpha-synuclein on dopamine homeostasis in a new human mesencephalic cell line. J Biol Chem. 2002;277:38884–38894. doi: 10.1074/jbc.M205518200. [DOI] [PubMed] [Google Scholar]
- 25.Cooper AA, et al. Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson's models. Science. 2006;313:324–328. doi: 10.1126/science.1129462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee HJ, Khoshaghideh F, Lee S, Lee SJ. Impairment of microtubule-dependent trafficking by overexpression of alpha-synuclein. Eur J Neurosci. 2006;24:3153–3162. doi: 10.1111/j.1460-9568.2006.05210.x. [DOI] [PubMed] [Google Scholar]
- 27.Martin LJ, et al. Parkinson's disease alpha-synuclein transgenic mice develop neuronal mitochondrial degeneration and cell death. J Neurosci. 2006;26:41–50. doi: 10.1523/JNEUROSCI.4308-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwata A, MiuraI S, Kanazawa, Sawada M, Nukina N. Alpha-synuclein forms a complex with transcription factor Elk-1. J Neurochem. 2001;77:239–252. doi: 10.1046/j.1471-4159.2001.t01-1-00232.x. [DOI] [PubMed] [Google Scholar]